Abstract
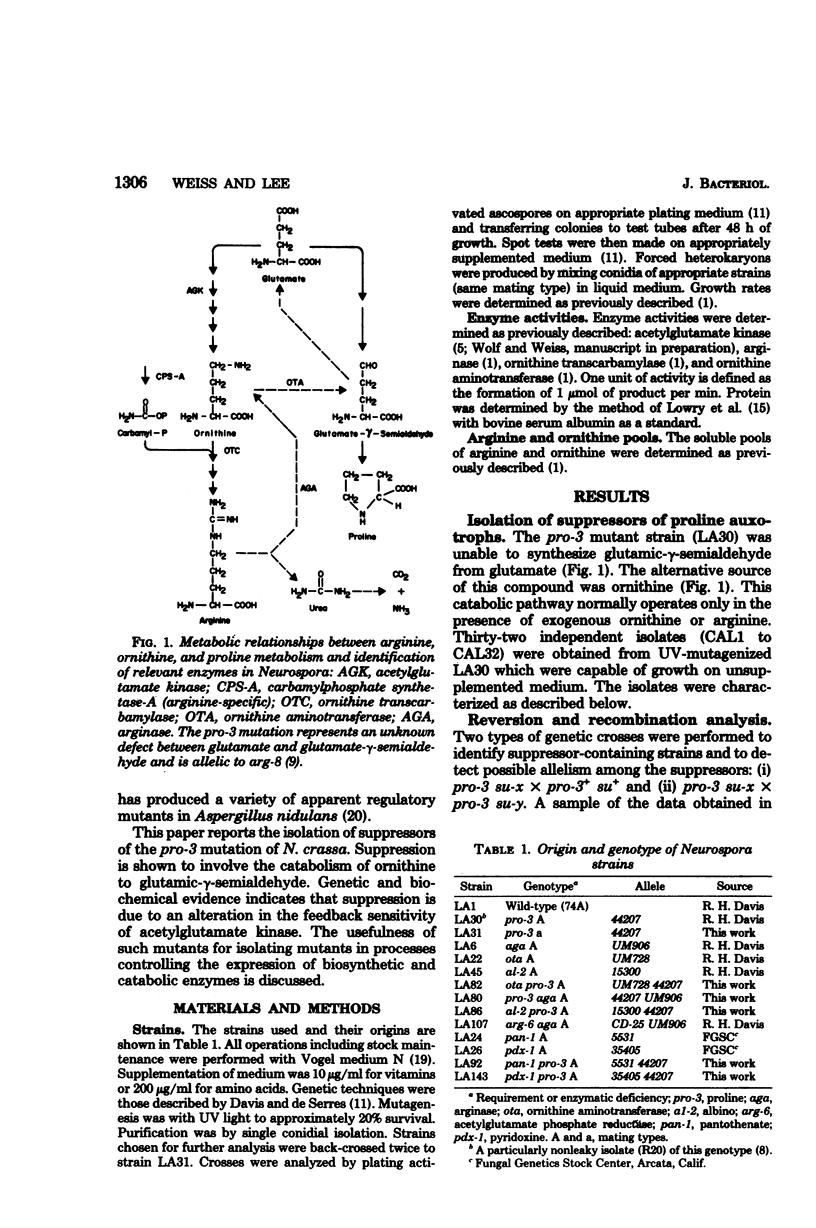
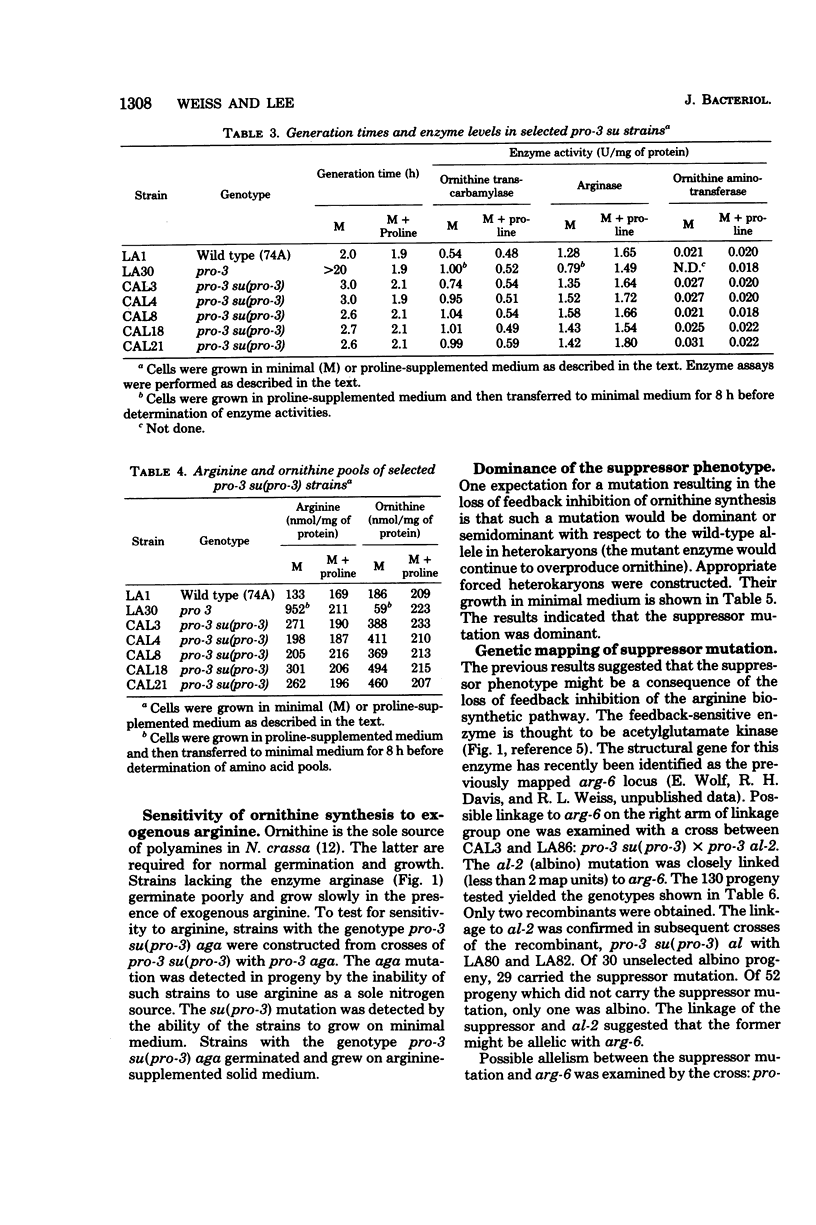
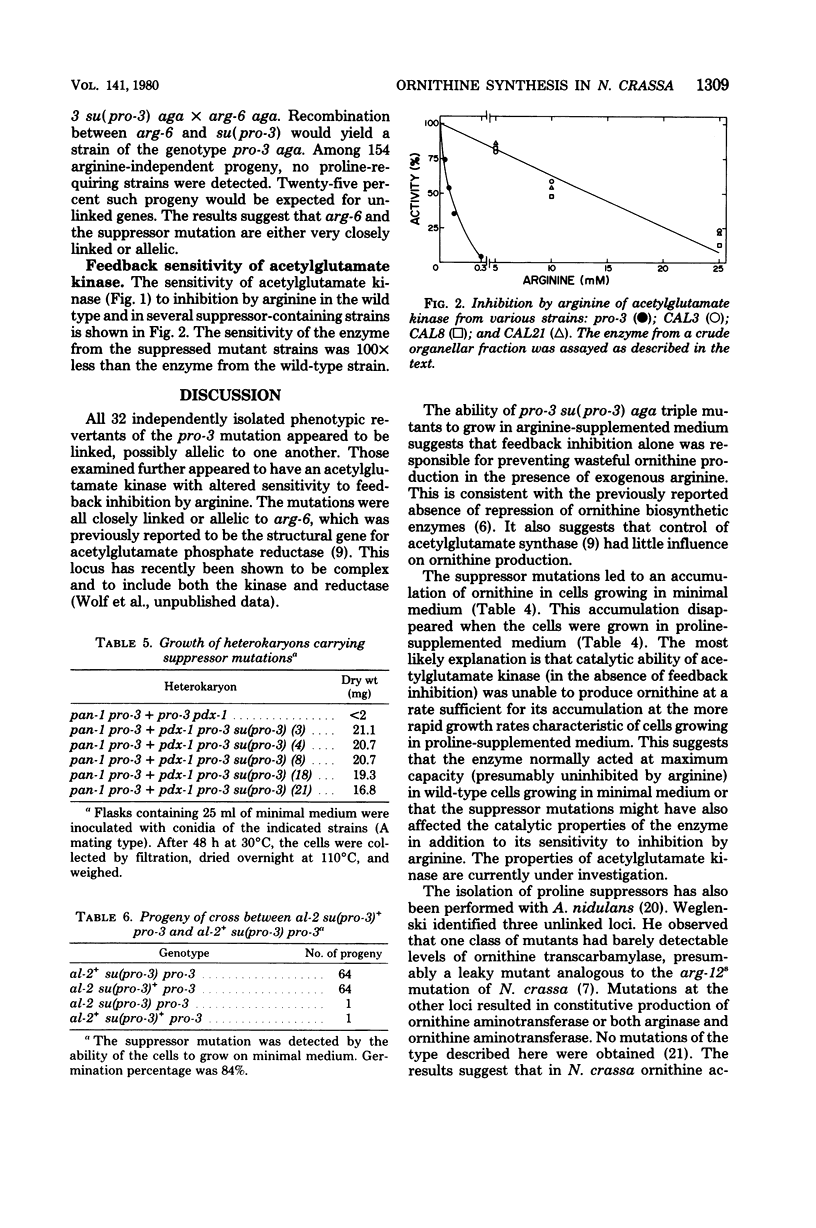
Thirty-two independent mutants were isolated which overcame the proline requirement of pro-3 mutations in Neurospora crassa. The mutations were not revertants, appeared to be allelic, were closely linked or allelic to arg-6, and in strains unable to degrade ornithine no longer suppressed the proline requirement. The suppressor mutations did not alter the levels of biosynthetic or catabolic enzymes, yet allowed accumulation of ornithine. Suppressed strains unable to degrade arginine still produced ornithine (as detected by growth) in arginine-supplemented medium. The results suggest that the suppressor mutants were impaired in the feedback inhibition of ornithine synthesis by arginine. The activity of the appropriate biosynthetic enzyme was less sensitive to inhibition by arginine. The potential usefulness of such mutations is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basabe J. R., Lee C. A., Weiss R. L. Enzyme assays using permeabilized cells of Neurospora. Anal Biochem. 1979 Jan 15;92(2):356–360. doi: 10.1016/0003-2697(79)90670-5. [DOI] [PubMed] [Google Scholar]
