Abstract
Study Objectives:
We previously reported that the microinjection of hypocretin (orexin) into the nucleus pontis oralis (NPO) induces a behavioral state that is comparable to naturally occurring active (rapid eye movement) sleep. However, other laboratories have found that wakefulness occurs following injections of hypocretin into the NPO. The present study tested the hypothesis that the discrepancy in behavioral state responses to hypocretin injections is due to the fact that hypocretin was not administered during the same states of sleep or wakefulness.
Design:
Adult cats were implanted with electrodes to record sleep and waking states. Hypocretin-1 (0.25 μL, 500mM) was microinjected into the NPO while the animals were awake or in quiet (non-rapid eye movement) sleep.
Measurements and Results:
When hyprocretin-1 was microinjected into the NPO during quiet sleep, active sleep occurred with a short latency. In addition, there was a significant increase in the time spent in active sleep and in the number of episodes of this state. On the other hand, the injection of hyprocretin-1 during wakefulness resulted not only in a significant increase in wakefulness, but also in a decrease in the percentage and frequency of episodes of active sleep.
Conclusions:
The present data demonstrate that the behavioral state of the animal dictates whether active sleep or wakefulness is induced following the injection of hypocretin. Therefore, we suggest that hypocretin-1 enhances ongoing states of wakefulness and their accompanying patterns of physiologic activity and that hypocretin-1 is also capable of promoting active sleep and the changes in various processes that occur during this state.
Citation:
Xi M; Chase MH. The injection of hypocretin-1 into the nucleus pontis oralis induces either active sleep or wakefulness depending on the behavioral state when it is administered. SLEEP 2010;33(9):1236-1243.
Keywords: Microinjection, orexin, wakefulness, REM sleep, nucleus pontis oralis
HYPOCRETIN-1 AND HYPOCRETIN-2 (ALSO KNOWN AS OREXIN-A AND OREXIN-B) ARE 2 NEUROPEPTIDES THAT ARE SYNTHESIZED BY A WELL-DEFINED GROUP of neurons that are located in the lateral hypothalamus.1–3 A deficiency in the functioning of the hypocretinergic system results in narcolepsy with cataplexy, which is characterized by a sudden loss of muscle tone (i.e., cataplexy) that is triggered by unexpected, strong emotional events in humans, dogs, and other species even though the subjects remain awake.4–7 The data suggest that the hypocretinergic system plays a crucial role in the regulation of not only wakefulness and motor facilitation, but also active (REM) sleep and its accompanying patterns of motor inhibition.
The nucleus pontis oralis (NPO) in the pontine tegmentum plays a key role in the generation of active sleep (AS) as well as wakefulness.8–12 Anatomic studies have shown that both hypocretin receptor-1 and receptor-2 are present in the NPO and that neurons within the NPO are innervated by a rich plexus of hypocretinergic fibers.13–16 We have reported that microinjections of either hypocretin-1 or hypocretin-2 into the NPO induce, with a short latency, a behavioral state that is comparable to naturally occurring AS.17,18 In addition, we have shown that the juxtacellular application of hypocretin-1 (which has a high affinity for both hypocretin receptor-1 and receptor-2) onto NPO neurons results in an increase in the discharge as well as the evoked and spontaneous excitatory synaptic activity of the neurons.17,19 These NPO cells are the putative effector neurons that comprise the active-sleep (AS) generator.8 These data indicate that hypocretinergic processes are involved in the generation of AS and that the NPO is a critical site of action of the hypocretinergic system with respect to the control of this state. On the other hand, recent studies from other laboratories have reported that comparable injections of hypocretin into the NPO result in extended periods of wakefulness, rather than inducement of AS.20,21 Consequently, we hypothesized that these “apparently” paradoxical findings are due to the fact that hypocretin was administered during different behavioral states in the 2 sets of studies. This hypothesis was based upon related state-dependent data, which were initially thought to be “discrepant,” but that revealed that excitatory stimuli that occur during wakefulness result in motor facilitation, although the identical stimuli, when applied during AS, promote profound motor inhibition. This phenomenon was named reticular response-reversal.22 Accordingly, in the present study, we compared, in chronically instrumented unanesthetized cats, the behavioral states that followed the microinjection of hypoctretin-1 into the NPO while the animals were awake with the states that arose when hypocretin-1 was injected during quiet sleep (QS).
MATERIAL AND METHODS
Animals and Surgical Procedures
Five adult cats (3.0-5.5 kg) were used in the present study. Animals, which were housed in a temperature- (22°C ± 1°C) and humidity- (50%-70%) controlled environment with a 12:12-hour light/dark cycle (lights on from 6:00-18:00), had ad libitum access to food and water. All procedures were in accord with the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996) and approved by the Animal Research Committee of the UCLA Office for the Protection of Research Subjects. The animals were prepared for monitoring of the states of sleep and wakefulness and for drug administration, as previously described.23 Briefly, each cat was anesthetized with isoflurane; using sterile surgical procedures, we implanted screw electrodes bilaterally in the calvarium overlying the frontal, parietal, and occipital cortices and into the orbital portion of the frontal bone to monitor the cortical electroencephalogram and electrooculogram, respectively. Wire electrodes for recording the electromyogram were inserted bilaterally into the dorsal neck muscles. A Winchester plug, which was connected to the implanted electrodes, and a chronic head-restraining device were bonded to the skull with acrylic cement. A hole, approximately 4 to 5 mm in diameter, was made in the calvarium overlying the cerebellar cortex and then covered with bonewax to provide subsequent access for a cannula that was used for the microinjection of drugs. Following the operations, the animals received systemically and topically administered antibiotics.
