The group of Dr. Jon A. Wolff reports results from a study examining the effects of a variety of parameters, such as rate and volume of injection, on the efficiency of hydrodynamic limb vein injection mediated gene transfer of luciferase in rats and nonhuman primates.
Abstract
The administration route is emerging as a critical aspect of nonviral and viral vector delivery to muscle, so as to enable gene therapy for disorders such as muscular dystrophy. Although direct intramuscular routes were used initially, intravascular routes are garnering interest because of their ability to target multiple muscles at once and to increase the efficiency of delivery and expression. For the delivery of naked plasmid DNA, our group has developed a hydrodynamic, limb vein procedure that entails placing a tourniquet over the proximal part of the target limb to block all blood flow and injecting the gene vector rapidly in a large volume so as to enable the gene vector to be extravasated and to access the myofibers. The present study was conducted in part to optimize the procedure in preparation for a human clinical study. Various injection parameters such as the effect of papaverine preinjection, tourniquet inflation pressure and duration, and rate of injection were evaluated in rats and nonhuman primates. In addition, the safety of the procedure was further established by determining the effect of the procedure on the neuromuscular and vascular systems. The results from these studies provide additional evidence that the procedure is well tolerated and they provide a foundation on which to formulate the procedure for a human clinical study.
Introduction
Muscle is an important target tissue for gene and other nucleic acid-based therapies, given its central role in muscular dystrophy and its ability to secrete therapeutic proteins for other disorders (Herweijer and Wolff, 2007). Accordingly, various delivery methods and vehicles to deliver genes and other nucleic acids to muscle are being developed and used in clinical trials. In addition to the type of delivery vehicle, the administration route is emerging as a critical aspect.
Early approaches used the direct, intramuscular route, in which the vector is injected via a syringe and needle directly into the muscle (Wolff et al., 1990). This route was first used with plasmid DNA (pDNA) and subsequently with other nonviral and viral vectors such as retroviral, adenoviral, and adeno-associated viral vectors (Davis et al., 1993; Ragot et al., 1993; Kessler et al., 1996; Fisher et al., 1997; Snyder et al., 1997; Monahan et al., 1998; Braun, 2008). However, for disorders such as muscular dystrophy that affect multiple muscles, each individual muscle would have to be injected. Furthermore, large muscles would require multiple injections. This is because muscle has a strong connective tissue that limits dispersion of the vector. The epimysium, a sheath that encases each muscle, is impermeable to the movement of vector between muscles. Within a muscle, the perimysium is a somewhat more permeable connective tissue sheath that surrounds fascicles (bundles) of 10–100 myofibers. Although the perimysium itself directly limits the contact of vectors with myofibers, another restricting element is the potential space between fascicles. It enlarges during intramuscular injection and serves as a sink for the injected vector (Wolff et al., 1992). Finally, the endomysium, the layer of connective tissue that surrounds each myofiber, is the most permeable enclosure but still provides another potential impediment to the vector contacting the myofiber.
An alternative approach to deliver gene vectors is to take advantage of muscle's rich vascular supply, as each myofiber is in close contact with capillaries (Lee and Schmid-Schonbein, 1995; Browning et al., 1996). However, muscle capillaries have a continuous, nonfenestrated endothelium with low permeability. Using pharmacologic agents and increased intravascular pressure, we first showed using an intraarterial route that naked pDNA could be efficiently expressed throughout the limb muscles of rodents and nonhuman primates (Zhang et al., 2001). Subsequently, we showed that an intravenous route also enabled high levels of foreign gene expression and small interfering RNA (siRNA) delivery (Hagstrom et al., 2004; Wells, 2004). The intravenous procedure, termed hydrodynamic limb vein (HLV) injection, is performed under conditions similar to those used for the Bier block procedure that is commonly used clinically for limb anesthesia during orthopedic surgery. The HLV procedure entails placing a tourniquet over the proximal part of the limb to block all blood flow and injecting the gene vector rapidly in a large volume so as to enable the gene vector to be extravasated and access the myofibers (Hagstrom et al., 2004). Presumably, the procedure enables egress of the pDNA by transiently disrupting the interendothelial cell junctions within the muscle vasculature (Vigen et al., 2007). Other groups have also reported the successful use of similar intraarterial and intravenous procedures to deliver plasmid DNA, adenoviral vectors, and adeno-associated viral vectors (Cho et al., 2000; Liang et al., 2004; Danialou et al., 2005; Su et al., 2005; Rodino-Klapac et al., 2007; Qiao et al., 2008; Suda et al., 2008; Toromanoff et al., 2008; Gregorevic et al., 2009). An advantage of the HLV procedure over the intraarterial procedure is that the HLV procedure is not as sensitive to the injection volume. With the intraarterial procedure in rats, lower volumes of injection solution resulted in much reduced expression from the injected pDNA. With HLV injection, however, volumes in the same range were similarly efficient (Budker et al., 1998; Hagstrom et al., 2004; Sebestyén et al., 2007). High levels of pDNA expression are obtained with the HLV procedure in nonhuman primates when the injection volume is sufficient to achieve the swelling necessary for efficient delivery to all limb muscles (Vigen et al., 2007).
In addition to efficiency of gene expression, safety is an important issue. Histological studies in rodents and nonhuman primates have shown that the HLV procedure caused transient muscle edema but no significant muscle damage (Hagstrom et al., 2004; Toumi et al., 2006). T2-weighted, magnetic resonance images in nonhuman primates also showed that the procedure caused transient muscle edema but no persistent muscle derangement such as a compartment syndrome (Vigen et al., 2007). Magnetic resonance angiography in nonhuman primates revealed vascular effects consistent with a transient effect on capillary permeability but no long-term abnormalities of concern (Vigen et al., 2007).
The present study was conducted in part to optimize the procedure in preparation for a human clinical study. Various injection parameters such as the effect of papaverine preinjection, tourniquet inflation pressure and duration, and rate of injection were evaluated. Given the larger number of rats that can be employed in a specific study, when possible the injection parameter was first studied in rats and then a few monkeys. Additional studies in monkeys were conducted to further address safety concerns with particular attention to the effect of the procedure on limb neuromuscular and vascular systems. The results from these studies provide a good basis for formulating the procedure for a human clinical study.
Materials and Methods
Animal studies
All procedures were carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals and were approved by the University of Wisconsin-Madison (Madison, WI), Mirus Bio (Madison, WI), and/or Roche Madison (Madison, WI) Animal Care and Use Committees. Optimization studies were performed in both rats and primates. The injection procedure was first tested and optimized in groups of rats and then repeated in a small number of rhesus monkeys. Studies to evaluate clinical safety were performed in rhesus monkeys. Details of the primate injections are shown in Table 1.
Table 1.
