Abstract
Primary hyperparathyroidism (HPT) results from the excessive secretion of parathyroid hormone from parathyroid tumors. While most HPT is sporadic, it is associated with a familial syndrome in a minority of cases. Study of these syndromes has helped define the pathophysiology of both familial and sporadic parathyroid neoplasms. Investigation of kindreds with multiple endocrine neoplasia type 1 (MEN1) and the hyperparathyroidism-jaw tumor syndrome led to the discovery of the tumor suppressor genes MEN1 and HRPT2. We now recognize that somatic mutations in MEN1 and HRPT2 tumor suppressor genes are frequent events in sporadic parathyroid adenomas and carcinomas, respectively. Parathyroid tumors in the MEN2A syndrome result from mutational activation of the RET oncogene. The CCND1/PRAD1 oncogene was discovered by analysis of sporadic parathyroid tumors. Studies of familial isolated hyperparathyroidism and analysis of chromosomal loss and gain in parathyroid tumors suggest that other genes relevant to parathyroid neoplasia await identification.
Keywords: hyperparathyroidism; parathyroid neoplasms; genes, tumor suppressor; oncogenes; multiple endocrine neoplasia; CDC73; CCND1; RET
Introduction
Primary hyperparathyroidism (HPT) is associated with a familial syndrome in a significant subset of patients [1]. Studies that have sought to define the molecular genetics behind these syndromes have led to a number of insights into the pathophysiology of parathyroid neoplasms. Germline inactivating mutations in the MEN1 and HRPT2 tumor suppressor genes have been strongly associated with familial parathyroid tumors [2-6]. Somatic mutations in these genes have also been demonstrated in sporadic parathyroid adenomas and carcinomas, respectively. Gain-of-function mutations affecting two oncogenes have also been implicated in the etiology of some benign parathyroid tumors. Although signaling mediated via the calcium-sensing receptor (CaSR) and vitamin D receptor (VDR) impact the hormonal function of normal and neoplastic parathyroid tissue, mutations in the genes encoding these receptors have not yet been linked to the development of sporadic parathyroid tumors. This article will review what is currently known regarding the molecular pathogenesis of parathyroid tumors.
Primary hyperparathyroidism: General concepts
Regulation of ionized calcium is achieved by secretion of parathyroid hormone (1-84) in response to changes in the ionized calcium within a relatively narrow physiologic range. Secretion of PTH is negatively regulated by the CaSR located on the surface of the parathyroid chief cells [7, 8]. PTH maintains the serum ionized calcium primarily by three mechanisms: stimulation of calcium reabsorption in the distal tubule of the kidney, stimulation of osteoclast resorption in the bone, and activation 25-hydroxyvitamin D 1-alpha hydroxylase in the proximal renal tubule, leading to synthesis of 1,25 dihydroxyvitamin D which in turn promotes increased calcium absorption in the small bowel.
Primary hyperparathyroidism is defined by elevation of serum ionized calcium in the setting of an inappropriate elevation of PTH [1]. Serum phosphorus is typically in the lower end of the normal range in HPT as a result of the phosphaturic action of PTH at the proximal renal tubule. Alkaline phosphatase and markers of bone formation and resorption are frequently elevated. Elevated serum chloride and decreased bicarbonate are also sometimes seen. The condition is asymptomatic in 70-80% of patients, and is frequently detected incidentally on routine chemistry panels. Common symptomatic manifestations include hypercalciuria, nephrolithiasis, osteoporosis and neuromuscular changes, such as fatigue, weakness and cognitive changes. Advanced disease is classically characterized by osteitis fibrosa cystica, a severe syndrome of skeletal demineralization [1, 2, 9]. Primary hyperparathyroidism occurs at all ages, but peaks in the sixth decade, with a female-to-male ratio between 2 and 3:1. Parathyroid adenomas have been associated with prior exposure to ionizing radiation. Increased incidence of adenomas is documented with doses as low as 0.5 Gy, especially when the exposure occurs in childhood.[10]
Parathyroid carcinoma is a rare cause of primary hyperparathyroidism, seen in less than 1% of cases [11, 12]. Parathyroid carcinoma can be difficult to diagnose, as many of the pathologic features are neither sensitive nor specific. Clinical findings suggestive of carcinoma may include a palpable neck mass, hoarseness, serum calcium greater than 3.5 mmol/L (14 mg/dL), and overt bone or kidney disease. Pathologic findings include fibrosis, increased mitotic activity, nuclear atypia, pleomorphism, invasion of surrounding tissues, distant metastases, and angio-lymphatic or perineural invasion.
Approximately 5 % of cases of primary hyperparathyroidism are associated with familial syndromes, but study of this group has provided great insight into the genetic and molecular changes that underlie the neoplastic transformation of parathyroid tissue (Table 1). The most common genetic syndromes associated with primary hyperparathyroidism are multiple endocrine neoplasia types 1 and 2A (MEN1, MEN-2A), the hyperparathyroidism-jaw tumor syndrome (HPT-JT), and familial isolated hyperparathyroidism (FIHP)[1-3, 9]. Familial hypocalciuric hypercalcemia (FHH) is a related, clinically benign syndrome resulting from heterozygous loss of function of the CaSR that does not correct with partial or subtotal parathyroidectomy (PTX) [7, 8]. These syndromes, their relation to parathyroid tumors and the molecular and genetic alterations that underlie them will be discussed in detail below (Table 1).
Table 1.