After recovery from the operation, all cats were adapted to the recording conditions on a daily basis for at least 2 weeks. By the end of the adaptation period, the animals exhibited stable spontaneous periods of wakefulness, QS, and AS.
Drug Administration
Following the period of adaptation, carbachol (0.25 μL, 22 mM in saline) was injected into the rostral pontine reticular formation (L: 2, P: 3, and H: −4)24 of each animal to determine the optimal stereotaxic coordinates for the most effective AS-induction site in the NPO. In experimental sessions, all of which were conducted between 10:00 and 16:00, hypocretin-1 (0.25 μL, 500 mM in saline; American Peptide Company, Sunnyvale, CA) was microinjected unilaterally into the NPO, as determined by the stereotaxic coordinates at which carbachol, in preliminary studies in each cat, induced long duration episodes of AS with a latency shorter than 4 minutes. In all cats, control solutions of saline (0.25 μL) were injected into the same site that received the injection of hypocretin-1 (saline sessions). Hypocretin-1 was selected in this study because it has a high affinity for both hypocretin receptor-1 and receptor-2, and is pharmacologically stable. We administered hypocretin-1 in the concentration of 500 mM because the results of a dose–response curve demonstrated that this dose (125 pmol) produces the shortest latency and the greatest increase in AS.17,18 The injection of hypocretin-1 (or saline) was carried out after the animals exhibited at least 2 spontaneous episodes of QS, which always occurred during the first hour of each experimental session. All injections were delivered over a period of 1 minute, using a 2-μL Hamilton syringe, which was connected to a remote-controlled hydraulic micropositioner so that the animals were not disturbed by the injection procedure. In each experimental session, only a single injection was carried out. A maximum of 5 injections were directed to the same site on either the left or the right side of the NPO in each animal. All experimental sessions, in each cat, were separated by at least 3 days. Control patterns of sleep and wakefulness were recorded after each injection session to ensure that the effects of the injections were reversible and that they did not produce any change in sleep or waking states from prior control values.
Polygraphic Recording and Data Analysis
Polygraphic records were recorded and then digitized using a Power Macintosh computer running SuperScope II software (GW Instruments, Somerville, MA). The behavioral states of wakefulness, QS, and AS were scored by analyzing each 15-second time segment of polygraphic records according to standard polygraphic and behavioral criteria.25 Two episodes of recording segments (i.e., 30 seconds) are required to establish the presence of a stable behavioral state of sleep or wakefulness. The following dependent variables were obtained in conjunction with each recording session: (1) percentage of time spent in wakefulness, QS, and AS; (2) latency to the onset of the first episode of wakefulness, QS and AS, as measured from the time of the beginning of the injection; (3) number of episodes of each behavioral state per hour (frequency); and (4) duration of each behavioral state. Experimental data are expressed as means ± SEM.
Analysis of variance for repeated measures and posthoc Scheffé F tests were applied to determine the statistical significance of the changes in individual variables (the percentages of wakefulness, QS, and AS) between drug (hypocretin-1) and control (saline) conditions over time. Accordingly, time was considered as a repeated-measure within-subject variable (3 levels corresponding to 3 hourly measures after injections) and treatment as a between-subject variable (2 levels corresponding to hypocretin-1 and saline). The statistical significance of the difference between sample means during the first hour following the injection of hypocretin-1 and saline was evaluated using the 2-tailed unpaired student's t-test. The criterion chosen to discard the null hypothesis was P < 0.05.
Histologic Determination of Microinjection Sites
After all of the microinjection studies were completed in each animal, the site of the last drug injection was marked with 0.5 μL of a 2% solution of Chicago sky blue dye in 0.5 M of Na acetate. The animal was then euthanized with an overdose of pentobarbital sodium (Nembutal) and perfused with saline followed by a solution of 10% formaldehyde. Serial sections of brainstem tissue were examined to verify the injection sites, which were all located within the NPO (Figure 1A).
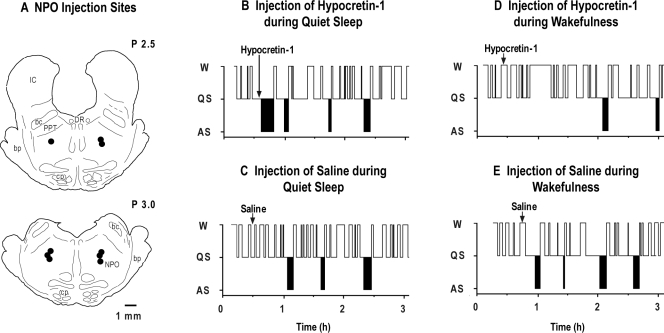
Figure 1.
Schematic presentation of the sites of hypocretin-1 injection in the pons of 5 cats (A). All injection sites, which are indicated by filled circles, are mapped onto coronal brainstem sections at levels P 2.5 and P 3.0. Hypnograms of the effects of (B) the injection of hypocretin-1 during quiet sleep (QS), (C) the injection of saline during QS, (D) the injection of hypocretin-1 during wakefulness (W), and (E) the injection of saline during W from a representative cat. The injection of hypocretin-1 (vertical arrow in B), which was carried out during QS, immediately induced an episode of active sleep (AS). In contrast, the injection of hypocretin-1 during W (vertical arrow in D) induced long-duration episodes of W and blocked the subsequent occurrence of AS for more than 1 hour. bc refers to the brachium conjunctivum; bp, brachium pontis; cp, cerebral peduncle; DR, dorsal raphe nucleus; IC, inferior colliculus; PPT, pedunculopontine tegmental nucleus.
