Abstract
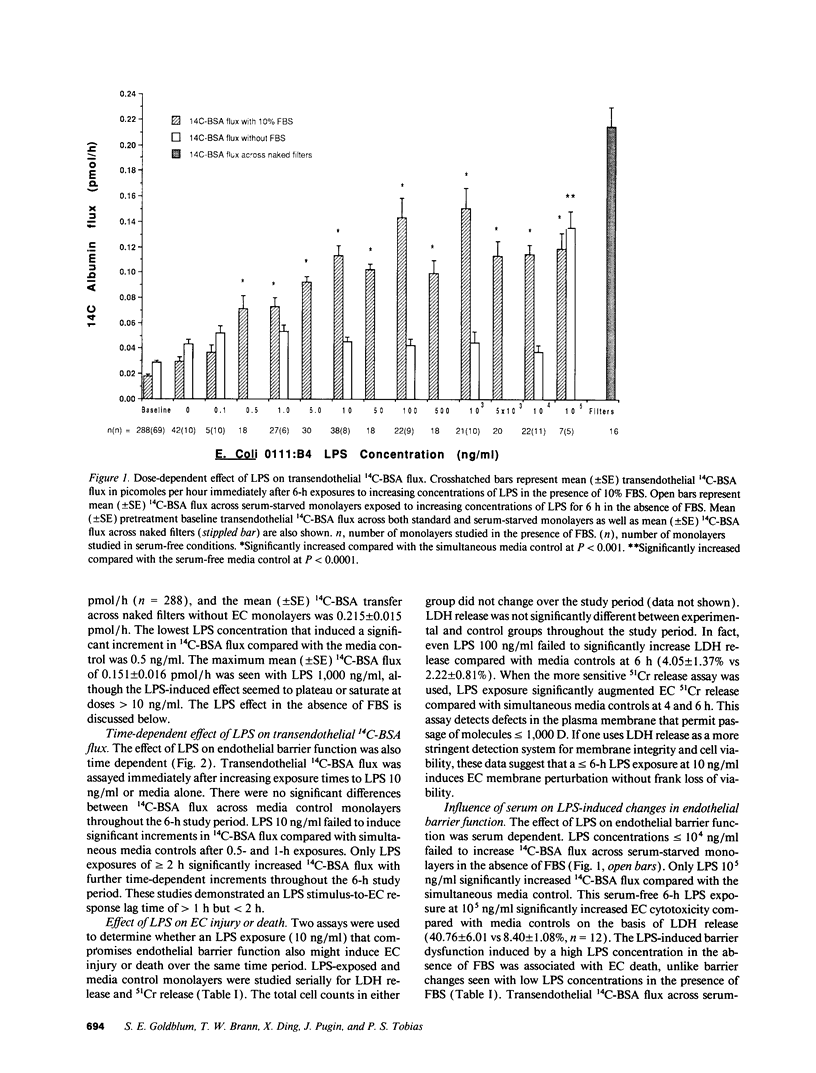