Primate Injection Conditions
| Study name | Age (years) | Weight (kg) | Limb | Inj. vol. (ml) | Inj. vol./ limb vol. | Tourniquet pressure (mmHg) | pDNA dose (mg) | Health problemsa |
|---|---|---|---|---|---|---|---|---|
| Papaverine study | 34 | 11.5 | Arm | 84 | 0.35 | 700 | 5 | None |
| Leg | 133 | 0.35 | 5 | None | ||||
| Arm | 84 | 0.35 | 5 | None | ||||
| Leg | 133 | 0.35 | 5 | None | ||||
| Tourniquet pressure study | 22 | 11.0 | Arm | 105 | 0.35 | 475 | 10 | None |
| Leg | 130 | 0.30 | 700 | 10 | None | |||
| Arm | 105 | 0.35 | 700 | 10 | None | |||
| Leg | 130 | 0.30 | 475 | 10 | None | |||
| Tourniquet pressure study | 6 | 9.2 | Arm | 124 | 0.35 | 700 | 10 | None |
| Leg | 142 | 0.35 | 475 | 10 | None | |||
| Arm | 124 | 0.35 | 700 | 10 | None | |||
| Leg | 142 | 0.35 | 475 | 10 | None | |||
| Injection rate study (0.5 ml/sec) | 2.5 | 4.0 | Arm | 63 | 0.45 | 450 | 11 | None |
| Leg | 90 | 0.45 | 16 | None | ||||
| Arm | 63 | 0.45 | 11 | None | ||||
| Leg | 90 | 0.45 | 16 | None | ||||
| Injection rate study (4.0 ml/sec) | 2.5 | 4.6 | Arm | 74 | 0.45 | 450 | 13 | None |
| Leg | 112 | 0.45 | 20 | None | ||||
| Arm | 74 | 0.45 | 13 | None | ||||
| Leg | 112 | 0.45 | 20 | None | ||||
| Tourniquet time study | 3 | 4.2 | Arm | 55 | 0.45 | 450 | 10 | None |
| Leg | 86 | 0.45 | 15 | None | ||||
| Arm | 55 | 0.45 | 10 | None | ||||
| Leg | 86 | 0.45 | 15 | None | ||||
| Tourniquet time study | 20 | 8.3 | Arm | 125 | 0.45 | 450 | 22 | None |
| Leg | 180 | 0.45 | 32 | None | ||||
| Arm | 125 | 0.45 | 22 | None | ||||
| Leg | 180 | 0.45 | 32 | None | ||||
| Exsanguination study | 20 | 6.5 | Arm | 113 | 0.45 | 450 | 21 | None |
| Leg | 130 | 0.45 | 24 | None | ||||
| Arm | 117 | 0.45 | 21 | None | ||||
| Laser Doppler studies | 3.5 | 6.5 | Arm | 85 | 0.45 | 450 | None | None |
| 3 | 4.0 | Arm | 56 | 0.45 | None | None | ||
| 2 | 3.3 | Arm | 45 | 0.40 | None | None | ||
| NCS-EMG studies | 5 | 7.2 | Arm | 96 | 0.40 | 450 | None | None |
| 4 | 6.5 | Arm | 72 | 0.40 | None | None | ||
| FMS studies | 4 | 6.5 | Arm | 90 | 0.45 | 450 | None | None |
| 4 | 7.0 | Arm | 90 | 0.45 | None | None | ||
| 5.5 | 7.0 | Arm | 99 | 0.45 | None | None | ||
| 5.5 | 8.5 | Arm | 120 | 0.45 | None | None |
Abbreviations: FMS, fine motor skill; Inj. vol., injection volume; NCS-EMG, nerve conduction study-electromyography; pDNA, plasmid DNA.
Post procedure health problems were defined as changes in overall activity, changes in the use of the injected limb, and any unresolved limb swelling or bruising.
Plasmid DNA solutions
The pCMV-Luc plasmid contains the human major immediate-early cytomegalovirus (CMV) enhancer/promoter; a chimeric human intron with a human β-globin 5′ splice site and immunoglobulin gene heavy chain variable region branch point, and a 3′ splice acceptor site optimized to consensus sequences for splicing in the 5′ untranslated region of the gene; a codon-optimized luciferase gene (Promega, Madison, WI); the late simian virus 40 (SV40) polyadenylation signal; the Tn5 promoter driving expression of the Tn5-based kanamycin resistance gene; and the ColE1 origin of replication.
All pDNA injection solutions were prepared in normal saline solution. The pDNA dose for all rat injections was 250 μg. For the rhesus monkey injections, the pDNA dose varied depending on the study and the size of the animal's limbs. Doses were less than the amount required for maximal expression so that differences in efficiency could be measured. For the safety studies that were performed in rhesus monkeys, the injection solution contained only saline so that the procedure could be evaluated without effects of pDNA. The injection solutions were at ambient temperature. In an unpublished study in rats we found that reporter gene expression was the same whether pDNA was delivered at ambient temperature or at 37°C.
pDNA delivery into rat hind limbs
The procedure for hydrodynamic limb vein injections in rats was previously described (Hagstrom et al., 2004). Briefly, female Sprague Dawley rats weighing 125 to 160 g (Harlan, Indianapolis, IN) were used for these studies. Animals were anesthetized with isoflurane (2–3%) and one hind limb was shaved and aseptically prepared for surgery. A latex tourniquet was wrapped tightly around the upper limb to occlude blood flow and secured in place with a hemostat clamp. The distal great saphenous vein was exposed through a small incision, a 25-gauge needle catheter was inserted into the vein, and a programmable syringe pump (model PHD 2000; Harvard Apparatus, Holliston, MA) was used to inject the pDNA solution at a rate of 10 ml/min. The needle was held in place and visualized during the injection to prevent the tip from puncturing the vein or moving during injection. After the injection, the tourniquet and catheter were removed, the incision was closed with suture, and each animal was given ketoprofen (5 mg/kg, intramuscular) for analgesia. Unless stated otherwise, the injection volume was 3.0 ml and the tourniquet was left on for 2 min after the injection. Rats were killed 5 or 7 days postinjection, all hind limb muscles were harvested, and luciferase activity was measured.
HLV delivery into primate limbs
Male rhesus monkeys weighing 3.3 to 11.5 kg and ranging in age from 2 to 34 years old were used for these studies (Table 1). Most animals had all four limbs injected during two procedures. One arm and one leg were injected on day 0 and the opposite arm and leg were injected on day 3. Anesthesia was induced with ketamine HCl (15 mg/kg, intramuscular) or alternatively a combination of ketamine HCl (7 mg/kg, intramuscular) and medetomidine (0.03 mg/kg, intramuscular) and then maintained with 2–3% isoflurane. Once anesthetized, the limbs to be injected were shaved and the animal was placed on a water-jacketed heating pad. The limb injection volume was determined by measuring the limb volume (area distal to the tourniquet) by fluid displacement and multiplying this volume by a number between 0.30 and 0.45, depending on the study. A 20-gauge (length, 3.8 cm) intravenous catheter was inserted into the distal cephalic (arm) or small saphenous vein (leg). The venipuncture site was located on the surface of the foot or hand or at the ankle or wrist. The catheter was flushed with saline, secured with tape, and connected to a syringe pump (OptiVantage DH injector; Mallinckrodt, Cincinnati, OH) that was preloaded with the pDNA injection solution. A pediatric-size tourniquet cuff (Delfi Medical Innovations, Vancouver, BC, Canada) was applied snugly to the limb just above the elbow or knee. Unless stated otherwise, the pDNA saline solution was injected immediately after tourniquet inflation to a pressure of 450 mmHg, at a rate of 2.0 ml/sec, and the tourniquet was left inflated for 2 min after the injection. Each animal was given buprenorphine (0.01 mg/kg, intramuscular) for analgesia at the start of the procedure. Immediately after injection, limbs were visually inspected and observations were recorded. Animals were monitored closely until they recovered from anesthesia and began to use their injected limbs to support themselves and move around their cages.
During some primate limb injections, the intravenous and intramuscular pressures were measured. For measuring intravenous pressure, a second catheter (22-gauge) was inserted into a vein adjacent to the injection catheter. For measuring intramuscular pressure, a 16-gauge catheter was inserted into a selected muscle (gastrocnemius or brachioradialis), the inner needle was removed and a fluid-filled, vented polyethylene tube (PE 50) was inserted through the catheter lumen into the muscle and flushed with saline. The 16-gauge catheter was then removed from the muscle so that only the PE tubing remained in the muscle (Danialou et al., 2005). The PE tubing and the intravenous pressure catheter were connected to a fluid-filled calibrated pressure transducer system (EasyGraf; LDS Test and Instruments, Middleton, WI) and the signal output was saved with a data acquisition system (PowerLab; ADInstruments, Colorado Springs, CO) for later analysis.