RESULTS
A total of 27 microinjections of hypocretin-1 and 20 control injections of saline were administered in 5 cats. Each animal received at least 5 injections of hypocretin-1 and 4 control injections of saline. Fourteen injections of hypocretin-1 and 10 control injections of saline were carried out during QS; 13 injections of hypocretin-1 and 10 control injections of saline were performed during wakefulness.
Figure 1B-E consists of a series of hypnograms that depict the effects of the microinjection of hypocretin-1 into the NPO during QS and wakefulness. In these representative figures, the injection of hypocretin-1 during QS induced AS with a short latency (Figure 1B). The hypocretin-induced state of AS and naturally occurring AS were indistinguishable on the basis of polygraphic recordings and behavioral observations. On the other hand, when hypocretin-1 was administered during the waking state, there was an increase in wakefulness following its administration (Figure 1D). Injections of saline during either QS or wakefulness did not result in any change in the states of QS, AS, or wakefulness (Figure 1C and E).
Time Period of the Effects Produced by Hypocretin-1 Administration During QS and Wakefulness
The analysis of variance for the percentage of time that the cats spent in AS and wakefulness during each of the first 3 hours following the injection of hypocretin-1 during QS revealed a significant time effect (AS: F2,44 = 21.53, P < 0.001; wakefulness: F2,44 = 10.53, P = 0.002). Similarly, there was a significant time effect with respect to the percentage of AS and wakefulness during each of the 3 postinjection hours following the injection of hypocretin-1 during wakefulness (AS: F2,44 = 20.44, P < 0.001; wakefulness: F2,44 = 15.61, P < 0.001). The results of a posthoc analysis (Scheffé test) demonstrated that, following its injection during QS, hypocretin-1 induced an increase in the time spent in AS during the first hour; this increase was significantly greater than the time spent in this state during the second or third postinjection hour (first hour: 26.1% ± 2.7% vs second hour: 11.1% ± 1.3% P < 0.001; first hour vs third hour: 12.3% ± 1.2%, P < 0.001). In addition, following the injection of hypocretin-1 during wakefulness, there was a significant increase in wakefulness during the first postinjection hour compared with the time spent in wakefulness during the second or third postinjection hour (first hour: 53.1% ± 3.3% vs second hour: 32.1% ± 2.8% P < 0.001; first hour vs third hour: 33.0% ± 2.9%, P < 0.001, posthoc Scheffé test). There were no significant changes in either sleep or wakefulness during the second or third postinjection hour following the injection of hypocretin-1 during QS or wakefulness, compared with saline control sessions, which indicates that the amount of sleep and wakefulness returned to control (saline) levels during the second and third postinjection hours of recording.
Because the effects produced by hypocretin-1 were limited to the first hour following its administration, as presented in the following sections, we conducted an analysis during this period of time of the specific changes in sleep and waking states that arose in conjunction with the administration of hypocretin-1 during QS and wakefulness.
Effects of Hypocretin-1 on Sleep and Wakefulness When Administered During QS
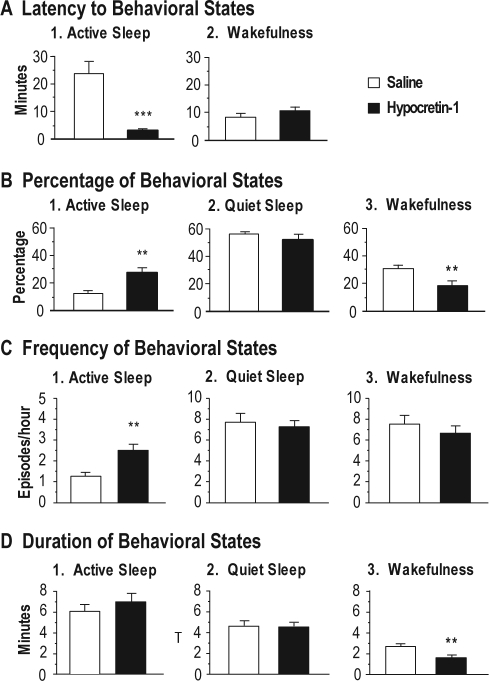
Figure 2A shows the mean latency to the onset of the first episode of AS and wakefulness following the application of hypocretin-1. Because injections were carried out during QS, there was no measure of the latency to this state. The mean latency to AS following the application of hypocretin-1 was 3.2 ± 0.4 minutes (n = 14), which was significantly shorter than that observed following saline injections (n = 10; df = 22, t = 5.78, P < 0.001, unpaired t-test).
Figure 2.
Effects of the injection of hypocretin-1 into the nucleus pontis oralis on the latency (A), percentage (B), frequency (C) and duration (D) of wakefulness, quiet sleep, and active sleep during the first hour following its injection during quiet sleep. The bars represent mean values; error bars indicate the SEM of each population. A: Graphs highlight the latency to the onset of the first episode of active sleep and wakefulness following injections of hypocretin-1 (n = 14) and saline (n = 10). Note that hypocretin-1 significantly reduced the mean latency to active sleep. B: Histograms illustrating the percentages of behavioral states following the injection of hypocretin-1, which significantly increased the time spent in active sleep and decreased the amount of wakefulness, compared with injections of saline. C: Graphs present the number of episodes of behavioral states per hour (frequency) following the injection of hypocretin-1 and saline. Injections of hypocretin-1 significantly increased the frequency of episodes of active sleep. D: Graphs showing that injections of hypocretin-1 significantly reduced the mean duration of episodes of wakefulness. Asterisks indicate the levels of statistical significance of the difference between means: **P < 0.01, ***P < 0.001 by unpaired t-test.