Genes implicated in syndromic and sporadic parathyroid tumorigenesis, and related syndromes
| Gene | Protein encoded |
Associated hyperparathyroid syndrome: main syndromic manifestations |
Features of syndromic parathyroid tumors |
Defect in sporadic parathyroid tumors |
|---|---|---|---|---|
| MEN1 | Menin | Multiple endocrine neoplasia type 1: anterior pituitary, parathyroid, enteropancreatic, foregut carcinoid tumors |
Multiple, asymmetric tumors typical (> 99% benign) |
Inactivation in ~25- 35% of benign tumors; mutation exceedingly rare in cancer |
| HRPT2/CDC73 | Parafibromin | Hyperparathyroidism-jaw tumor syndrome: fibro-osseous jaw, parathyroid, uterine tumors; renal cysts |
Single tumor common (~15% malignant) |
Inactivation in ~70% of cancers; mutation rare in sporadic adenomas |
| CASR | Calcium- sensing receptor |
Familial hypocalciuric hypercalcemia (FHH) with heterozygous inactivation; neonatal severe hyperparathyroidism (NSHPT) with homozygous inactivation |
FHH: near-normal size and surgical pathology; altered serum calcium set-point for PTH release NSHPT: Marked enlargement of multiple glands |
Decreased expression common; mutation exceedingly rare |
| RET | c-Ret | Multiple endocrine neoplasia type 2A: medullary thyroid cancer, pheochromocytoma, parathyroid tumors |
Single tumor common (> 99% benign) |
Mutation exceedingly rare |
| CCND1/PRAD1 | Cyclin D1 | NA | NA | Overexpression results from DNA rearrangement involving PTH gene |
NA, not applicable
Tumor suppressors and the two-hit hypothesis
An important model for tumor development was proposed by Alfred Knudson from his epidemiologic analysis of retinoblastoma nearly 40 years ago [13]. Sporadic retinoblastoma is much more common than familial cases, yet the latter has a much earlier age of onset and more frequently affects both eyes. The “two-hit” hypothesis of neoplasia as proposed by Knudson suggests that two events (or “hits”) in an affected cell confer a selective growth advantage resulting in clonal expansion of its progeny. Knudson’s concept can be updated in accordance with current data. In many hereditary tumor syndromes the first event or “hit” is an inherited mutation in one allele of a tumor suppressor gene in the germline DNA that is therefore present in all the cells of the affected offspring. The earlier age of onset and tendency for bilateral or multifocal disease in familial tumor syndromes is explained by the greater likelihood of an individual cell acquiring a “second hit”, i.e. a somatic second mutation in the same gene. The second-hit, that inactivates the wild-type allele, most often results from DNA rearrangement or large, subchromosomal or even chromosomal, deletion. Such DNA loss can be recognized by loss-of heterozygosity (LOH) of DNA markers (such as polymorphic microsatellite repeats or single nucleotide polymorphisms) in the vicinity of the pertinent tumor suppressor gene. Parathyroid tumors in the context of the familial syndromes MEN1 and HPT-JT have been associated with bi-allelic loss of function of the MEN1 and HRPT2 tumor suppressor genes, respectively. In the majority of patients, an inactivating germline mutation of the implicated gene can be demonstrated.
Multiple endocrine neoplasia type 1 and the MEN1 gene
Multiple endocrine neoplasia type 1 is the most common familial cause of primary hyperparathyroidism, accounting for approximately 2% of all cases [14]. Overall, the syndrome is rare, with a prevalence of 2-3 per 100,000. It is characterized by a predisposition to develop endocrine tumors in pituitary, parathyroid and enteropancreatic endocrine cells, although tumors in several other endocrine and non-endocrine tissues are also associated with the syndrome [15].
Primary hyperparathyroidism is the most common endocrine component of MEN1, demonstrating greater than 90% penetrance by age 50 years. In contrast to sporadic disease, there is no female preponderance, and it typically presents in the second to fourth decade of life. Disease is usually multiglandular and has a high rate of recurrence following apparent surgical cure [16, 17].
MEN1 has an autosomal dominant inheritance pattern. The tumor susceptibility results from germline inactivation of one allele of the MEN1 gene on chromosome 11q13, a 9.8 kb gene consisting of 10 exons, that encodes the 610 amino acid protein, menin [18]. More than 400 different germline mutations have been discovered in patients and families with MEN1. Mutations are scattered throughout the coding region, but no definite genotype-phenotype correlation has been described. The majority of germline MEN1 mutations are either nonsense or missense point mutations or insertions or deletions that cause frameshift. Most known mutations would be expected to inactivate the menin protein. About 20-30% of patients with MEN1 do not have an identified germline mutation. It is hypothesized that these patients have either a mutation in a non-coding region of MEN1 that is not detected by current mutation screening techniques, or mutations in as yet unrecognized genes that affect the transcription or action of menin. The vast majority of tumors in MEN1 patients have been shown to have a somatic mutation of the second wild-type allele [4, 5].
Mouse models of MEN1 have been generated with inactivating mutations of Men1, the mouse homolog of MEN1, resulting in parathyroid tumors or hyperplasia, pancreatic tumors (most commonly insulinoma), and anterior pituitary tumors. In the mouse models, thyroid and adrenal medullary tumors are also commonly seen. Loss of heterozygosity at the Men1 locus is demonstrated in the majority of tumors [19-21].
Somatic MEN1 mutation has been demonstrated in various series of sporadic parathyroid adenomas, with frequencies ranging from 3-35% for a mutation in at least one allele [22-25]. In studies that looked at loss of heterozygosity at 11q13 in sporadic adenomas, the frequency ranged from 26-37%. A small percentage of patients with apparently sporadic parathyroid adenomas are demonstrated to harbor a germline mutation of MEN1 [26-32]. Since HPT is usually the earliest and most penetrant feature of MEN1, kindreds may rarely be assigned a provisional diagnosis of familial isolated primary hyperparathyroidism if only younger MEN1 mutation carriers are considered at the time of family ascertainment (see below).
Although the association of MEN1 mutation with both sporadic and familial parathyroid adenomas has been well documented, association with parathyroid carcinoma is rare. At least two cases of parathyroid carcinoma have been reported in MEN1 patients, one with concurrent parathyroid adenoma, and the other with bilateral carcinoma [33, 34].
Molecular functions of menin
Menin, the protein encoded by the MEN1 gene, is a predominantly nuclear protein that expressed throughout the body. It lacks homology to other proteins that might provide insight into its mechanism as a tumor suppressor. Based on its associations with other proteins, it appears that menin has roles in cellular proliferation, regulation of gene transcription, DNA replication and repair, and control of the cell cycle. The pathways and interactions described below involving menin, however, remain to be proven clinically important or relevant to parathyroid tumorigenesis.
Menin can function as a suppressor of transcription through its interaction with the AP-1/Jun-Fos family of transcription factors [35]. Menin binds JunD, and when menin binding is disrupted, JunD changes from a growth suppressor to a growth promoter [36]. Menin’s action as a JunD corepressor involves recruitment of a histone deacetylase complex [37].
Menin also associates with a histone methyltransferase (HMT) complex containing homologs of the yeast Set1 assembly [38]. Menin’s HMT activity increases expression of the cyclin-dependent kinase inhibitors (CDKI) p27(Kip1) and p18(Ink4c), to suppress cell growth (cf. Fig. 1) [39, 40]. Interestingly, germline mutation of p27 or other CDKI including p15(Ink4b), p18, and p21(WAF1) can be a rare cause of tumor syndromes with similarities to MEN1 [41, 42].