Blood samples were collected before and 24 hr after HLV injections. For each blood collection procedure, animals were sedated with ketamine HCl (15 mg/kg, intramuscular) or a combination of ketamine HCl (7 mg/kg, intramuscular) and medetomidine (0.03 mg/kg, intramuscular). Blood was collected from the cephalic or small saphenous vein and samples were analyzed in a clinical laboratory for creatine phosphokinase (CPK) and other blood chemistries.
Monkeys were killed on day 7 and all limb muscles below the knee or elbow were harvested to measure luciferase expression. As previously listed, 21 muscles were harvested from the arms and 15 muscles were harvested from the legs (Hagstrom et al., 2004). Large muscles were divided into multiple smaller pieces and each muscle was weighed and then immediately frozen in liquid nitrogen. Limb muscles that were injected on day 0 gave “7-day” expression and limbs that were injected on day 3 gave “4-day” expression. As previously described, luciferase expression in muscle increases on average 3.1-fold between day 4 and day 7 (Vigen et al., 2007). To allow direct comparison between both time points, therefore, the day 4 luciferase data had to be adjusted by multiplying the measured values by this factor. The actual day 4 luciferase measurements as well as the day 7 equivalent values are shown.
Papaverine and saline preinjections
Studies were performed in rats and one rhesus monkey, using preinjections of papaverine (100 μg/ml) or saline. The tourniquet was applied to the limb and the preinjection solution was delivered intravenously through the injection catheter 2 min before the pDNA. The preinjection volume was 1.5 ml in the rat hind limb, 20 ml in the monkey arm, and 30 ml in the monkey leg. Only animals in the papaverine studies received papaverine preinjections.
Limb exsanguination
In rats, limb exsanguination was performed by elevating the rat's hind limb above the level of the heart for 3 min and then wrapping a latex bandage around the limb to force more blood out of the vasculature. With the limb elevated and the bandage applied, the tourniquet was attached to the upper limb. Once the tourniquet was attached, the limb was lowered, the latex bandage was removed, and the pDNA was injected.
For HLV injections into an exsanguinated monkey limb, the injection catheter was inserted into the vein and then the limb was elevated above the heart for 3 min. Thereafter, the tourniquet was applied and inflated, the limb was lowered, and the pDNA injection was performed. To avoid the risk of moving or pulling on the injection catheter, the monkey limb was not wrapped.
Luciferase assay: rat and primate
For the luciferase assays, lysis buffer (0.1% Triton X-100, 1 mM dithiothreitol, 100 mM potassium phosphate; pH 7.8) was added to each muscle sample (15 ml of lysis buffer per gram of muscle) and homogenized with a PRO 200 homogenizer (Pro Scientific, Oxford, CT). Homogenates were mixed and then centrifuged at 1860 × g for 10 min at 4°C. Aliquots of the supernatant (or dilutions there of ) were analyzed for luciferase activity with a luminometer (Veritas; Turner Biosystems, Sunnyvale, CA). Relative light units were converted to nanograms, using a standard curve (Promega), and the appropriate correction for dilution was applied. The total luciferase in nanograms per gram limb muscle was calculated by summing the nanograms of luciferase in each muscle group and dividing this by the total weight of the limb muscles.
Laser Doppler imaging, nerve conduction studies, and resting electromyography
A laser Doppler imager (LDI-VR; Moor Instruments, Wilmington, DE) was used to assess limb perfusion in primates. Laser Doppler image (LDI) scans were obtained just before injection and then 15 min, 30 min, 45 min, 60 min, 4 hr, 8 hr, 24 hr, 30 hr, and 48 hr postinjection. Nerve conduction studies (NCS) and resting electromyography (EMG) were performed with a VikingSelect EMG/NCS system (Viasys Healthcare, Madison, WI). Anesthesia and limb preparation for these procedures were the same as for limb injection. See the online supplement (at www.liebertonline.com/hum) for additional information.
Fine and gross motor skill
Animals were transferred into a behavior observation cage with a Plexiglas board mounted on the front of the cage. The Plexiglas board had two openings for the arms and a box mounted in the middle with two recessed wells for holding food treats. Once trained, the animals would put an arm through the opening and use their fingers to pull food out of the wells. For this study, we used pieces of grapes that fit down into the well so that the animals had to be able to pinch the grape with their fingers in order to retrieve it. Before the start of the study, animals were trained on average 4 days/week for 3 weeks to become comfortable with the task. The training and the actual testing were performed early in the morning, when the animals were hungry and motivated to pick up the food. For this study, the left arm of each animal was injected and evaluated. To persuade the animal to use just the left hand for the food pick-up task we covered the opening for the right hand. For the timed test, food was placed in the two wells and then a timer was started as soon as the animal's hand moved through the opening to grab the grape piece. The timer was stopped once the animal had retrieved both grapes and had pulled his hand back into the cage. Animals were tested repeatedly 5 to 10 times over a 5-min period on each test day. Animals were tested on day −6 and day 0 before injection and on days 1, 4, 7, 14, and 21 after injection.
Gross motor skill was evaluated by using a rating system. The rating was done by observing the animals as they moved about their cages and handled a large section of fruit or an enrichment toy that contained food. The following scoring system was used: 0, normal: uses both hands equally; 1, uses both hands but favors the uninjected hand; 2, uses the injected hand for walking and moving around but not for taking treats; 3, does not use the injected hand. Animals were evaluated on the same days as the fine motor skill testing.
Statistical analysis
The average, standard deviation and standard error of the mean (SEM) were calculated. The figures show the error as the SEM unless otherwise indicated. Statistical analyses were performed with Prism (GraphPad Software, San Diego, CA). The p values were calculated by paired or unpaired two-tailed t test or by analysis of variance.
Results
Papaverine preinjection
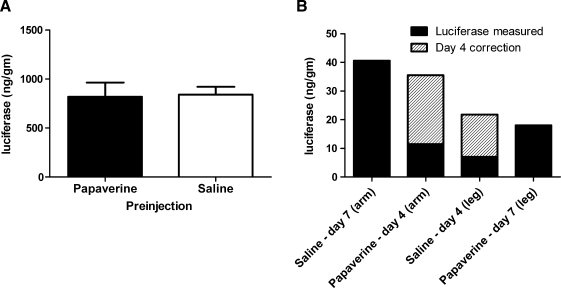
In a previous publication that first described the HLV procedure, injections were performed using a papaverine preinjection similar to the technique used for arterial pDNA delivery (Hagstrom et al., 2004). To determine whether papaverine enhances intravenous delivery of pDNA, we performed studies in rats and a single rhesus monkey (Fig. 1A and B). In the rat study, one group of animals received a papaverine preinjection and another group of animals received a saline preinjection (Fig. 1A). Each rat was then given an HLV injection of pCMV-Luc. Luciferase expression was the same in both groups (p = 0.680). Papaverine did not enhance luciferase expression. In the monkey study, two limbs were preinjected with papaverine and two limbs were preinjected with saline (Fig. 1B). For each limb, 5 mg of pCMV-Luc was injected 2 min after the preinjection. In rats and monkey, expression in papaverine-preinjected limbs was overall the same as in saline-preinjected limbs. Eliminating the papaverine preinjection simplifies the procedure and eliminates concerns about potential systemic effects of papaverine.
FIG. 1.