The percentage of time that the cats spent in sleep and waking states following the injection of hypocretin-1 during QS is shown in Figure 2B. Compared with saline injections, injections of hypocretin-1 significantly increased the amount of AS by 99.2% (df = 22, t = 3.72, P = 0.002, unpaired t-test). This increase was accompanied by a significant decrease of 34.2% in the time spent in wakefulness (df = 22, t = 3.06, P = 0.006, unpaired t-test). There was no statistically significant difference in the time spent in QS following the injection of hypocretin-1 compared with control injections.
The effects of the injection of hypocretin-1 into the NPO during QS on the frequency of sleep and waking states are shown in the Figure 2C. Following the injection of hypocretin-1, the frequency of AS increased significantly (df = 22, t = 0.56, P = 0.002, unpaired t-test). The frequency of episodes of wakefulness or QS did not change significantly following the injection of hypocretin-1. The mean duration of the AS episodes that occurred following the injection of hypocretin-1 was not significantly different from that observed following the injection of saline, although there was a tendency for an increase in the duration of episodes of AS in conjunction with the administration of hypocretin-1 (Figure 2D; df = 22, t = 1.12, P = 0.276, unpaired t-test).
Effects of Hypocretin-1 on Sleep and Wakefulness When Administered During Wakefulness
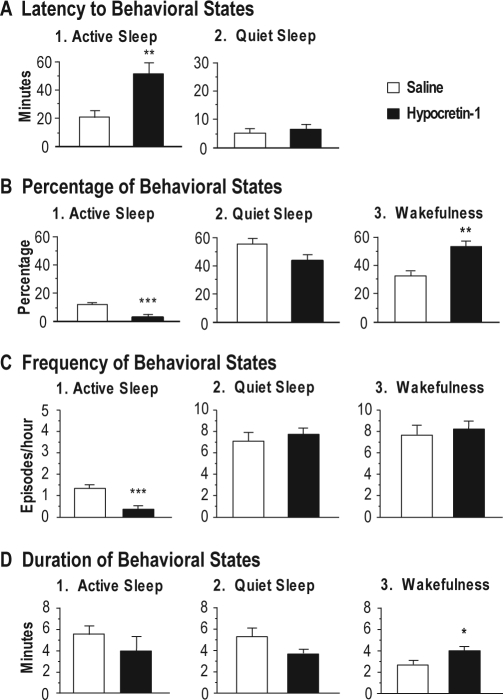
The latency to the onset of the first episode of AS and QS following the application of hypocretin-1 during waking states is shown in Figure 3A. The mean latency to AS following the application of hypocretin-1 during waking states was significantly longer than that observed following saline injections (df = 17, t = 3.30, P = 0.042, unpaired t-test).
Figure 3.
Effects of hypocretin-1 injections into the nucleus pontis oralis on the latency (A), percentage (B), frequency (C) and duration (D) of wakefulness, quiet sleep, and active sleep during the first hour following injections that were carried out while the animals were awake. Bars represent mean values; error bars indicate the SEM of each population. A: Graphs show the latency to the onset of the first episode of active sleep and quiet sleep following injections of hypocretin-1 (n = 13) and saline (n = 10). Injections of hypocretin-1 significantly increased the mean latency to active sleep. Because injections were carried out during wakefulness, there was no measure of the latency to wakefulness. B: Histograms illustrate the percentages of behavioral states observed following the injection of hypocretin-1, which significantly increased the percentage of time spent in wakefulness and decreased active sleep, compared with saline injections. C: Graphs present the number of episodes of behavioral states per hour (frequency) following the injection of hypocretin-1 and saline. Note that injections of hypocretin-1 significantly reduced the frequency of episodes of active sleep. D: Graphs showing that hypocretin-1 significantly increased the mean duration of episodes of wakefulness. Asterisks indicate the levels of statistical significance of the difference between means: *P < 0.05, **P < 0.01, ***P < 0.001 by unpaired t-test.
An increase in wakefulness occurred when hypocretin-1 was injected into the NPO while the animals were awake. Figure 3B presents the effects of hypocretin-1, when injected during wakefulness, on the percentage of time that the cats spent in sleep and waking states following the injection. Compared with saline injections, injections of hypocretin-1 during wakefulness significantly increased the time spent in this state by 58.5% (df = 21, t = 4.37, P < 0.001, unpaired t-test). In addition, there was a corresponding decrease in AS by 81.5% (df = 21, t = 6.23, P < 0.001, unpaired t-test). There was a tendency toward a decrease in QS, although the mean percentage of time spent in this state was not significantly different from that observed following the injection of saline.
The injection of hypocretin-1 into the NPO during wakefulness also resulted in a change in the frequency of sleep and waking states, as shown in Figure 3C. Thus, following the injection of hypocretin-1, the frequency of episodes of AS decreased significantly (df = 21, t = 4.09, P < 0.001, unpaired t-test). On the other hand, the frequency of episodes of either wakefulness or QS did not change significantly following the injection of hypocretin-1. The mean duration of episodes of wakefulness following the injection of hypocretin-1 during wakefulness was significantly longer than that observed following the injection of saline (Figure 3D; df = 21, t = 1.97, P = 0.042, unpaired t-test).
