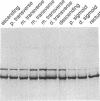
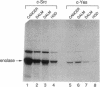
Abstract
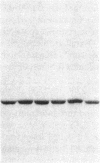
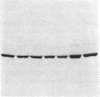
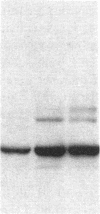
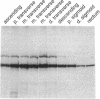
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon with a high incidence of colon cancer. Dysplasia is a precursor to carcinoma and a predictor of malignant potential; epithelia containing high-grade or severe dysplasia is most likely to develop cancer. The cellular oncogene c-src and its viral homologue v-src (the transforming gene of Rous sarcoma virus) encode 60-kD cytoplasmic, membrane-associated protein tyrosine kinases. For the viral protein or transforming mutants of the cellular protein (Src), a close correlation exists between elevated tyrosine kinase activity and malignant transformation of cells. Previously, we and others observed elevated Src activity in sporadic colon carcinomas and benign adenomas at greatest risk for developing cancer (those with large size, villous architecture, and/or severe dysplasia). Here we report that Src activity and protein abundance are also elevated in neoplastic UC epithelia. Activity is highest in malignant and severely dysplastic epithelia, and 6-10-fold higher in mildly dysplastic than in nondysplastic epithelia. Thus, Src activity is elevated in premalignant UC epithelia, which is at greatest risk for developing cancer. The data suggest that activation of the src proto-oncogene is an early event in the genesis of UC colon cancer.
Full text
PDF



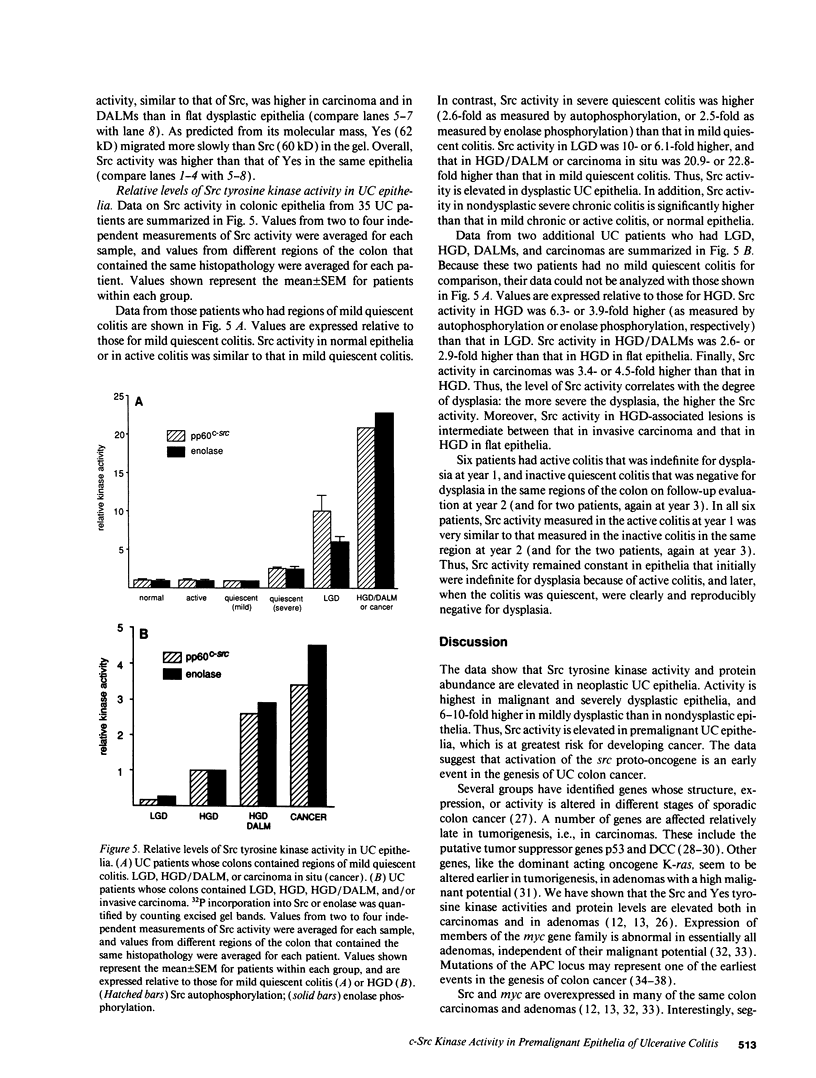


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTLER V. B., COLLER F. A. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954 Jun;139(6):846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Bell S. M., Kelly S. A., Hoyle J. A., Lewis F. A., Taylor G. R., Thompson H., Dixon M. F., Quirke P. c-Ki-ras gene mutations in dysplasia and carcinomas complicating ulcerative colitis. Br J Cancer. 1991 Jul;64(1):174–178. doi: 10.1038/bjc.1991.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bolen J. B. Signal transduction by the SRC family of tyrosine protein kinases in hemopoietic cells. Cell Growth Differ. 1991 Aug;2(8):409–414. [PubMed] [Google Scholar]
- Bolen J. B., Thompson P. A., Eiseman E., Horak I. D. Expression and interactions of the Src family of tyrosine protein kinases in T lymphocytes. Adv Cancer Res. 1991;57:103–149. doi: 10.1016/s0065-230x(08)60997-5. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., DeSeau V., Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., Deseau V., Rosen N. Analysis of pp60c-src in human colon carcinoma and normal human colon mucosal cells. Oncogene Res. 1987 Jul;1(2):149–168. [PubMed] [Google Scholar]
- Burmer G. C., Levine D. S., Kulander B. G., Haggitt R. C., Rubin C. E., Rabinovitch P. S. c-Ki-ras mutations in chronic ulcerative colitis and sporadic colon carcinoma. Gastroenterology. 1990 Aug;99(2):416–420. doi: 10.1016/0016-5085(90)91024-z. [DOI] [PubMed] [Google Scholar]
- Burmer G. C., Rabinovitch P. S., Haggitt R. C., Crispin D. A., Brentnall T. A., Kolli V. R., Stevens A. C., Rubin C. E. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992 Nov;103(5):1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Hutchinson M. A., Eckhart W. Structural and functional modification of pp60c-src associated with polyoma middle tumor antigen from infected or transformed cells. Mol Cell Biol. 1985 Oct;5(10):2647–2652. doi: 10.1128/mcb.5.10.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kamps M. P., Meisler A. I., Pipas J. M., Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989 Jun;83(6):2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Mamajiwalla S., Skolnick S. A., Eckhart W., Burgess D. R. Intestinal crypt cells contain higher levels of cytoskeletal-associated pp60c-src protein tyrosine kinase activity than do differentiated enterocytes. Oncogene. 1993 Apr;8(4):1033–1039. [PubMed] [Google Scholar]