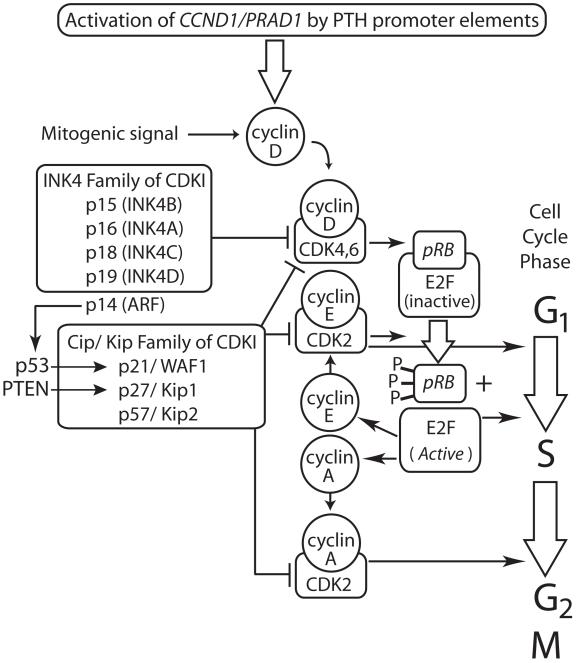
Figure 1. Role of cyclin D1, product of the CCND1/PRAD1 proto-oncogene, in cell cycle regulation.
Chromosomal rearrangement in a subset of sporadic parathyroid adenomas, that positions the CCND1/PRAD1 proto-oncogene (normally activated by mitogenic signals) under the influence of parathyroid hormone gene promoter/enhancer elements [83-85], stimulates transcription of cyclin D1. Cyclin D expression is physiologically upregulated by mitogenic signals. Enhanced cyclin D expression results in increased complex formation between cyclin D and cyclin-dependent kinases 4 (CDK4) and 6 (CDK6). The retinoblastoma gene product, pRB, in its unphosphorylated state, normally binds to and sequesters the E2F family of transcription factors. Successive phosphorylation of pRB by CDK4 and CDK6 (bound to cyclin D) and CDK2 (bound to cyclin E) inhibits its ability to bind and sequester E2F. Upon its release from pRB, E2F becomes transcriptionally active and switches on multiple genes important for nucleotide synthesis, DNA replication and cell cycle progression from the G1 phase into the S phase, including cyclin E. E2F-stimulated synthesis of cyclin A drives CDK2-mediated progression from S to G2. Members of both the INK4 and Cip/Kip families of CDK inhibitors (CDKI) inhibit the function of cyclin D/ CDK4/6 complexes while members of the Cip/Kip family also inhibit cyclin E/ CDK2 and cyclin A/CDK2 complexes. The products of P53 and PTEN can strongly induce the expression of certain CDKI as shown. The CDKI also function in other phases of the cell cycle not shown here.
Distinct from its role as a tumor suppressor in multiple endocrine tissues, menin is an essential co-factor in the pathogenesis of leukemia in which it promotes homeobox (Hox) gene expression through its interactions with lens epithelium-derived growth factor [43] and oncogenic fusion proteins containing mixed-lineage leukemia HMT activity [44, 45]. Deregulation of Hox genes has been demonstrated in both MEN1-associated and sporadic parathyroid adenomas [46].
Menin also interacts with Smad3, a member of the transforming growth factor-beta (TGF-beta) pathway, to promote gene transcription. In parathyroid tissue, TGF-beta inhibits cell proliferation and PTH production [47]. Menin inactivation antagonizes TGF-beta mediated growth inhibition and increases PTH levels [48].
Menin appears to have a role in DNA replication and repair. Investigators have demonstrated menin association with proteins such as the activator of S-phase kinase (ASK), the forkhead transcription factor CHES1 and human telomerase reverse transcriptase (hTERT) [49-51].
More recent research has focused on a possible role for menin in regulation of transcription by nuclear receptors, most interestingly a possible role of vitamin D receptor regulation in parathyroid adenomas [52].
Hyperparathyroidism-jaw tumor syndrome and the HRPT2 gene
HPT-JT is a rare autosomal dominant familial cancer syndrome manifested by primary hyperparathyroidism, ossifying tumors of the maxilla and mandible that are histologically distinct from the osteoclastic brown tumors of primary hyperparathyroidism, and less commonly renal cysts and/or uterine tumors [53-55]. Primary hyperparathyroidism is usually the presenting manifestation. Parathyroid carcinoma is present in approximately 15% of those with HPT.
A germline inactivating mutation of the HRPT2 gene can be demonstrated in more than half of cases [56]. The HRPT2 gene encodes the protein parafibromin, which consists of 531 amino acids and has weak homology to the yeast protein Cdc73p [56]. Mutations in HRPT2 are scattered throughout the coding region, and most are predicted to cause inactivation of the protein product [57]. Somatic mutation of HRPT2 is uncommon in sporadic parathyroid adenomas [58]. In contrast, mutations of HRPT2 are frequently seen in apparently sporadic cases of parathyroid carcinoma [59-61]. Some 20% of patients with apparently sporadic parathyroid cancer may harbor germline HRPT2 mutations, suggesting that such cases may in fact represent undiagnosed HPT-JT [61]. Germline HRPT2 mutation is a rare cause of familial isolated primary hyperparathyroidism (see below).
Molecular functions of parafibromin
Parafibromin is a ubiquitously expressed protein whose function as a tumor suppressor is not well understood. It is the human homolog of the yeast Cdc73 protein, which in both yeast and humans is part of the RNA polymerase II-regulatory Paf1 complex. The Paf1 complex associates with RNA polymerase II and appears to have roles in gene transcription mediated by histone methylation in the promoter and coding regions of specific genes [62]. In human cell lines, endogenous parafibromin represses expression of MYC that encodes the c-Myc proto-oncogene. Interference with MYC expression blocks the proliferative effects of parafibromin knockdown [63]. Parafibromin has a nuclear localization signal, and mutation of this region blocks nuclear targeting. Overexpression of wild-type, but not NLS-mutant parafibromin, can induce apoptosis in transfected cells [64]. Parafibromin is also expressed in the cytoplasm, where it interacts with the actin binding proteins actinin-2 and actinin-3, that are involved in organization of the cytoskeleton [65]. Recent studies suggest that dysregulation of several microRNAs may contribute to the pathogenesis of parathyroid cancers harboring HRPT2 mutation [66].