Comparison of the Effects Produced by Hypocretin-1 Administration During QS and Wakefulness
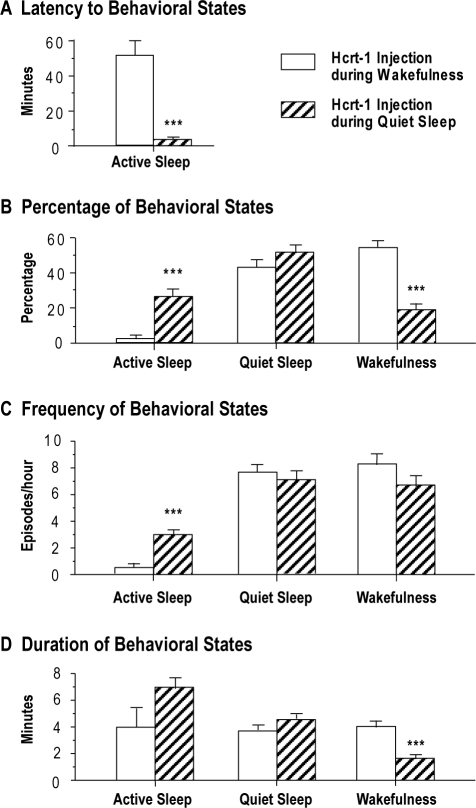
Figure 4A shows that the mean latency to AS following the application of hypocretin-1 during QS was significantly shorter than that observed following the injection of hypocretin-1 during wakefulness (df = 21, t = 7.43, P < 0.001, unpaired t-test).
Figure 4.
Comparisons of the effects of hypocretin-1 (Hcrt-1) injections into the nucleus pontis oralis on the latency (A), percentage (B), frequency (C), and duration (D) of wakefulness, quiet sleep, and active sleep following the injection of Hcrt-1 during quiet sleep or wakefulness. Bars represent the mean values; error bars indicate the SEM of each population. A: Graphs show the latency to the onset of the first episode of active sleep following injections of Hcrt-1 during either quiet sleep (n = 14) or wakefulness (n = 13). Injections of Hcrt-1 during wakefulness significantly increased the mean latency to active sleep. B: Histograms illustrating the percentages of behavioral states following the injection of Hcrt-1 during quiet sleep, which significantly increased the percentage of time spent in active sleep, compared with the percentages following the injection of Hcrt-1 during wakefulness. In contrast, the animals spent a significantly greater the percentage of time in wakefulness following injections of Hcrt-1 during this state, compared with the time spent in wakefulness following injections during quiet sleep. C: Graphs present the frequency of episodes of behavioral states following the injection of Hcrt-1 either during quiet sleep or wakefulness. Injections of Hcrt-1 during quiet sleep significantly increased the frequency of episodes of active sleep. D: Graphs show that injections of Hcrt-1 during wakefulness resulted in a significant increase in the mean duration of episodes of wakefulness. Asterisks indicate the levels of statistical significance of the difference between means: ***P < 0.001 by unpaired t-test.
Figure 4B presents the percentage of time spent in sleep and wakefulness following hypocretin-1 injections during QS, compared with the time in these states that occurred when hypocretin-1 was administered while the animals were awake. The cats spent significantly more time in AS following the injection of hypocretin-1 during QS (26.1% ± 2.7%), compared with the amount of AS that occurred following injections during wakefulness (2.3% ± 1.2%; df = 25, t = 7.76, P < 0.001, unpaired t-test). On the other hand, when hypocretin-1 was injected during wakefulness, the animals spent significantly more time in this state (53.1% ± 3.3%), compared with the amount of wakefulness that occurred when injections were carried out during QS (19.9% ± 2.5%, df = 25, t = 8.12, P < 0.001, unpaired t-test).
The frequency of episodes of AS also increased significantly following the injection of hypocretin-1 during QS compared with during wakefulness (Figure 4C; df = 25, t = 6.97, P < 0.001, unpaired t-test). In 7 out of 14 experimental sessions in which hypocretin-1 was injected into the NPO during QS, 3 or more episodes of AS occurred during the first postinjection hour. On the other hand, in 7 of 13 experimental sessions in which hypocretin-1 was injected during wakefulness, no episodes of AS occurred during this period. The mean duration of episodes of wakefulness following the injection of hypocretin-1 during QS was significantly shorter than that following the injection of hypocretin-1 during the waking state (Figure 4D; df = 25, t = 6.98, P < 0.001, unpaired t-test). In addition, there was a tendency toward an increase in the duration of episodes of AS following the injection of hypocretin-1 during QS, compared with during wakefulness, although this increase did not reach statistical significance.