- Bechet J., Greenson M., Wiame J. M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):31–39. doi: 10.1111/j.1432-1033.1970.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Wesseling A. C. Cross-pathway regulation: histidine-mediated control of histidine, tryptophan, and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):893–898. doi: 10.1128/jb.119.3.893-898.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybis J. J., Davis R. H. Acetylglutamate kinase: a feedback-sensitive enzyme of arginine biosynthesis in Neurospora. Biochem Biophys Res Commun. 1974 Sep 23;60(2):629–634. doi: 10.1016/0006-291x(74)90287-3. [DOI] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Bowman B. J., Weiss R. L. Intracellular compartmentation and transport of metabolites. J Supramol Struct. 1978;9(4):473–488. doi: 10.1002/jss.400090403. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Compartmentation and regulation of fungal metabolism: genetic approaches. Annu Rev Genet. 1975;9:39–65. doi: 10.1146/annurev.ge.09.120175.000351. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Utilization of exogenous and endogenous ornithine by Neurospora crassa. J Bacteriol. 1968 Aug;96(2):389–395. doi: 10.1128/jb.96.2.389-395.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux J. C., Urrestarazu L. A., Wiame J. M. Arginine metabolism in Saccharomyces cerevisiae: subcellular localization of the enzymes. J Bacteriol. 