In Drosophila, the parafibromin analog Hyrax is a member of the Wnt/wingless pathway. It is essential for embryonic development, with roles in proliferation, differentiation, apoptosis and cell survival. It is involved in the movement of beta-catenin to the nucleus to drive transcription of Wnt target genes [57]. Parafibromin also has an apparent role in mammalian embryonic development as well. Homozygous parafibromin null mice die in utero, and conditional knockout of parafibromin in adult mice results in cachexia and death [67].
Familial hypocalciuric hypercalcemia and the CASR gene
FHH is an autosomal dominant trait usually causing mild HPT with relative hypocalciuria; hypercalcemia in FHH is highly penetrant at all ages, even in the perinatal period [68]. FHH cases almost always remain hypercalcemic following partial or subtotal PTX. Most cases of FHH result from a heterozygous loss-of-function mutation in the CASR gene on the long arm of chromosome 3 that encodes the CaSR [69-71]. Homozygous or compound heterozygous inheritance of two inactive CASR alleles classically results in neonatal severe hyperparathyroidism [69-71]. A germline missense mutation in the CaSR has recently been described however that causes an FHH phenotype in homozygotes but normocalcemia in most heterozygotes [72]. In addition two undiscovered genes have been implicated in rare kindreds with FHH; 1 gene at chromosome 19p [73] and 1 gene at 19q [74]. Somatic inactivation of CASR has not been found in sporadic parathyroid adenomas [75, 76], even though significant loss of CASR expression, not due to allelic loss, has been documented in such tumors and very likely contributes to their altered calcium set point for PTH release [77].
Familial isolated hyperparathyroidism
Familial isolated hyperparathyroidism (FIHP) is a clinically-defined syndrome in kindreds with HPT but lacking the specific features of MEN1, HPT-JT or FHH. The majority of FIHP patients lack germline mutation of MEN1, HRPT2 or CASR [78, 79]. A distinct genetic etiology has not been defined, although a genomic screen of seven FIHP families has identified a suggestive 1.7 Mb region on chromosome 2 [80].
Oncogenes in parathyroid neoplasia
Oncogenes derive from naturally occurring genes called proto-oncogenes that positively regulate cell growth and/or proliferation. Oncogenes represent mutationally activated or overexpressed forms of proto-oncogenes that can induce neoplasia.
Germline activating mutations in the RET (REarranged during Transfection) protooncogene are associated with three different endocrine tumor syndromes associated with thyroid C-cells: multiple endocrine neoplasia type 2A (MEN2A) and type 2B (MEN2B) syndromes, and familial medullary thyroid cancer (FMTC). RET encodes c-Ret, a widely expressed transmembrane protein tyrosine kinase. Different germline activating mutations in RET can result in the different disease phenotypes. MEN2A, whose spectrum of disease manifestations includes medullary thyroid carcinoma (MTC), pheochromocytoma, and HPT due to one or multiple parathyroid adenomas, results from missense mutation of a cysteine residue at codon 634 in about 85% of cases [81]. HPT in MEN2A is usually mild, resembles sporadic HPT in its clinical presentation, and is almost always due to benign tumors. Parathyroid tumors are not part of the MEN2B or FMTC disease pattern. Interestingly RET/PTC gene rearrangements, involving the tyrosine kinase domain encoded in the 3′ region of RET, are frequently found in papillary thyroid cancer (PTC), especially those associated with radiation exposure [82].
The CCND1 or PRAD1 (parathyroid adenomatosis 1) oncogene was discovered during the molecular characterization of several large sporadic parathyroid adenomas harboring DNA re-arrangements that involved the PTH gene locus on chromosome 11 [83-85]. The PRAD1 oncogene in sporadic parathyroid tumor samples was identified downstream of a breakpoint resulting from pericentromeric inversion of chromosome 11 DNA [85]. The chromosomal rearrangement positions the 5′ PTH gene regulatory region (normally located at 11p15) just upstream of the 11q13 region containing the PRAD1 protooncogene [83-85]. The PRAD1 oncogene was recognized to be a member of the cyclin family on the basis of sequence homology [85] and it was later re-named cyclin D1 (CCND1).
Cyclins are key regulators of a class of kinases (cyclin-dependent kinases, CDKs) that govern progression of cells through the cell cycle (Fig. 1). Increased expression of cyclin D1 (and other cyclin D isoforms) enhances transcription of multiple genes required for DNA synthesis and cell cycle progression (Fig. 1). CCND1/PRAD1 is overexpressed in some 20 to 40% of sporadic parathyroid adenomas and in an even higher percentage of parathyroid cancers [86-89]. Activating missense mutations in the cyclin D1 coding region have not been found in sporadic parathyroid adenomas [90]. No somatic chromosomal rearrangements involving CCND1/PRAD1 have been reported in parathyroid carcinoma, nor have germline chromosomal translocations or rearrangements involving CCND1/PRAD1 been identified in any familial form of primary hyperparathyroidism.
Potential role of other genes in parathyroid neoplasia
Mutations in several candidate genes, chosen because of their known importance in the regulation of parathyroid cell growth or hormonal secretion, have been examined for a possible role in parathyroid tumor formation. No somatic mutations in CASR have yet been found in studies of sporadic parathyroid adenomas and parathyroid cancers. Mutations in neither the vitamin D receptor nor the vitamin D activating enzyme 25-hydroxyvitamin D-1alpha-hydroxylase have so far been found in molecular analyses of sporadic parathyroid tumors [91, 92].
It is highly likely that the dysregulation of other genes, besides those discussed above, can initiate or promote parathyroid tumor formation. As noted above, the susceptibility to parathyroid neoplasia in the majority of FIHP kindreds appears to result from the germline mutation of genes not currently recognized for a role in parathyroid disease: among 76 families initially considered as FIHP in 5 clinical studies that investigated for germline MEN1, CASR and HRPT2 gene mutation, 53 families or nearly 70% had no currently recognized syndromic etiology [78, 79, 93-95].
The loss or gain of specific regions of chromosomal DNA detected by techniques such as comparative genomic hybridization (CGH) also suggests the existence of currently unidentified parathyroid tumor suppressors and oncogenes. Several investigators have found recurrent loss of chromosomal DNA at the 1p, 6q, 9p, and 13q loci in benign or malignant parathyroid tumors indicating the potential presence there of novel parathyroid tumor suppressor genes [96-99]. The presence of currently unknown oncogenes at 9q, 16p, 19p, and Xq is suggested by a convergence of results from several laboratories demonstrating specific chromosomal gain at these loci in parathyroid adenomas or cancers [96, 98-100].