DISCUSSION
In the present study, we demonstrated that the behavioral state of the animal, at the time of the administration of hypocretin-1 into the NPO, determined whether wakefulness or AS was induced. If the cat was in QS at the time when hypocretin was injected, the AS state arose with a short latency. The hypocretin-induced state of AS was comparable to naturally occurring AS on the basis of polygraphic recordings (see Figure 2 in Xi et al.17). In addition, there was a significant increase in the percentage of time spent in AS and a corresponding reduction in wakefulness. Furthermore, the mean latency to AS following the application of hypocretin-1 during QS was significantly shorter than that observed following the injection of hypocretin-1 during wakefulness. Finally, following the injection of hypocretin-1 during QS, there was a significant increase in the number of episodes of AS. In contrast, if the animal was awake at the time of injection, there was a significant increase in the percentage of time spent in wakefulness and a comparable reduction in the amount and number of episodes of AS. These data support our hypothesis that not only does hypocretin act to promote an ongoing state of wakefulness and its accompanying patterns of somatomotor activation,26 but that hypocretin is also capable of producing AS and the inhibition of somatomotor activity that occurs during this state.27
Other laboratories have recently reported that injections of hypocretin into the NPO result in an increase in wakefulness and a suppression of AS in the rat and cat,20,21 which initially appeared to contradict our previously reported findings that AS arose following the administration of hypocretin into the NPO.17,18 The present study was conducted to determine if these apparently “contradictory” findings could be explained on the basis that the state of the animals was different when hypocretin was injected in 2 sets of studies. We conclude that the present study provides strong evidence that the state of the animal at the time of the injection of hypocretin is the critical factor that is responsible for determining whether AS or wakefulness is induced. Accordingly, these results resolve previous “apparently” paradoxical findings that different behavioral states arise when hypocretin is injected into the NPO.17,18,20,21
The present findings, which reveal that different behavioral responses occur depending on the ongoing state of sleep and wakefulness, are similar to other state-dependent phenomenon that we described a number of years ago, which we named reticular response-reversal.28 This phenomenon is one in which there is a striking reversal in various physiologic patterns of activity to exteroceptive and interoceptive inputs that are governed by the ongoing state of sleep or wakefulness. Our first description of the phenomenon of reticular response-reversal was based upon our finding that somatomotor responses to excitatory afferents were either facilitated or inhibited depending on the behavioral states of the animal. We reported that electrical stimulation of the NPO results in motor excitation during wakefulness, whereas the identical stimulus elicits a diametrically opposite motor drive, inhibition, during AS.29,30 We determined that the state of the animal at the time of stimulation of the NPO determines whether the resultant motor drive is excitatory or inhibitory. In the present study, injections of hypocretin-1 were directed to the same region of the pontine reticular formation, i.e., the NPO, that we believe is responsible for generating the state of AS as well as the processes that result in reticular response-reversal. Therefore, we suggest that the phenomenon of reticular response-reversal, together with the present results, provide additional support for the concept that the state of the animal is critically important when evaluating the results of state-related experiments.
A great number of studies have presented complementary data indicating that the NPO is the critical brainstem region that is ultimately responsible for the generation of AS, somatomotor atonia, and other AS-dependent patterns of physiologic activity.8–12 Anatomically, hypocretinergic fibers project to cells within the NPO14,15; in addition, cells in the NPO contain both hypocretin receptor-1 and receptor-2.13,16,31 Microdialysis experiments have shown that the release of hypocretin in the hypothalamus is greater during AS, compared with QS, although the highest levels are present during wakefulness.32 Kiyashchenko et al.33 reported that the microinjection of hypocretin into the pontine reticular formation produces motor inhibition in decerebrated rats, which is consistent with our observation that the microinjection of hypocretin into the NPO elicits an AS-like state and its accompanying pattern of motor inhibition. Although unit-recording studies in the rat and mouse have not shown that hypocretinergic cells discharge during AS,34–36 the studies of Steininger et al.37 and Alam et al.38 described electrophysiologically identified AS-on neurons and waking-on/AS-on neurons in the hypocretinergic zone of the hypothalamus, although the neurotransmitter phenotype of these recorded cells was not determined. Using c-fos immunocytochemistry as a marker of neuronal activity, we discovered that a group of hypocretinergic neurons in the lateral hypothalamus is activated during carbachol-induced AS.39
Our prior electrophysiologic data also demonstrate that hypocretin excites neurons of the NPO.17 We therefore suggest that a preponderance of the evidence indicates that the hypocretinergic system plays an important role in AS-related processes and that the NPO is one of critical sites of action for the hypocretinergic system with respect to the control of AS. In addition, NPO neurons are a key component of the brainstem–spinal-cord inhibitory system that is responsible for promoting muscle atonia during AS; these cells also are components of the “neuronal gate” that converts excitatory drives to motoneurons that occur during wakefulness into powerful inhibitory inputs during AS, which was our original finding that led to our description of the phenomenon of reticular response-reversal.28–30 Therefore, we suggest that the change in behavioral state (i.e., AS), induced by the injection of hypocretin into the NPO during QS, results from the activation of the same population of NPO neurons that is normally responsible for the generation of AS and the accompanying inhibition of muscle activity.11
In the present study, the administration of hypocretin into the NPO during wakefulness promoted wakefulness and suppressed AS. These results agree with findings in the cat and the rat that hypocretin-1 produces an increase in wakefulness and a reduction in AS when it is injected into the NPO while the animals are awake.20,21 A large body of evidence demonstrates that the hypocretinergic system is critically involved in promoting wakefulness and that it also activates somatomotor functions that normally accompany this state.40–44 Since the NPO is an established component of the ascending reticular activating system that is responsible for promoting arousal and motor activation,45 and the hypocretins are “excitatory” neurotransmitters, we suggest that hypocretinergic projections to the NPO constitute an important contribution to the mesopontine system that is responsible for the generation and maintenance of wakefulness. On the other hand, the present data also demonstrate that hypocretin produces an entirely different response, which is the induction of AS, when it is injected into the NPO during QS. These data indicate that hypocretinergic synaptic contacts on neurons in the NPO also participate in promoting AS and the inhibition of motor activity that accompanies this state. When pathologies occur, such as in cataplexy, wherein there is a significant decrease in the functioning of the hypocretinergic system,5,6,46 we hypothesize that there is a corresponding reduction in hypocretinergic activation of NPO neurons, which results in a striking reduction in motor activity during wakefulness and a relative increase in motor activity during AS, in a manner that is coherent with the phenomena of reticular response-reversal. This hypothesis is supported by the fact that cataplectic humans and canines exhibit a decrease in motor activity during wakefulness as well as a relative increase in somatomotor tone during AS.43,47–49
Hypocretin levels are low or undetectable in narcoleptics with cataplexy.50,51 However, the hypocretinergic system is intact (i.e., hypocretin levels in the cerebrospinal fluid are normal) in narcoleptics without cataplexy.50,51 Since there is no difference in the latency to AS and the amount of AS in both narcoleptics with and without cataplexy,52,53 it is clear that the hypocretinergic system does not have a significant impact on either parameter of AS. Nevertheless, there is a population of hypocretinergic neurons that are activated during AS,39 and unit-recording studies indicate that these neurons discharge selectively during the phasic periods of AS.34,35 Therefore, we suggest that the promotion of AS following the injection of hypocretin into the AS generator in the NPO reflects inputs of hypocretinergic origin that are normally associated with the phasic periods of AS.