Effect of papaverine and saline preinjections on luciferase expression in rat and rhesus monkey limbs. (A) Rats had either a preinjection of 1.5 ml of papaverine solution (n = 9) or saline (n = 7) followed 2 min later by a 3.0-ml hydrodynamic limb vein (HLV) injection of pDNA (250 μg of pCMV-Luc). Animals were killed 5 days later and limb muscles were analyzed for luciferase activity. Average luciferase in each group is shown. (B) One rhesus monkey had preinjections of papaverine (two limbs) or saline (two limbs) followed by HLV injections containing 5 mg of pCMV-Luc. The total injection volume (papaverine or saline and pDNA solution) was 0.35 ml/ml limb volume and the tourniquet pressure was 700 mmHg. The total luciferase in each injected limb is shown, as well as the day 7 equivalent for the day 4 samples (see Materials and Methods).
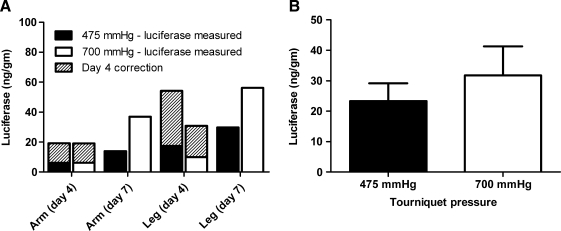
Tourniquet inflation pressure
In the primate HLV procedures, an inflatable tourniquet was used to occlude the vasculature of the limb. The tourniquet was applied snugly around the limb above the knee or elbow and then inflated to the desired pressure. The tourniquet pressure was sufficiently high to block blood flow and to prevent outflow of the pDNA solution. Tourniquet pressures in early studies were very high (700 to 1000 mmHg). These high tourniquet pressures ensured that the injection solution stayed in the limb and extravasated into the muscle tissue. As the procedure was further developed and optimized, it became evident that the high tourniquet pressure could pose a safety issue and could potentially damage limb nerves and or muscles. Although we have not observed any signs of tourniquet-induced damage, it seemed prudent to investigate the feasibility of using a lower tourniquet pressure (Fig. 2). The tourniquet used for these procedures was the same as the one designed for the Bier block procedure. In the Bier block, a tourniquet is applied to a limb and inflated to a pressure above the patient's systolic pressure. Clinical devices designed to inflate and monitor tourniquet pressures generally have a maximal inflation pressure of 475 mmHg (although some have a maximum of 600 mmHg). For these tourniquet pressure studies, two rhesus monkeys had two limbs injected using a high (700 mmHg) tourniquet pressure and two limbs injected using a lower (475 mmHg) tourniquet pressure. Figure 2A shows the average luciferase expression in the individual limbs. The total average luciferase expression (Fig. 2B) was slightly higher with a tourniquet pressure of 700 mmHg but not significantly different than with 475 mmHg (p = 0.4422). The lower tourniquet pressure can thus be applied without significantly affecting transferred gene expression levels. Some of the data presented in Fig. 2 were previously reported (Vigen et al., 2007).
FIG. 2.
Comparison of high (700 mmHg) and lower (475 mmHg) tourniquet pressures. Two monkeys each had two limbs injected at a tourniquet pressure of 475 mmHg (n = 4 limbs) and two limbs injected at a tourniquet pressure of 700 mmHg (n = 4 limbs). The pCMV-Luc dose for each injection was 10 mg and the injection volume was 0.30 to 0.35 ml/ml limb volume. (A) The total luciferase in each injected limb is shown, as well as the day 7 equivalent for the day 4 samples (see Materials and Methods). (B) The average total luciferase at each tourniquet pressure with the day 4 correction.
Rate of injection
In previous studies, we investigated the optimal injection rate for delivering pDNA intravenously to rat limbs (Hagstrom et al., 2004). In those studies, which used a papaverine preinjection, we found that expression increased steadily with the injection rate up to 10 ml/min and then began to decline at higher rates. Even though we have eliminated the preinjection, there is no reason to believe that the optimal injection rate would change. We have used the 10-ml/min rate without papaverine for numerous studies and have consistently obtained high levels of reporter gene expression. Thus, all of the rat studies presented here are with the 10-ml/min (0.17-ml/sec) injection rate.
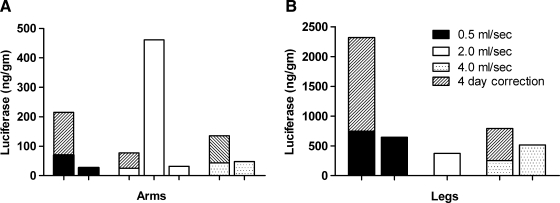
For rhesus monkey limb injections, our first studies were performed with an injection rate of 2.0 ml/sec (120 ml/min). We have routinely used this injection rate and obtained high levels of reporter gene expression. A slower injection rate would require the tourniquet to remain in place longer whereas a faster rate would shorten the tourniquet time. To further investigate this parameter, we performed limb injections using a slower rate of 0.5 ml/sec and a faster rate of 4.0 ml/sec (Fig. 3). For comparison, we used luciferase data from other studies that followed (the exsanguination and tourniquet time studies, Table 1) and that used a rate of 2.0 ml/sec. The limb injection volumes were all 0.45 ml/ml limb volume and the pCMV-Luc dose was between 10 and 20 mg per injection (∼180 μg/ml in the injection solution). Luciferase expression in the limbs was variable but overall similar at each rate. There was no trend toward higher or lower expression with faster or slower rates. In this study and some others we found that expression was lower in the arms than in the legs. One explanation for this is that we used very small animals and it was difficult to achieve a good snug tourniquet fit on the little arms, and therefore pDNA solution may have leaked out of the limb during the injection.
FIG. 3.
Effect of injection rate on luciferase expression in rhesus monkey limbs. Two rhesus monkeys were used specifically to evaluate the injection rate. One animal had all four limbs injected at a rate of 0.5 ml/sec and a second animal had all four limbs injected at a rate of 4.0 ml/sec. Injections from other studies (see exsanguination and tourniquet time studies in Table 1) that used an injection rate of 2.0 ml/sec and otherwise the same injection conditions are shown for comparison. For each limb the injection volume was 0.45 ml/ml limb volume and contained pCMV-Luc at 180 μg/ml. Limb total luciferase expression is shown with the day 4 correction (see Materials and Methods). (A) Total luciferase in the arms. (B) Total luciferase in the legs.
Tourniquet time: postinjection
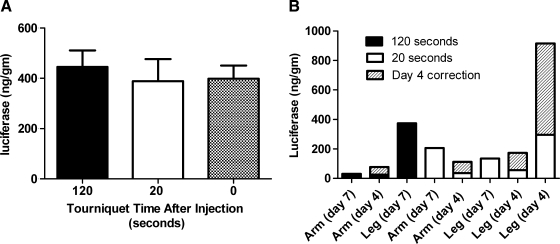
During HLV procedures, a tourniquet is used to block blood flow into and out of the limb. The tourniquet is tightened or inflated just before injection and left on for up to 2 min after the injection. The assumption had been that expression improved if the tourniquet was left inflated after injection, allowing the pDNA to stay in the extravascular space and in contact with the myofibers. Studies were conducted in rats and two rhesus monkeys to evaluate the effect that postinjection tourniquet time has on reporter gene expression (Fig. 4).
FIG. 4.
Postinjection tourniquet time versus luciferase expression. (A) Three groups of rats (n = 4) had HLV injections of 250 μg of pCMV-Luc. The tourniquet was removed at 120 sec, 20 sec, or immediately after injection. Animals were killed 7 days after injection and limb muscles were analyzed for luciferase activity. (B) Two monkeys had HLV injections in all four limbs. The pDNA concentration was 180 μg/ml and the injection volume was 0.45 ml/ml limb volume. The postinjection tourniquet time was either 20 sec (five limbs) or 120 sec (three limbs). The total luciferase in each limb is shown with the day 4 correction (see Materials and Methods).