Practice Points
The cornerstone of treatment of primary hyperparathyroidism is surgical.
Bilateral neck exploration with excision of adenoma is the classic approach, although minimally invasive surgery guided by non-invasive imaging and intra-operative PTH monitoring is gaining favor in non-familial cases.
Subtotal parathyroidectomy is indicated in familial syndromes, such as MEN1 and FIHP
The surgical approach in HPT-JT is controversial because of the increased risk of parathyroid cancer, but subtotal parathyroidectomy with close postoperative biochemical monitoring for recurrence is currently recommended over prophylactic total parathyroidectomy
En bloc resection is recommended as primary treatment for parathyroid carcinoma.
Medical therapy with calcimimetics is useful for patients with primary hyperparathyroidism who are poor surgical candidates, or have non-localizable tumors or inoperable disease (although approved in the European Union, such use of the calcimimetic cinacalcet for benign primary hyperparathyroidism is currently off-label in the United States)
Research agenda
Diagnostic reagents based on the expression of parafibromin need further development to increase the ability to distinguish benign from malignant parathyroid tumors on surgical specimens
Additional chemo- and/or immunotherapies for inoperable parathyroid cancer deserve further development
Novel tumor susceptibility genes indicated by recurrent patterns of loss or gain of DNA in parathyroid tumors or by linkage in FIHP kindreds should be identified
Summary
The vast majority of primary hyperparathyroidism, the metabolic disease that results from hypersecretion of hormone from parathyroid tumors, is sporadic. Study of uncommon familial syndromes has nevertheless helped to define the pathophysiology of both familial and sporadic parathyroid neoplasms. The tumor suppressor genes MEN1 and HRPT2 were discovered through the genetic analysis of kindreds with multiple endocrine neoplasia type 1 and the hyperparathyroidism-jaw tumor syndrome. Somatic mutations in MEN1 and HRPT2 are frequent events in the clonal development of sporadic parathyroid adenomas and carcinomas, respectively. Menin, encoded by MEN1, and parafibromin, encoded by HRPT2, are components of distinct transcriptional regulatory and histone modifying protein complexes and likely play roles in additional pathways that affect cell growth and proliferation. The role of the CCND1/PRAD1 oncogene in sporadic parathyroid tumors highlights the importance of cell cycle dysregulation in neoplastic transformation. The phenotypic expressions of RET oncogene mutation in multiple endocrine neoplasia type 2A include benign parathyroid tumors. The current difficulty in distinguishing benign from malignant parathyroid tumors based on surgical pathologic analysis may be overcome by improved diagnostic reagents based on the expression of parafibromin. Additional medical therapies for parathyroid cancer not amenable to surgery await development. Clinical genetic analysis of kindreds with familial isolated hyperparathyroidism and molecular genetic studies of recurrent patterns of chromosomal loss and gain in parathyroid tumors suggest that novel genes that predispose to parathyroid neoplasia await identification.
Acknowledgements
The authors thank Dr. Stephen J. Marx for critical reading of the manuscript and our colleagues Drs. Lee S. Weinstein, Monica C. Skarulis and Sunita K. Agarwal of the National Institute of Diabetes and Digestive and Kidney Diseases for their ongoing support and encouragement. The Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest, or competing financial or personal relationships that could inappropriately influence the content of this article.
REFERENCES
- 1.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–58. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 2.Westin G, Bjorklund P, Akerstrom G. Molecular genetics of parathyroid disease. World J Surg. 2009;33:2224–33. doi: 10.1007/s00268-009-0022-6. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JL. Molecular alterations in hereditary and sporadic thyroid and parathyroid diseases. Adv Anat Pathol. 2009;16:23–32. doi: 10.1097/PAP.0b013e3181915f7d. [DOI] [PubMed] [Google Scholar]
- 4.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–75. doi: 10.1038/nrc1610. * [DOI] [PubMed] [Google Scholar]
- 5.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 6.DeLellis RA. Parathyroid carcinoma: an overview. Adv Anat Pathol. 2005;12:53–61. doi: 10.1097/01.pap.0000151319.42376.d4. [DOI] [PubMed] [Google Scholar]
- 7.Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. * [DOI] [PubMed] [Google Scholar]
- 8.Brown EM, Pollak M, Seidman CE, et al. Calcium-ion-sensing cell-surface receptors. N Engl J Med. 1995;333:234–40. doi: 10.1056/NEJM199507273330407. [DOI] [PubMed] [Google Scholar]
- 9.Marx SJ. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med. 2000;343:1863–75. doi: 10.1056/NEJM200012213432508. [DOI] [PubMed] [Google Scholar]
- 10.Schneider AB, Gierlowski TC, Shore-Freedman E, et al. Dose-response relationships for radiation-induced hyperparathyroidism. J Clin Endocrinol Metab. 1995;80:254–7. doi: 10.1210/jcem.80.1.7829622. [DOI] [PubMed] [Google Scholar]
- 11.Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197–205. * [PubMed] [Google Scholar]
- 12.Ruda JM, Hollenbeak CS, Stack BC., Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359–72. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Knudson AG., Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold A, Marx SJ. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7th edn American Society for Bone and Mineral Research; Washington, DC: 2008. Familial Hyperparathyroidism (Including MEN, FHH, and HPT-JT) pp. 361–366. [Google Scholar]
- 15.Schussheim DH, Skarulis MC, Agarwal SK, et al. Multiple endocrine neoplasia type 1: new clinical and basic findings. Trends Endocrinol.Metab. 2001;12:173–178. doi: 10.1016/s1043-2760(00)00372-6. [DOI] [PubMed] [Google Scholar]