1978 Mar;133(3):1096–1107. doi: 10.1128/jb.133.3.1096-1107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martinoia E., Heck U., Boller T., Wiemken A., Matile P. Some properties of vacuoles isolated from Neurospora crassa slime variant. Arch Microbiol. 1979 Jan 16;120(1):31–34. doi: 10.1007/BF00413268. [DOI] [PubMed] [Google Scholar]
- Messenguy F. Regulation of arginine biosynthesis in Saccharomyces cerevisiae: isolation of a cis-dominant, constitutive mutant for ornithine carbamoyltransferase synthesis. J Bacteriol. 1976 Oct;128(1):49–55. doi: 10.1128/jb.128.1.49-55.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca G., Mora J. Nitrogen regulation of arginase in Neurospora crassa. J Bacteriol. 1977 Sep;131(3):719–725. doi: 10.1128/jb.131.3.719-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weglenski P. Genetical analysis of proline mutants and their suppressors in Aspergillus nidulans. Genet Res. 1966 Dec;8(3):311–321. doi: 10.1017/s001667230001017x. [DOI] [PubMed] [Google Scholar]
- Weglenski P. The mechanism of action of proline suppressors in Aspergillus nidulans. J Gen Microbiol. 1967 Apr;47(1):77–85. doi: 10.1099/00221287-47-1-77. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Control of arginine utilization in Neurospora. J Bacteriol. 1977 Feb;129(2):866–873. doi: 10.1128/jb.129.2.866-873.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]