In rats, we investigated three tourniquet times. The tourniquet was left on for 2 min or 20 sec or removed immediately after the injection. Limbs had similar amounts of luciferase expression with no significant difference between any of the tourniquet times (p = 0.8666) (Fig. 4A).
In monkeys, one animal had two limbs injected with the tourniquet remaining inflated for 2 min after the injection and two limbs injected with the tourniquet slowly released 20 sec after the injection. The second animal had one limb injected with the 2-min postinjection tourniquet time and three limbs injected with the tourniquet released after 20 sec. Luciferase expression in the limbs was variable, but overall the data do not indicate that a longer tourniquet time enhances expression (Fig. 4B).
Studies in rats and monkeys showed no benefit for longer tourniquet times, and shortening the tourniquet time may improve the safety of the HLV procedure.
Limb exsanguination studies
To further optimize the HLV injection procedure, we investigated whether draining the intravascular blood from the limb before injection would have a beneficial effect on pDNA delivery or the safety of the procedure. Draining intravascular limb blood (“limb exsanguination”) is a routine step in the Bier block procedure and is performed by elevating the limb and wrapping it to gently squeeze blood out of the vasculature (Watt and Kuipers, 1979). Limb exsanguination could potentially improve the HLV procedure by preventing the extravasation of blood into the muscle tissue and by reducing the amount of endogenous DNases that could break down the injected pDNA.
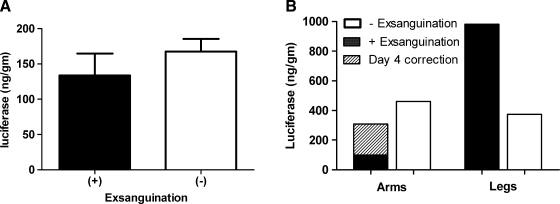
Limb exsanguination studies were performed in rats and monkeys (Fig. 5). In the rat study, one group of rats underwent limb exsanguination before the HLV injection and a second group of rats received HLV injections with no limb exsanguination (Fig. 5A). The rats used for these studies weighed more than those used in the other rat studies and thus the amount of luciferase expression per muscle mass was lower. There was no significant difference with or without limb exsanguination (p = 0.3380).
FIG. 5.
Luciferase expression with and without limb exsanguination. (A) Two groups of rats were injected with 250 μg of pCMV-Luc with (n = 4) or without (n = 6) limb exsanguination. Limb muscles were harvested 7 days after injection. (B) One rhesus monkey had two limbs exsanguinated before injection and one limb injected without exsanguination. Also shown is luciferase expression in a leg of a monkey in another study, injected under the same conditions without limb exsanguination. The injection volume was 0.45 ml/ml limb volume and the pDNA (pCMV-Luc) concentration was 180 μg/ml. Total luciferase in the arms and legs with the day 4 correction is shown.
To evaluate limb exsanguination in the primate, one arm and one leg were exsanguinated before injection and one arm was injected without exsanguination (Fig. 5B). The other leg of this monkey was not injected because of technical problems with the catheter venipuncture. Expression in the leg of another monkey injected without limb exsanguination and under the same injection conditions (tourniquet time study) was used for comparison. Expression in the limbs injected in this study was variable but overall no higher with exsanguination. Studies performed in both rats and rhesus monkey indicated that exsanguination did not substantially increase luciferase expression.
Creatine phosphokinase
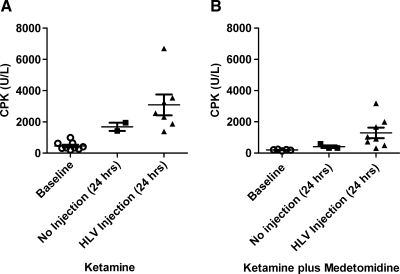
In several previous publications, we reported that serum CPK was transiently elevated in primates after the HLV injection procedure (Hagstrom et al., 2004; Vigen et al., 2007). We assumed that this elevation was a reflection of the muscle effects caused entirely by the HLV procedure. While conducting other primate studies that did not involve HLV injections, we found that CPK values were regularly elevated 24 hr postprocedurally. Because we were using ketamine intramuscularly as a sedative for all primate procedures, we decided to investigate whether ketamine alone causes CPK elevation 24 hr after administration (Fig. 6). We also tested a combination of ketamine plus medetomidine because this mixture, which contains a lower dose of ketamine, is known to cause less muscle toxicity (Sun et al., 2003). For this investigation, animals were given an intramuscular injection of ketamine HCl (15 mg/kg intramuscular) alone or a combination of ketamine HCl (7 mg/kg, intramuscular) and medetomidine (0.03 mg/kg). Once the animals were sedated, a baseline blood sample was collected. Some of the animals then received an HLV injection into one limb while they were still sedated. Twenty-four hours later, animals were again sedated with the same drugs and another blood sample was collected. Ketamine alone caused higher CPK levels than the combination of ketamine plus medetomidine. Ketamine-sedated animals had elevated CPK levels of 1689 U/liter on average (Fig. 6A) 24 hr after administration whereas the ketamine plus medetomidine-sedated animals had an average of 455 U/liter (Fig. 6B), which is within the normal range. Twenty-four hours after HLV injection, the ketamine-only animals had an average CPK level of 3092 U/liter, which was 2.4-fold higher (p = 0.0297) than that of the ketamine plus medetomidine-sedated animals, which had an average CPK level of 1325 U/liter. Much of the transient increase in serum CPK that was previously observed was due to the sedation protocol and not the HLV procedure. The modest increase in CPK levels resulting from the HLV injection further demonstrates the safety of the procedure.
FIG. 6.
Creatine phosphokinase (CPK) levels after administration of ketamine or ketamine plus medetomidine. Rhesus monkeys were sedated with either (A) ketamine (15 mg/kg, intramuscular) or (B) ketamine (7 mg/kg) and medetomidine (0.03 mg/kg, intramuscular) together. Baseline blood samples were collected soon after sedation. Some animals received an HLV injection and others were recovered immediately after the blood collection. Twenty-four hours later animals were again sedated with the same drugs and another blood sample was collected. Individual CPK levels, group averages, and SEM are shown (n = 2–9). (A) Monkeys sedated with ketamine. (B) Monkeys sedated with a combination of ketamine and medetomidine.
Intravenous and intramuscular pressure
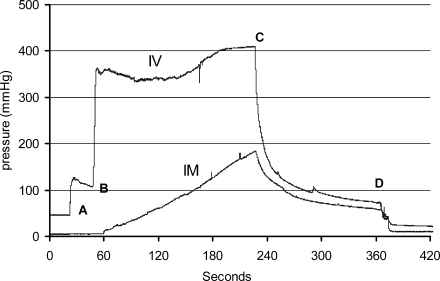
We measured intravenous and intramuscular pressures during 12 primate HLV injections. The changes in intravenous and intramuscular pressure and the peak pressures were similar from injection to injection. The average peak intravenous pressure was 391 ± 32 mmHg and the average peak intramuscular pressure was 145 ± 18 mmHg. A representative pressure tracing of an injection is shown in Fig. 7. In this injection, the intravenous pressure increased to approximately 120 mmHg just before the injection, when the tourniquet was inflated and the catheter was flushed. As soon as the injection began, the intravenous pressure increased rapidly. The pressure plateaued at approximately 350 mmHg and then gradually increased to 400 mmHg by the end of the injection. As soon as the injection ended, the intravenous pressure rapidly decreased and returned to a pressure similar to that preinjection. The intramuscular pressure gradually increased during the injection, peaking at 175 mmHg near the end of the injection. After the injection, intramuscular pressure decreased and returned to baseline levels once the tourniquet was deflated and removed from the limb. In summary, we observed no prolonged elevated intravenous and intramuscular pressures that could potentially damage limb tissues.