- 16.Rizzoli R, Green J, Marx SJ. Primary hyperparathyroidism in familial multiple endocrine neoplasia type I. Long-term follow-up of serum calcium levels after parathyroidectomy. Am J Med. 1985;78:467–474. doi: 10.1016/0002-9343(85)90340-7. [DOI] [PubMed] [Google Scholar]
- 17.Hellman P, Skogseid B, Oberg K, et al. Primary and reoperative parathyroid operations in hyperparathyroidism of multiple endocrine neoplasia type 1. Surgery. 1998;124:993–999. [PubMed] [Google Scholar]
- 18.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. * [DOI] [PubMed] [Google Scholar]
- 19.Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–23. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libutti SK, Crabtree JS, Lorang D, et al. Parathyroid gland-specific deletion of the mouse Men1 gene results in parathyroid neoplasia and hypercalcemic hyperparathyroidism. Cancer Res. 2003;63:8022–8. [PubMed] [Google Scholar]
- 21.Harding B, Lemos MC, Reed AA, et al. Multiple Endocrine Neoplasia Type 1 (MEN1) knockout mice develop parathyroid, pancreatic, pituitary and adrenal tumours with hypercalcaemia, hypophosphataemia and hypercorticosteronaemia. Endocr Relat Cancer. 2009;16:1313–1327. doi: 10.1677/ERC-09-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miedlich S, Krohn K, Lamesch P, et al. Frequency of somatic MEN1 gene mutations in monoclonal parathyroid tumours of patients with primary hyperparathyroidism. Eur J Endocrinol. 2000;143:47–54. doi: 10.1530/eje.0.1430047. [DOI] [PubMed] [Google Scholar]
- 23.Uchino S, Noguchi S, Sato M, et al. Screening of the Men1 gene and discovery of germ-line and somatic mutations in apparently sporadic parathyroid tumors. Cancer Res. 2000;60:5553–7. [PubMed] [Google Scholar]
- 24.Scarpelli D, D’Aloiso L, Arturi F, et al. Novel somatic MEN1 gene alterations in sporadic primary hyperparathyroidism and correlation with clinical characteristics. J Endocrinol Invest. 2004;27:1015–21. doi: 10.1007/BF03345303. [DOI] [PubMed] [Google Scholar]
- 25.Vierimaa O, Villablanca A, Alimov A, et al. Mutation analysis of MEN1, HRPT2, CASR, CDKN1B, and AIP genes in primary hyperparathyroidism patients with features of genetic predisposition. J Endocrinol Invest. 2009;32:512–8. doi: 10.1007/BF03346498. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet. 1997;6:1169–75. doi: 10.1093/hmg/6.7.1169. [DOI] [PubMed] [Google Scholar]
- 27.Carling T, Correa P, Hessman O, et al. Parathyroid MEN1 gene mutations in relation to clinical characteristics of nonfamilial primary hyperparathyroidism. J Clin Endocrinol Metab. 1998;83:2960–3. doi: 10.1210/jcem.83.8.4977. [DOI] [PubMed] [Google Scholar]
- 28.Farnebo F, Teh BT, Kytola S, et al. Alterations of the MEN1 gene in sporadic parathyroid tumors. J Clin Endocrinol Metab. 1998;83:2627–30. doi: 10.1210/jcem.83.8.4846. [DOI] [PubMed] [Google Scholar]
- 29.Cetani F, Pardi E, Giovannetti A, et al. Genetic analysis of the MEN1 gene and HPRT2 locus in two Italian kindreds with familial isolated hyperparathyroidism. Clin Endocrinol (Oxf) 2002;56:457–64. doi: 10.1046/j.1365-2265.2002.01502.x. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka C, Uchino S, Noguchi S, et al. Biallelic inactivation by somatic mutations of the MEN1 gene in sporadic parathyroid tumors. Cancer Lett. 2002;175:175–9. doi: 10.1016/s0304-3835(01)00729-7. [DOI] [PubMed] [Google Scholar]
- 31.Dwight T, Nelson AE, Theodosopoulos G, et al. Independent genetic events associated with the development of multiple parathyroid tumors in patients with primary hyperparathyroidism. Am J Pathol. 2002;161:1299–306. doi: 10.1016/S0002-9440(10)64406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi Y, Nowak NJ, Pacchia AL, Morrison C. Chromosome 11 genomic changes in parathyroid adenoma and hyperplasia: array CGH, FISH, and tissue microarrays. Genes Chromosomes Cancer. 2008;47:639–48. doi: 10.1002/gcc.20565. [DOI] [PubMed] [Google Scholar]
- 33.Dionisi S, Minisola S, Pepe J, et al. Concurrent parathyroid adenomas and carcinoma in the setting of multiple endocrine neoplasia type 1: presentation as hypercalcemic crisis. Mayo Clin Proc. 2002;77:866–9. doi: 10.4065/77.8.866. [DOI] [PubMed] [Google Scholar]
- 34.Shih RY, Fackler S, Maturo S, et al. Parathyroid carcinoma in multiple endocrine neoplasia type 1 with a classic germline mutation. Endocr Pract. 2009;15:567–72. doi: 10.4158/EP09045.CRR1. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal SK, Guru SC, Heppner C, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–52. doi: 10.1016/s0092-8674(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal SK, Novotny EA, Crabtree JS, et al. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci U S A. 2003;100:10770–5. doi: 10.1073/pnas.1834524100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Lee JE, Cho EJ, et al. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res. 2003;63:6135–9. [PubMed] [Google Scholar]
- 38.Hughes CM, Rozenblatt-Rosen O, Milne TA, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–97. doi: 10.1016/s1097-2765(04)00081-4. * [DOI] [PubMed] [Google Scholar]
- 39.Milne TA, Hughes CM, Lloyd R, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–54. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karnik SK, Hughes CM, Gu X, et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005;102:14659–64. doi: 10.1073/pnas.0503484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A. 2006;103:15558–63. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94:1826–34. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46. doi: 10.1016/j.ccr.2008.05.003. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama A, Wang Z, Wysocka J, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–49. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen YX, Yan J, Keeshan K, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A. 2006;103:1018–23. doi: 10.1073/pnas.0510347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen HC, Rosen JE, Yang LM, et al. Parathyroid tumor development involves deregulation of homeobox genes. Endocr Relat Cancer. 2008;15:267–75. doi: 10.1677/ERC-07-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaji H, Canaff L, Lebrun JJ, et al. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A. 2001;98:3837–42. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sowa H, Kaji H, Kitazawa R, et al. Menin inactivation leads to loss of transforming growth factor beta inhibition of parathyroid cell proliferation and parathyroid hormone secretion. Cancer Res. 2004;64:2222–8. doi: 10.1158/0008-5472.can-03-3334. [DOI] [PubMed] [Google Scholar]