Although the neuronal (cellular)-level mechanisms that mediate the dual state-dependent effects of hypocretin in the NPO have not been elucidated, it is possible that the generation of AS and wakefulness occurs as a result of different levels of activity during sleep and wakefulness in neuronal structures/neurotransmitter systems that project to the NPO. For example, hypocretin, when injected during wakefulness, may facilitate the discharge of waking-on NPO neurons that are already excited by serotonergic projections from the dorsal raphe and noradrenergic inputs from the locus coeruleus, since neurons of these cell groups project to the NPO and exhibit their highest activity levels during the waking state.54–57 On the other hand, since there is a dramatic decrease in discharge of the preceding groups of monoaminergic neurons during QS, compared with wakefulness,55–58 it is possible that the potential for activation of AS-on cells in the NPO is enhanced following the administration of hypocretin during QS by monoaminergic disinhibition.59
We have previously reported that the effects of the injection of hypocretin-1 on sleep and waking states are not significantly different from those produced by hypocretin-2.17 Hypocretin-1 is known to have an equally high affinity for both hypocretin receptor-1 and receptor-2, whereas hypocretin-2 has a 10-fold higher affinity for hypocretin receptor-2 than for hypocretin receptor-1.3 Based on our data and the difference in the affinities of hypocretin receptor-1 and receptor-2, we suggest that hypocretinergic processes, acting on hypocretin receptor-2 rather than hypocretin receptor-1 in the NPO, may play a more important role in the control of AS and wakefulness. On the other hand, molecular and behavioral studies using hypocretin-receptors knock-out mice suggest that, although both receptors are involved in the regulation of wakefulness and sleep, hypocretin receptor-2 is more important for the maintenance of wakefulness, whereas hypocretin receptor-1 may be principally involved in the regulation of AS.60,61 Future experiments using specific hypocretin-receptor antagonists would be useful in determining the receptor subtype or subtypes involved.
In summary, the present findings demonstrate that AS, as well as muscle atonia, can be induced with a short latency following the microinjection of hypocretin-1 into the NPO during QS, whereas prolonged periods of wakefulness arise following the microinjection of hypocretin-1 into the NPO when the animal is awake. These data highlight the importance of the behavioral state of the animal at the time of the administration of hypocretin. In addition, the present findings support our hypothesis that hypocretin has dual functions that are expressed according to the phenomenon of reticular response-reversal, which consist of the promotion of wakefulness and motor activity, as well as the generation of AS and motor inhibition.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the following grant from the U.S. Public Health Service: MH 43362. We thank Mr. Oscar Ramos, Mr. Trent Wenzel, Ms. Ricki-Leigh Malaguti, and Ms. Emi Koda for their excellent technical assistance.