FIG. 7.
Intravascular (IV) and intramuscular (IM) pressure recording during HLV injection. In rhesus monkeys, intravenous pressure was measured through a catheter inserted into a vein adjacent to the injection catheter and intramuscular pressure was measured through a fluid-filled catheter inserted directly into a muscle. The tourniquet was applied to the leg just before the start of the data collection. “A” indicates when the injection catheter was flushed and the tourniquet was inflated to 450 mmHg. “B” indicates when the pDNA injection was started at a rate of 0.5 ml/min. “C” indicates the end of the pDNA injection. “D” indicates the point at which the tourniquet was deflated and removed from the limb.
Laser Doppler imaging: safety
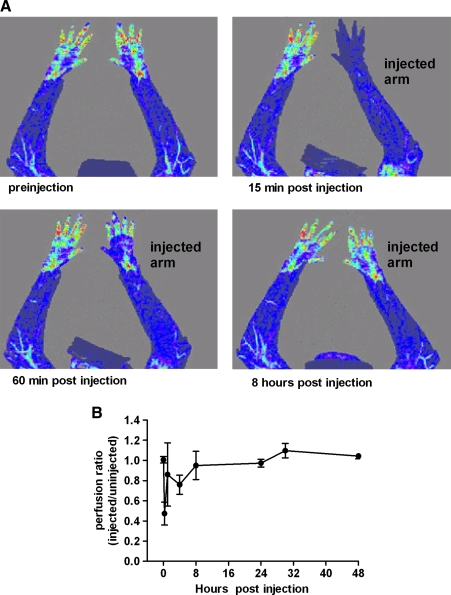
During HLV injections, fluid extravasates out of the vasculature and causes tissue swelling. A potential safety concern about this procedure is that the tourniquet compression or the swelling itself could interfere with blood flow and tissue perfusion after the injection is complete. To evaluate limb perfusion, we conducted a primate study in which we measured perfusion before and after injection with a laser Doppler imager (Fig. 8). LDI scans were performed on both arms before injection and at multiple time points between 15 min and 48 hr postinjection. For the HLV injections, one arm from each animal was injected with saline. The perfusion in the injected limbs was expressed as a perfusion ratio that compared the injected arm with the uninjected arm (see the online supplement at www.liebertonline.com/hum). Figure 8A shows representative LDI images from a single animal. In this example, perfusion was low immediately after injection but improved by 1 hr and fully recovered by 24 hr. Animals were continuously anesthetized from preinjection to 1 hr postinjection. After the 1-hr postinjection LDI scan, animals were taken back to their cages and allowed to move around and use the injected limb. This physical activity appeared to help reduce swelling and improve blood flow to the injected limb. Figure 8B shows the average limb perfusion ratio for all three animals at selected time points. Compared with preinjection, the perfusion ratios at 15 min, 1 hr, and 8 hr postinjection were reduced by an average 53 ± 13, 15 ± 33, and 6 ± 11%, respectively. The perfusion ratios for these arms were, however, not significantly different at 4 hr (p = 0.1025) or 8 hr (p = 0.6603) postinjection. Between 24 and 48 hr, limb perfusion in the injected and uninjected limbs was the same for all animals, demonstrating the absence of any vascular changes.
FIG. 8.
Laser Doppler imaging of rhesus arms before and after injection. LDI scans were performed on anesthetized animals immediately before HLV injection of saline and at multiple time points between 15 min and 48 hr postinjection. The injection volume was 0.40 ml/ml limb volume and the injection rate was 2.0 ml/sec. The tourniquet was inflated to a pressure of 450 mmHg and left inflated for 2 min after the injection. (A) LDI of preinjection and early time points after injection are shown in one representative animal. Areas of low blood flow appear blue whereas areas of high blood flow appear yellow or red. (B) The LDI perfusion ratios of the injected limbs compared with the contralateral uninjected limbs (n = 3) are shown for each time point.
Nerve conduction studies and resting electromyography: safety
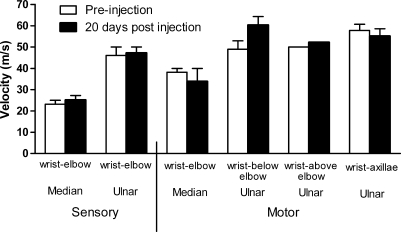
One of the safety concerns about the HLV procedure is the potential that tourniquet pressure or the injection could cause neuromuscular damage. To further evaluate neuromuscular function, we performed sensory and motor nerve conduction studies (NCS) and resting EMG tests (see the online supplement at www.liebertonline.com/hum) on two rhesus monkeys before and 20 days after HLV saline injection into an arm (Fig. 9). NCS were performed to evaluate the ability of the nerves to conduct electrical signals and resting EMG was performed to look for indications of muscle denervation or inflammation. The postinjection evaluation was done on day 20 because it can take at least 2 weeks for nerve degeneration to occur after injury (Chaudhry and Cornblath, 1992). For the HLV injections, one arm from each animal was injected with saline. The NCS was conducted immediately before the needle EMG study at both time points. The latencies and amplitudes collected during the NCS varied somewhat between the animals and time points, but the average calculated sensory nerve and motor nerve conduction velocities were similar (Fig. 9). Overall these data indicate that the nerves were not damaged by the procedure. The needle EMG data showed that the insertional activity of the resting muscle was normal and there were no spikes, waves, or fasciculations at preinjection or 20 days after injection (data not shown).
FIG. 9.
Sensory and motor nerve conduction velocities. Sensory and motor nerve (ulnar and median nerves) conduction studies were performed in two rhesus monkeys before and 20 days after HLV saline injection. The injection volume was 0.40 ml/ml limb volume and the injection rate was 2.0 ml/sec. The tourniquet was inflated to a pressure of 450 mmHg and left inflated for 2 min after the injection. Sensory nerve conduction studies were performed by electrically stimulating a nerve and then recording directly from it or from one of its branches. Ulnar and median sensory nerve velocities between the wrist and the elbow are shown. Motor nerve conduction studies were performed by electrically stimulating along the ulnar or median nerves and then recording from the muscle innervated. Ulnar and median motor nerve velocities along the ulnar and median nerves are shown. Average velocities and standard deviations are shown.
Fine motor skill testing: safety
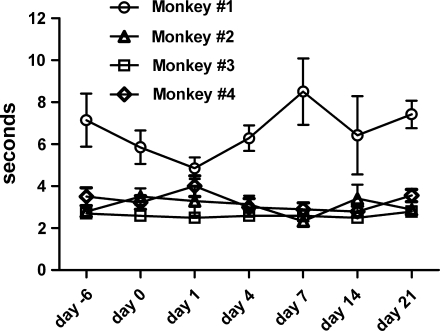
To evaluate whether the HLV injection procedure affects function of the hand, we performed gross and fine motor skill testing on four rhesus monkeys (Emborg et al., 1998; Emborg, 2004) (Fig. 10). Animals were tested before and after injection. For the HLV limb injections we used saline without pDNA. The gross motor rating score was zero (normal, uses both hands equally) at each evaluation time point for each animal tested; there was no change (data not shown). During each evaluation, animals willingly used the injected hand to move around their cages or to manipulate a large section of fruit or an enrichment toy containing food. During the fine motor skill testing, we found that three of the animals had similar food retrieval times and that the fourth animal was consistently slower, possibly because he appeared not to like grape skins. Figure 10 shows that the average food retrieval times for each individual animal did not change after injection.