- 49.Lin SY, Elledge SJ. Multiple tumor suppressor pathways negatively regulate telomerase. Cell. 2003;113:881–9. doi: 10.1016/s0092-8674(03)00430-6. [DOI] [PubMed] [Google Scholar]
- 50.Busygina V, Kottemann MC, Scott KL, et al. Multiple endocrine neoplasia type 1 interacts with forkhead transcription factor CHES1 in DNA damage response. Cancer Res. 2006;66:8397–403. doi: 10.1158/0008-5472.CAN-06-0061. [DOI] [PubMed] [Google Scholar]
- 51.Schnepp RW, Hou Z, Wang H, et al. Functional interaction between tumor suppressor menin and activator of S-phase kinase. Cancer Res. 2004;64:6791–6. doi: 10.1158/0008-5472.CAN-04-0724. [DOI] [PubMed] [Google Scholar]
- 52.Dreijerink KM, Varier RA, van Nuland R, et al. Regulation of vitamin D receptor function in MEN1-related parathyroid adenomas. Mol Cell Endocrinol. 2009;313:1–8. doi: 10.1016/j.mce.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 53.Jackson CE, Norum RA, Boyd SB, et al. Hereditary hyperparathyroidism and multiple ossifying jaw fibromas: a clinically and genetically distinct syndrome. Surgery. 1990;108:1006–1012. [PubMed] [Google Scholar]
- 54.Bradley KJ, Hobbs MR, Buley ID, et al. Uterine tumours are a phenotypic manifestation of the hyperparathyroidism-jaw tumour syndrome. J Intern Med. 2005;257:18–26. doi: 10.1111/j.1365-2796.2004.01421.x. [DOI] [PubMed] [Google Scholar]
- 55.Chen JD, Morrison C, Zhang C, et al. Hyperparathyroidism-jaw tumour syndrome. J Intern Med. 2003;253:634–42. doi: 10.1046/j.1365-2796.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 56.Carpten JD, Robbins CM, Villablanca A, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32:676–80. doi: 10.1038/ng1048. * [DOI] [PubMed] [Google Scholar]
- 57.Newey PJ, Bowl MR, Thakker RV. Parafibromin--functional insights. J Intern Med. 2009;266:84–98. doi: 10.1111/j.1365-2796.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 58.Krebs LJ, Shattuck TM, Arnold A. HRPT2 mutational analysis of typical sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2005;90:5015–7. doi: 10.1210/jc.2005-0717. [DOI] [PubMed] [Google Scholar]
- 59.Howell VM, Haven CJ, Kahnoski K, et al. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet. 2003;40:657–63. doi: 10.1136/jmg.40.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cetani F, Pardi E, Borsari S, et al. Genetic analyses of the HRPT2 gene in primary hyperparathyroidism: germline and somatic mutations in familial and sporadic parathyroid tumors. J Clin Endocrinol Metab. 2004;89:5583–91. doi: 10.1210/jc.2004-0294. [DOI] [PubMed] [Google Scholar]
- 61.Shattuck TM, Valimaki S, Obara T, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–9. doi: 10.1056/NEJMoa031237. * [DOI] [PubMed] [Google Scholar]
- 62.Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, et al. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol. 2005;25:612–20. doi: 10.1128/MCB.25.2.612-620.2005. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin L, Zhang JH, Panicker LM, Simonds WF. The parafibromin tumor suppressor protein inhibits cell proliferation by repression of the c-myc proto-oncogene. Proc Natl Acad Sci U S A. 2008;105:17420–5. doi: 10.1073/pnas.0710725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin L, Czapiga M, Nini L, et al. Nuclear localization of the parafibromin tumor suppressor protein implicated in the hyperparathyroidism-jaw tumor syndrome enhances its proapoptotic function. Mol Cancer Res. 2007;5:183–93. doi: 10.1158/1541-7786.MCR-06-0129. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal SK, Simonds WF, Marx SJ. The parafibromin tumor suppressor protein interacts with actin-binding proteins actinin-2 and actinin-3. Mol Cancer. 2008;7:65. doi: 10.1186/1476-4598-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbetta S, Vaira V, Guarnieri V, et al. Differential expression of microRNAs in human parathyroid carcinomas compared with normal parathyroid tissue. Endocr Relat Cancer. 2009 doi: 10.1677/ERC-09-0134. E-pub date 2009/11/21; doi 10.1677/ERC-09-0134. [DOI] [PubMed] [Google Scholar]
- 67.Wang P, Bowl MR, Bender S, et al. Parafibromin, a component of the human PAF complex, regulates growth factors and is required for embryonic development and survival in adult mice. Mol Cell Biol. 2008;28:2930–40. doi: 10.1128/MCB.00654-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marx SJ, Attie MF, Levine MA, et al. The hypocalciuric or benign variant of familial hypercalcemia: clinical and biochemical features in fifteen kindreds. Medicine (Baltimore) 1981;60:397–412. doi: 10.1097/00005792-198111000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Pollak MR, Brown EM, Chou Y-HW, et al. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 70.Brown EM. Familial hypocalciuric hypercalcemia and other disorders with resistance to extracellular calcium. Endocrinol Metab Clin North Am. 2000;29:503–522. doi: 10.1016/s0889-8529(05)70148-1. [DOI] [PubMed] [Google Scholar]
- 71.Hendy GN, D’Souza-Li L, Yang B, et al. Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum.Mutat. 2000;16:281–296. doi: 10.1002/1098-1004(200010)16:4<281::AID-HUMU1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 72.Lietman SA, Tenenbaum-Rakover Y, Jap TS, et al. A novel loss-of-function mutation, Gln459Arg, of the calcium-sensing receptor gene associated with apparent autosomal recessive inheritance of familial hypocalciuric hypercalcemia. J Clin Endocrinol Metab. 2009;94:4372–9. doi: 10.1210/jc.2008-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heath H, III, Jackson CE, Otterud B, Leppert MF. Genetic linkage analysis in familial benign (hypocalciuric) hypercalcemia: evidence for locus heterogeneity. Am J Hum Genet. 1993;53:193–200. [PMC free article] [PubMed] [Google Scholar]