- Cartwright C. A., Meisler A. I., Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Cowan W. M., Hunter T., Eckhart W. pp60c-src expression in the developing rat brain. Proc Natl Acad Sci U S A. 1988 May;85(10):3348–3352. doi: 10.1073/pnas.85.10.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Kaplan P. L., Hunter T., Eckhart W. Alterations in pp60c-src accompany differentiation of neurons from rat embryo striatum. Mol Cell Biol. 1987 May;7(5):1830–1840. doi: 10.1128/mcb.7.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Compton C., Cheng E., Fromowitz F., Viola M. V. c-Ki-ras mutations in dysplastic fields and cancers in ulcerative colitis. Gastroenterology. 1992 Jun;102(6):1983–1987. doi: 10.1016/0016-5085(92)90323-q. [DOI] [PubMed] [Google Scholar]
- Ciclitira P. J., Macartney J. C., Evan G. Expression of c-myc in non-malignant and pre-malignant gastrointestinal disorders. J Pathol. 1987 Apr;151(4):293–296. doi: 10.1002/path.1711510409. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- D'Emilia J. C., Mathey-Prevot B., Jaros K., Wolf B., Steele G., Jr, Summerhayes I. C. Preneoplastic lesions induced by myc and src oncogenes in a heterotopic rat colon. Oncogene. 1991 Feb;6(2):303–309. [PubMed] [Google Scholar]
- Ekbom A., Helmick C., Zack M., Adami H. O. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990 Nov 1;323(18):1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Finley G. G., Schulz N. T., Hill S. A., Geiser J. R., Pipas J. M., Meisler A. I. Expression of the myc gene family in different stages of human colorectal cancer. Oncogene. 1989 Aug;4(8):963–971. [PubMed] [Google Scholar]
- Foss F. M., Veillette A., Sartor O., Rosen N., Bolen J. B. Alterations in the expression of pp60c-src and p56lck associated with butyrate-induced differentiation of human colon carcinoma cells. Oncogene Res. 1989;5(1):13–23. [PubMed] [Google Scholar]
- Garcia R., Parikh N. U., Saya H., Gallick G. E. Effect of herbimycin A on growth and pp60c-src activity in human colon tumor cell lines. Oncogene. 1991 Nov;6(11):1983–1989. [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Joslyn G., Carlson M., Thliveris A., Albertsen H., Gelbert L., Samowitz W., Groden J., Stevens J., Spirio L., Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991 Aug 9;66(3):601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem M. F., Meisler A. I., Finley G. G., Bryce W. H., Jones M. O., Tribby I. I., Pipas J. M., Koski R. A. Distribution of cells expressing myc proteins in human colorectal epithelium, polyps, and malignant tumors. Cancer Res. 1992 Nov 1;52(21):5853–5864. [PubMed] [Google Scholar]
- Meltzer S. J., Mane S. M., Wood P. K., Resau J. H., Newkirk C., Terzakis J. A., Korelitz B. I., Weinstein W. M., Needleman S. W. Activation of c-Ki-ras in human gastrointestinal dysplasias determined by direct sequencing of polymerase chain reaction products. Cancer Res. 1990 Jun 15;50(12):3627–3630. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Park J., Meisler A. I., Cartwright C. A. c-Yes tyrosine kinase activity in human colon carcinoma. Oncogene. 1993 Oct;8(10):2627–2635. [PubMed] [Google Scholar]
- Peltomäki P., Aaltonen L. A., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Green J. S., Jass J. R., Weber J. L., Leach F. S. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993 May 7;260(5109):810–812. doi: 10.1126/science.8484120. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Rosen N., Bolen J. B., Schwartz A. M., Cohen P., DeSeau V., Israel M. A. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986 Oct 15;261(29):13754–13759. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Yin J., Harpaz N., Tong Y., Huang Y., Laurin J., Greenwald B. D., Hontanosas M., Newkirk C., Meltzer S. J. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology. 1993 Jun;104(6):1633–1639. doi: 10.1016/0016-5085(93)90639-t. [DOI] [PubMed] [Google Scholar]