FIG. 10.
Fine motor food retrieval times before and after injection. Four rhesus monkeys had fine motor skill timed tests performed on the indicated days before and after HLV injection of saline. The injection volume was 0.45 ml/ml limb volume and the injection rate was 2.0 ml/sec. The tourniquet was inflated to a pressure of 450 mmHg and left inflated for 2 min after the injection. For each test, animals repeated the food retrieval task 5 to 10 times over a 5-min period. The average food retrieval time at each time point is shown. Statistical analysis of the data showed that there was no variation in each animal's food retrieval times over the course of the study (p = 0.415 for monkey 1, p = 0.632 for monkey 2, p = 0.961 for monkey 3, and p = 0.263 for monkey 4).
Primate HLV experience in toto
The data presented in this paper were collected from limb injections in 17 rhesus monkeys. In the course of our research to date, we have performed HLV injection in 47 rhesus monkeys and 2 cynomolgus monkeys for a total of 184 limb injections. Depending on the study, animals underwent injections into a single limb or multiple limbs. In some studies limbs were injected repeatedly up to six times (our unpublished data). All animals recovered quickly from the procedure without any obvious side effects or physical deficits.
Discussion
This study evaluated the effects of a variety of injection conditions on pDNA-derived luciferase expression levels to optimize delivery in preparation for a human clinical study. Two important injection parameters are the rate of injection and the volume of the injection. As we previously noted (Hagstrom et al., 2004; Sebestyén et al., 2007), the expression levels achieved by the HLV procedure are tolerant of changes in these two parameters, in contrast to the more sensitive intraarterial procedure. The results in the present study in both rats and monkeys are consistent with the robustness of the procedure, a key attribute for its progression to human trials.
An important objective of this study was to simplify the procedure by eliminating unnecessary conditions. Simplicity is an important aspect of a clinical procedure. In this regard, it was important to determine whether papaverine preinjection was required for optimal expression. A concern was that the failure of the tourniquet could lead to leakage of papaverine out of the limb and into the systemic circulation, where it could produce adverse effects such as arrhythmia. Neither in rats nor in monkeys did papaverine appear critical for efficient luciferase expression, thus substantially simplifying and increasing the safety of the procedure. Exsanguination of the limb before the injection appeared to be unnecessary, but the sample size for primates was too small to be conclusive. Exsanguination was beneficial for rAAV6 delivery in dogs, but this benefit may have been due to reduced exposure of the viral vector to antibodies in the blood and thus not pertain to pDNA delivery (Gregorevic et al., 2009).
The tourniquet inflation pressure was another parameter that was varied to increase the safety margin of the procedure. During the initial development of the procedure we used a tourniquet inflation pressure of 700 to 1000 mmHg to ensure complete occlusion. During consideration of the procedure for a human trial, however, we realized that exceeding 475 mmHg of pressure was not recommended for the tourniquet devices used during the Bier block procedure. Although there is no direct correlation between tourniquet pressure and risk of nerve damage, it is still considered an important factor. Therefore testing of the HLV procedure was done using a pressure of 450 or 475 mmHg, which did not substantially attenuate expression levels. It is important to realize that the tourniquet pressure is only one aspect that determines whether complete occlusion is achieved. The fit of the tourniquet around the limb is of importance as well. Given the particular anatomy and small size of the monkey limb, we would expect even better occlusion in the larger human limb for which the tourniquets are designed.
Another factor affecting the risk of nerve damage from a tourniquet is the time that the tourniquet is inflated. Our present study showed that leaving the tourniquet inflated after injection is not required. During a Bier block procedure for limb anesthesia, a tourniquet may be in place for more than 1 hr. Even under these conditions the risk of nerve damage is extremely rare. In contrast, HLV procedures can be completed in approximately 5 min from inflating the tourniquet to releasing the pressure after the injection. EMG and hand function studies indicated that the HLV procedure did not cause any nerve damage.
Muscle damage is another safety concern. In addition to our previous studies (Toumi et al., 2006; Vigen et al., 2007) this study provided data relevant to this concern. We previously noted increased CPK values postinjection, which were attributed to either actual “muscle damage” or a transient, nonlethal disruption of the sarcolemma. However, it appears that the ketamine injected for monkey sedation contributed substantially to overall CPK levels and that the HLV procedure itself raises CPK levels less than we previously thought. Tracings of the intramuscular pressure provided additional data relevant to potential muscle toxicity. After injection, intramuscular pressure quickly returned to baseline levels, indicating that vascular blood flow supplying the limb muscles is not likely to become compromised by increased intramuscular pressure. These data suggest that a compartment syndrome is unlikely to occur.
Given that anesthesia is required for the animals during the procedure, we were unable to monitor whether the procedure is painful during the injection. However, persistent pain from the procedure is unlikely, based on the following observations. The monkeys did not appear to be in pain after their anesthesia wore off. Immediately afterward, they were active and did not avoid use of their injected limbs. Pain is likely to result from either the tourniquet pressure or increased intravascular and intramuscular pressure. Given that these increased pressures were present only during the injection, pain should not persist afterward. Short-lived approaches to attenuate pain may be required just during the injection.
Damage to the vascular system is another safety concern. A previous magnetic resonance imaging study did not detect any vascular changes of concern after HLV injection (Vigen et al., 2007). The present study used another technique, laser Doppler imaging, to evaluate limb blood perfusion and did not observe any persistent effects postinjection.
Studies addressing transfection efficiency show that one HLV injection of naked pDNA can transfect up to 40% of large groups of muscles in the primate limb (Hagstrom et al., 2004; and our unpublished data). A single HLV injection of β-galactosidase-expressing plasmid into mice and rats transfects nearly 30% of muscle fibers throughout the injected limbs (our unpublished data). Limitations of the HLV delivery procedure are that it is locoregional and not systemic, and that transfection efficiencies vary for different muscles and are lower for large plasmids such as those expressing the full-length dystrophin gene.
In conclusion, on the basis of the studies present here and our experience involving 184 limb injections in nonhuman primates, the HLV procedure appears to be safe and robust without any significant toxic effects on the neuromuscular and vascular systems. Human clinical studies are warranted to evaluate its ability to safely deliver naked pDNA and viral vectors.
Supplementary Material
Acknowledgments
The authors thank Larry Whitesell, Jacob Griffin, Mark Noble, Tracie Milarch, Mavis Eldridge, and Linda Goth for technical assistance, and Mark Pithan for electromyography and nerve conduction evaluations. This study was funded by the Association Française contre les Myopathies. This publication was made possible in part by grant P51 RR000167 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center (WNPRC), University of Wisconsin-Madison. This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
Author Disclosure Statement
J.O. Hegge, C.I. Wooddell, G. Zhang, J.E. Hagstrom, M.G. Sebestyén, and J.A. Wolff are employed by Roche Madison, which owns patents for the hydrodynamic limb vein delivery procedure. T. Huss is employed by Transgene, which conducted a phase I clinical trial for MyoDys plasmid delivery by intramuscular injection.