- 74.Lloyd SE, Pannett AA, Dixon PH, et al. Localization of familial benign hypercalcemia, Oklahoma variant (FBHOk), to chromosome 19q13. Am J Hum Genet. 1999;64:189–195. doi: 10.1086/302202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hosokawa Y, Pollak MR, Brown EM, Arnold A. Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors. J Clin Endocrinol Metab. 1995;80:3107–10. doi: 10.1210/jcem.80.11.7593409. [DOI] [PubMed] [Google Scholar]
- 76.Cetani F, Pinchera A, Pardi E, et al. No evidence for mutations in the calcium-sensing receptor gene in sporadic parathyroid adenomas. J Bone Miner Res. 1999;14:878–82. doi: 10.1359/jbmr.1999.14.6.878. [DOI] [PubMed] [Google Scholar]
- 77.Farnebo F, Enberg U, Grimelius L, et al. Tumor-specific decreased expression of calcium sensing receptor messenger ribonucleic acid in sporadic primary hyperparathyroidism. J Clin Endocrinol Metab. 1997;82:3481–6. doi: 10.1210/jcem.82.10.4300. [DOI] [PubMed] [Google Scholar]
- 78.Simonds WF, James-Newton LA, Agarwal SK, et al. Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 2002;81:1–26. doi: 10.1097/00005792-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Simonds WF, Robbins CM, Agarwal SK, et al. Familial isolated hyperparathyroidism is rarely caused by germline mutation in HRPT2, the gene for the hyperparathyroidism-jaw tumor syndrome. J Clin Endocrinol Metab. 2004;89:96–102. doi: 10.1210/jc.2003-030675. [DOI] [PubMed] [Google Scholar]
- 80.Warner JV, Nyholt DR, Busfield F, et al. Familial isolated hyperparathyroidism is linked to a 1.7 Mb region on chromosome 2p13.3-14. J Med Genet. 2006;43:e12. doi: 10.1136/jmg.2005.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276:1575–9. * [PubMed] [Google Scholar]
- 82.Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol. 2002;13:3–16. doi: 10.1385/ep:13:1:03. [DOI] [PubMed] [Google Scholar]
- 83.Arnold A, Kim HG, Gaz RD, et al. Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest. 1989;83:2034–40. doi: 10.1172/JCI114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenberg CL, Kim HG, Shows TB, et al. Rearrangement and overexpression of D11S287E, a candidate oncogene on chromosome 11q13 in benign parathyroid tumors. Oncogene. 1991;6:449–53. [PubMed] [Google Scholar]
- 85.Motokura T, Bloom T, Kim HG, et al. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350:512–5. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 86.Hsi ED, Zukerberg LR, Yang WI, Arnold A. Cyclin D1/PRAD1 expression in parathyroid adenomas: an immunohistochemical study. J Clin Endocrinol Metab. 1996;81:1736–9. doi: 10.1210/jcem.81.5.8626826. [DOI] [PubMed] [Google Scholar]
- 87.Hemmer S, Wasenius VM, Haglund C, et al. Deletion of 11q23 and cyclin D1 overexpression are frequent aberrations in parathyroid adenomas. Am J Pathol. 2001;158:1355–62. doi: 10.1016/S0002-9440(10)64086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tominaga Y, Tsuzuki T, Uchida K, et al. Expression of PRAD1/cyclin D1, retinoblastoma gene products, and Ki67 in parathyroid hyperplasia caused by chronic renal failure versus primary adenoma. Kidney Int. 1999;55:1375–83. doi: 10.1046/j.1523-1755.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- 89.Vasef MA, Brynes RK, Sturm M, et al. Expression of cyclin D1 in parathyroid carcinomas, adenomas, and hyperplasias: a paraffin immunohistochemical study. Mod Pathol. 1999;12:412–6. [PubMed] [Google Scholar]
- 90.Hosokawa Y, Tu T, Tahara H, et al. Absence of cyclin D1/PRAD1 point mutations in human breast cancers and parathyroid adenomas and identification of a new cyclin D1 gene polymorphism. Cancer Lett. 1995;93:165–70. doi: 10.1016/0304-3835(95)03805-7. [DOI] [PubMed] [Google Scholar]
- 91.Samander EH, Arnold A. Mutational analysis of the vitamin D receptor does not support its candidacy as a tumor suppressor gene in parathyroid adenomas. J Clin Endocrinol Metab. 2006;91:5019–21. doi: 10.1210/jc.2006-1543. [DOI] [PubMed] [Google Scholar]
- 92.Lauter K, Arnold A. Analysis of CYP27B1, encoding 25-hydroxyvitamin D-1alpha-hydroxylase, as a candidate tumor suppressor gene in primary and severe secondary/tertiary hyperparathyroidism. J Bone Miner Res. 2009;24:102–4. doi: 10.1359/JBMR.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warner J, Epstein M, Sweet A, et al. Genetic testing in familial isolated hyperparathyroidism: unexpected results and their implications. J Med Genet. 2004;41:155–60. doi: 10.1136/jmg.2003.016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villablanca A, Calender A, Forsberg L, et al. Germline and de novo mutations in the HRPT2 tumour suppressor gene in familial isolated hyperparathyroidism (FIHP) J Med Genet. 2004;41:e32. doi: 10.1136/jmg.2003.012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cetani F, Pardi E, Ambrogini E, et al. Genetic analyses in familial isolated hyperparathyroidism: implication for clinical assessment and surgical management. Clin Endocrinol. 2006;64:146–152. doi: 10.1111/j.1365-2265.2006.02438.x. [DOI] [PubMed] [Google Scholar]
- 96.Palanisamy N, Imanishi Y, Rao PH, et al. Novel chromosomal abnormalities identified by comparative genomic hybridization in parathyroid adenomas. J Clin Endocrinol Metab. 1998;83:1766–70. doi: 10.1210/jcem.83.5.4806. [DOI] [PubMed] [Google Scholar]
- 97.Agarwal SK, Schrock E, Kester MB, et al. Comparative genomic hybridization analysis of human parathyroid tumors. Cancer Genet Cytogenet. 1998;106:30–6. doi: 10.1016/s0165-4608(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 98.Farnebo F, Kytölä S, Teh BT, et al. Alternative genetic pathways in parathyroid tumorigenesis. J Clin Endocrinol Metab. 1999;84:3775–3780. doi: 10.1210/jcem.84.10.6057. [DOI] [PubMed] [Google Scholar]
- 99.Kytola S, Farnebo F, Obara T, et al. Patterns of chromosomal imbalances in parathyroid carcinomas. Am J Pathol. 2000;157:579–86. doi: 10.1016/S0002-9440(10)64568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia JL, Tardio JC, Gutierrez NC, et al. Chromosomal imbalances identified by comparative genomic hybridization in sporadic parathyroid adenomas. Eur J Endocrinol. 2002;146:209–13. doi: 10.1530/eje.0.1460209. [DOI] [PubMed] [Google Scholar]