REFERENCES
- 1.Zhang JH, Sampogna S, Morales FR, Chase MH. Orexin (hypocretin)-like immunoreactivity in the cat hypothalamus: a light and electron microscopic study. Sleep. 2001;24:67–76. doi: 10.1093/sleep/24.1.67. [DOI] [PubMed] [Google Scholar]
- 2.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 4.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 5.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 6.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol. 2003;13:340–51. doi: 10.1111/j.1750-3639.2003.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. New York: Plenum Press; 2005. [Google Scholar]
- 9.Siegel JM. Brainstem mechanisms generating REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: WB Saunders; 2000. pp. 112–33. [Google Scholar]
- 10.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–88. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 11.Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–84. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 12.Jones BE. Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience. 1991;40:637–56. doi: 10.1016/0306-4522(91)90002-6. [DOI] [PubMed] [Google Scholar]
- 13.Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 14.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JH, Sampogna S, Morales FR, Chase MH. Distribution of hypocretin (orexin) immunoreactivity in the feline pons and medulla. Brain Res. 2004;995:205–17. doi: 10.1016/j.brainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88:176–82. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 17.Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–8. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- 18.Xi MC, Chase MH. Neuronal mechanisms of active (rapid eye movement) sleep induced by microinjections of hypocretin into the nucleus pontis oralis of the cat. Neuroscience. 2006;140:335–42. doi: 10.1016/j.neuroscience.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Hypocretinergic facilitation of synaptic activity of neurons in the nucleus pontis oralis of the cat. Brain Res. 2003;976:253–8. doi: 10.1016/s0006-8993(03)02566-6. [DOI] [PubMed] [Google Scholar]
- 20.Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–64. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno-Balandran E, Garzon M, Bodalo C, Reinoso-Suarez F, de Andres I. Sleep-wakefulness effects after microinjections of hypocretin 1 (orexin A) in cholinoceptive areas of the cat oral pontine tegmentum. Eur J Neurosci. 2008;28:331–41. doi: 10.1111/j.1460-9568.2008.06334.x. [DOI] [PubMed] [Google Scholar]
- 22.Babb MI, Chase MH. Masseteric and digastric reflex activity during conditioned sensorimotor rhythm. Electroencephalogr Clin Neurophysiol. 1974;36:357–65. doi: 10.1016/0013-4694(74)90185-0. [DOI] [PubMed] [Google Scholar]
- 23.Yamuy J, Mancillas JR, Morales FR, Chase MH. C-fos expression in the pons and medulla of the cat during carbachol-induced active sleep. J Neurosci. 1993;13:2703–18. doi: 10.1523/JNEUROSCI.13-06-02703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berman AL. A cytoarchitectonic atlas with stereotaxic coordinates. Madison: Univ. of Wisconsin Press; 1968. The brainstem of the cat. [Google Scholar]
- 25.Ursin R, Sterman M. Manual for standardized scoring of sleep and waking states in adult cats. Los Angeles: University of California; 1981. [Google Scholar]
- 26.Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–45. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamuy J, Fung SJ, Xi M, Chase MH. State-dependent control of lumbar motoneurons by the hypocretinergic system. Exp Neurol. 2010;221:335–45. doi: 10.1016/j.expneurol.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chase MH, Babb M. Masseteric reflex response to reticular stimulation reverses during active sleep compared with wakefulness or quiet sleep. Brain Res. 1973;59:421–6. doi: 10.1016/0006-8993(73)90284-9. [DOI] [PubMed] [Google Scholar]
- 29.Chandler SH, Nakamura Y, Chase MH. Intracellular analysis of synaptic potentials induced in trigeminal jaw-closer motoneurons by pontomesencephalic reticular stimulation during sleep and wakefulness. J Neurophysiol. 1980;44:372–82. doi: 10.1152/jn.1980.44.2.372. [DOI] [PubMed] [Google Scholar]
- 30.Fung SJ, Boxer PA, Morales FR, Chase MH. Hyperpolarizing membrane responses induced in lumbar motoneurons by stimulation of the nucleus reticularis pontis oralis during active sleep. Brain Res. 1982;248:267–73. doi: 10.1016/0006-8993(82)90584-4. [DOI] [PubMed] [Google Scholar]
- 31.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–97. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 32.Kiyashchenko LI, Mileykovskiy BY, Maidment N, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–6. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiyashchenko LI, Mileykovskiy BY, Lai YY, Siegel JM. Increased and decreased muscle tone with orexin (hypocretin) microinjections in the locus coeruleus and pontine inhibitory area. J Neurophysiol. 2001;85:2008–16. doi: 10.1152/jn.2001.85.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–70. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 37.Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999;840:138–47. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- 38.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–31. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypothalamic neurons that contain hypocretin (orexin) express c-fos during active wakefulness and carbachol-induced active sleep. Sleep Res Online. 2001;4:25–32. [Google Scholar]
- 40.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 41.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 42.Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Curr Opin Pharmacol. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol. 2004;55:125–48. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilduff TS. Hypocretin/orexin: maintenance of wakefulness and a multiplicity of other roles. Sleep Med Rev. 2005;9:227–30. doi: 10.1016/j.smrv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 46.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 47.Wittig R, Zorick F, Piccione P, Sicklesteel J, Roth T. Narcolepsy and disturbed nocturnal sleep. Clin Electroencephalogr. 1983;14:130–4. doi: 10.1177/155005948301400306. [DOI] [PubMed] [Google Scholar]
- 48.Mitler MM, Dement WC. Sleep studies on canine narcolepsy: pattern and cycle comparisons between affected and normal dogs. Electroencephalogr Clin Neurophysiol. 1977;43:691–9. doi: 10.1016/0013-4694(77)90084-0. [DOI] [PubMed] [Google Scholar]
- 49.Dauvilliers Y, Rompre S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30:844–9. doi: 10.1093/sleep/30.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 51.Dauvilliers Y, Baumann CR, Carlander B, et al. CSF hypocretin-1 levels in narcolepsy, Kleine-Levin syndrome, and other hypersomnias and neurological conditions. J Neurol Neurosurg Psychiatry. 2003;74:1667–73. doi: 10.1136/jnnp.74.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassetti C, Aldrich MS. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;120:1423–35. doi: 10.1093/brain/120.8.1423. [DOI] [PubMed] [Google Scholar]
- 53.Aldrich MS. Diagnostic aspects of narcolepsy. Neurology. 1998;50:S2–7. doi: 10.1212/wnl.50.2_suppl_1.s2. [DOI] [PubMed] [Google Scholar]
- 54.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–50. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 55.Chu NS, Bloom FE. Activity patterns of catecholamine-containing pontine neurons in the dorso-lateral tegmentum of unrestrained cats. J Neurobiol. 1974;5:527–44. doi: 10.1002/neu.480050605. [DOI] [PubMed] [Google Scholar]
- 56.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–8. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 57.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–75. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 59.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–7. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–58. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 61.Willie JT, Chemelli RM, Sinton CM, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–30. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]