References
- Braun S. Muscular gene transfer using nonviral vectors. Curr. Gene Ther. 2008;8:391–405. doi: 10.2174/156652308786070998. [DOI] [PubMed] [Google Scholar]
- Browning J. Hogg N. Gobe G. Cross R. Capillary density in skeletal muscle of Wistar rats as a function of muscle weight and body weight. Microvasc. Res. 1996;52:281–287. doi: 10.1006/mvre.1996.0065. [DOI] [PubMed] [Google Scholar]
- Budker V. Zhang G. Danko I. Williams P. Wolff J. The efficient expression of intravascularly delivered DNA in rat muscle. Gene Ther. 1998;5:272–276. doi: 10.1038/sj.gt.3300572. [DOI] [PubMed] [Google Scholar]
- Chaudhry V. Cornblath D.R. Wallerian degeneration in human nerves: Serial electrophysiological studies. Muscle Nerve. 1992;15:687–693. doi: 10.1002/mus.880150610. [DOI] [PubMed] [Google Scholar]
- Cho W.K. Ebihara S. Nalbantoglu J. Gilbert R. Massie B. Holland P. Karpati G. Petrof B.J. Modulation of Starling forces and muscle fiber maturity permits adenovirus-mediated gene transfer to adult dystrophic (mdx) mice by the intravascular route. Hum. Gene Ther. 2000;11:701–714. doi: 10.1089/10430340050015608. [DOI] [PubMed] [Google Scholar]
- Danialou G. Comtois A.S. Matecki S. Nalbantoglu J. Karpati G. Gilbert R. Geoffroy P. Gilligan S. Tanguay J.F. Petrof B.J. Optimization of regional intraarterial naked DNA-mediated transgene delivery to skeletal muscles in a large animal model. Mol. Ther. 2005;11:257–266. doi: 10.1016/j.ymthe.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Davis H.L. Demeneix B.A. Quantin B. Coulombe J. Whalen R.G. Plasmid DNA is superior to viral vectors for direct gene transfer into adult mouse skeletal muscle. Hum. Gene Ther. 1993;4:733–740. doi: 10.1089/hum.1993.4.6-733. [DOI] [PubMed] [Google Scholar]
- Emborg M.E. Evaluation of animal models of Parkinson's disease for neuroprotective strategies. J. Neurosci. Methods. 2004;139:121–143. doi: 10.1016/j.jneumeth.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Emborg M.E. Ma S.Y. Mufson E.J. Levey A.I. Taylor M.D. Brown W.D. Holden J.E. Kordower J.H. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J. Comp. Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- Fisher K.J. Jooss K. Alston J. Yang Y. Haecker S.E. High K. Pathak R. Raper S.E. Wilson J.M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- Gregorevic P. Schultz B.R. Allen J.M. Halldorson J.B. Blankinship M.J. Meznarich N.A. Kuhr C.S. Doremus C. Finn E. Liggitt D. Chamberlain J.S. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol. Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom J.E. Hegge J. Zhang G. Noble M. Budker V. Lewis D.L. Herweijer H. Wolff J.A. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol. Ther. 2004;10:386–398. doi: 10.1016/j.ymthe.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Herweijer H. Wolff J.A. Gene therapy progress and prospects: Hydrodynamic gene delivery. Gene Ther. 2007;14:99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- Kessler P.D. Podsakoff G.M. Chen X. McQuiston S.A. Colosi P.C. Matelis L.A. Kurtzman G.J. Byrne B.J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Schmid-Schonbein G.W. Biomechanics of skeletal muscle capillaries: Hemodynamic resistance, endothelial distensibility, and pseudopod formation. Ann. Biomed. Eng. 1995;23:226–246. doi: 10.1007/BF02584425. [DOI] [PubMed] [Google Scholar]
- Liang K.W. Nishikawa M. Liu F. Sun B. Ye Q. Huang L. Restoration of dystrophin expression in mdx mice by intravascular injection of naked DNA containing full-length dystrophin cDNA. Gene Ther. 2004;11:901–908. doi: 10.1038/sj.gt.3302239. [DOI] [PubMed] [Google Scholar]
- Monahan P.E. Samulski R.J. Tazelaar J. Xiao X. Nichols T.C. Bellinger D.A. Read M.S. Walsh C.E. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- Qiao C. Li J. Zheng H. Bogan J. Li J. Yuan Z. Zhang C. Bogan D. Kornegay J. Xiao X. Hydrodynamic limb vein injection of AAV8 canine myostatin propeptide gene in normal dogs enhances muscle growth. Hum. Gene Ther. 2009;20:1–10. doi: 10.1089/hum.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragot T. Vincent N. Chafey P. Vigne E. Gilgenkrantz H. Couton D. Cartaud J. Briand P. Kaplan J.C. Perricaudet M. Kahn A. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Rodino-Klapac L.R. Janssen P.M. Montgomery C.L. Coley B.D. Chicoine L.G. Clark K.R. Mendell J.R. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J. Transl. Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebestyén M.G. Hegge J.O. Noble M.A. Lewis D.L. Herweijer H. Wolff J.A. Progress toward a nonviral gene therapy protocol for the treatment of anemia. Hum. Gene Ther. 2007;18:269–285. doi: 10.1089/hum.2006.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R.O. Spratt S.K. Lagarde C. Bohl D. Kaspar B. Sloan B. Cohen L.K. Danos O. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum. Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- Su L.T. Gopal K. Wang Z. Yin X. Nelson A. Kozyak B.W. Burkman J.M. Mitchell M.A. Low D.W. Bridges C.R. Stedman H.H. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112:1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- Suda T. Suda K. Liu D. Computer-assisted hydrodynamic gene delivery. Mol. Ther. 2008;16:1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- Sun F.J. Wright D.E. Pinson D.M. Comparison of ketamine versus combination of ketamine and medetomidine in injectable anesthetic protocols: Chemical immobilization in macaques and tissue reaction in rats. Contemp. Top. Lab. Anim. Sci. 2003;42:32–37. [PubMed] [Google Scholar]
- Toromanoff A. Cherel Y. Guilbaud M. Penaud-Budloo M. Snyder R.O. Haskins M.E. Deschamps J.Y. Guigand L. Podevin G. Arruda V.R. High K.A. Stedman H.H. Rolling F. Anegon I. Moullier P. Le Guiner C. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol. Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- Toumi H. Hegge J. Subbotin V. Noble M. Herweijer H. Best T.M. Hagstrom J.E. Rapid intravascular injection into limb skeletal muscle: A damage assessment study. Mol. Ther. 2006;13:229–236. doi: 10.1016/j.ymthe.2005.07.699. [DOI] [PubMed] [Google Scholar]
- Vigen K.K. Hegge J.O. Zhang G. Mukherjee R. Braun S. Grist T.M. Wolff J.A. Magnetic resonance imaging-monitored plasmid DNA delivery in primate limb muscle. Hum. Gene Ther. 2007;18:257–268. doi: 10.1089/hum.2006.115. [DOI] [PubMed] [Google Scholar]
- Watt J.M. Kuipers A. Limb exsanguination for Bier's block. Anaesthesia. 1979;34:376. doi: 10.1111/j.1365-2044.1979.tb04960.x. [DOI] [PubMed] [Google Scholar]
- Wells D.J. Opening the floodgates: Clinically applicable hydrodynamic delivery of plasmid DNA to skeletal muscle. Mol. Ther. 2004;10:207–208. doi: 10.1016/j.ymthe.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Wolff J.A. Malone R.W. Williams P. Chong W. Acsadi G. Jani A. Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wolff J.A. Dowty M.E. Jiao S. Repetto G. Berg R.K. Ludtke J.J. Williams P. Slautterback D.B. Expression of naked plasmids by cultured myotubes and entry of plasmids into T tubules and caveolae of mammalian skeletal muscle. J. Cell Sci. 1992;103:1249–1259. doi: 10.1242/jcs.103.4.1249. [DOI] [PubMed] [Google Scholar]
- Zhang G. Budker V. Williams P. Subbotin V. Wolff J.A. Efficient expression of naked DNA delivered intraarterially to limb muscles of nonhuman primates. Hum. Gene Ther. 2001;12:427–438. doi: 10.1089/10430340150504046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.