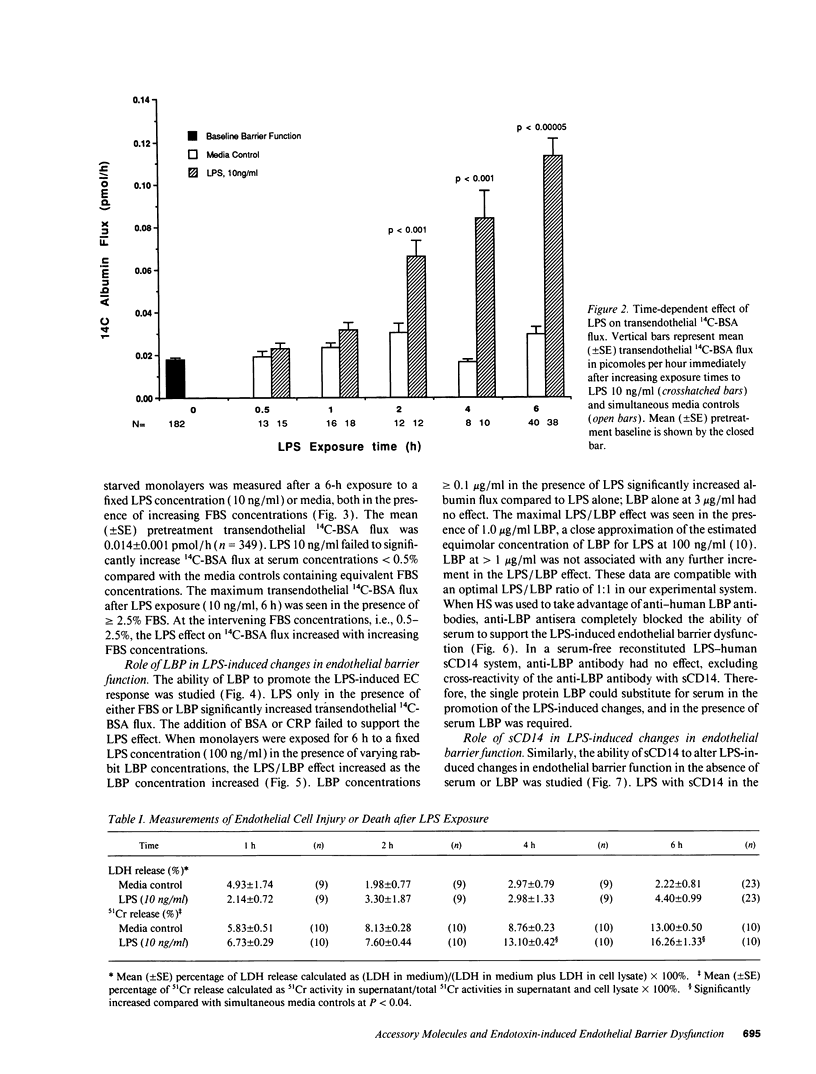
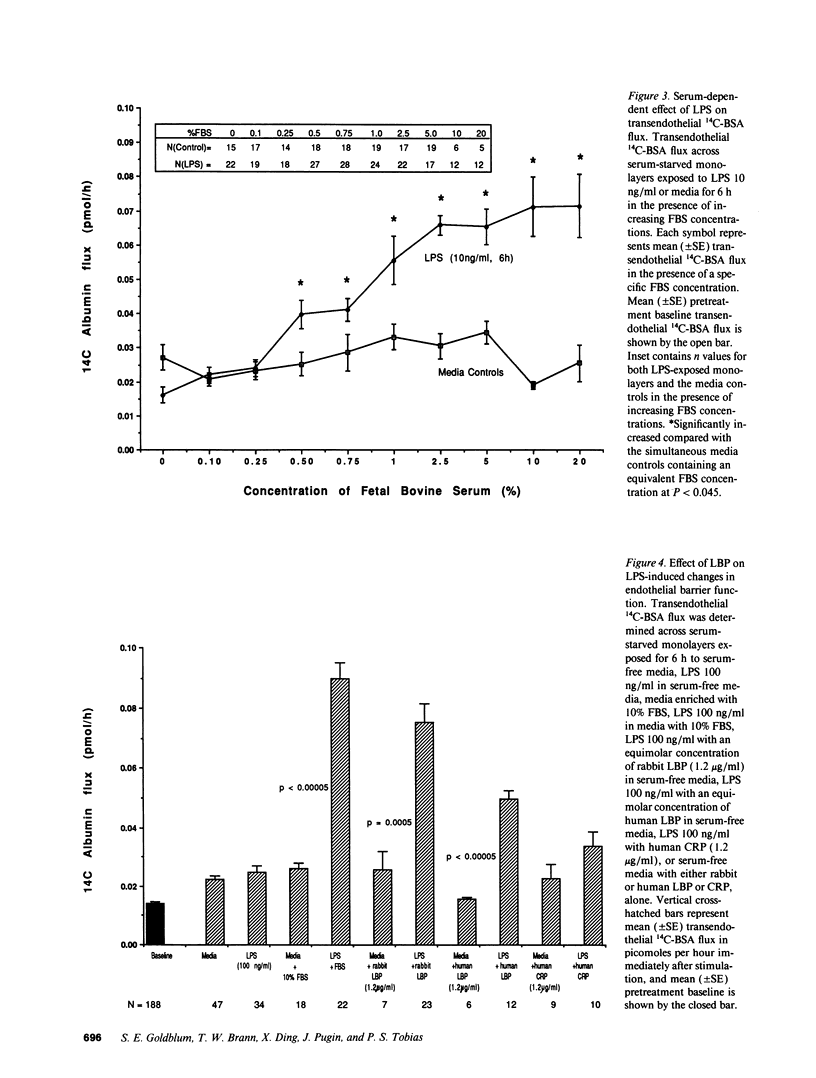
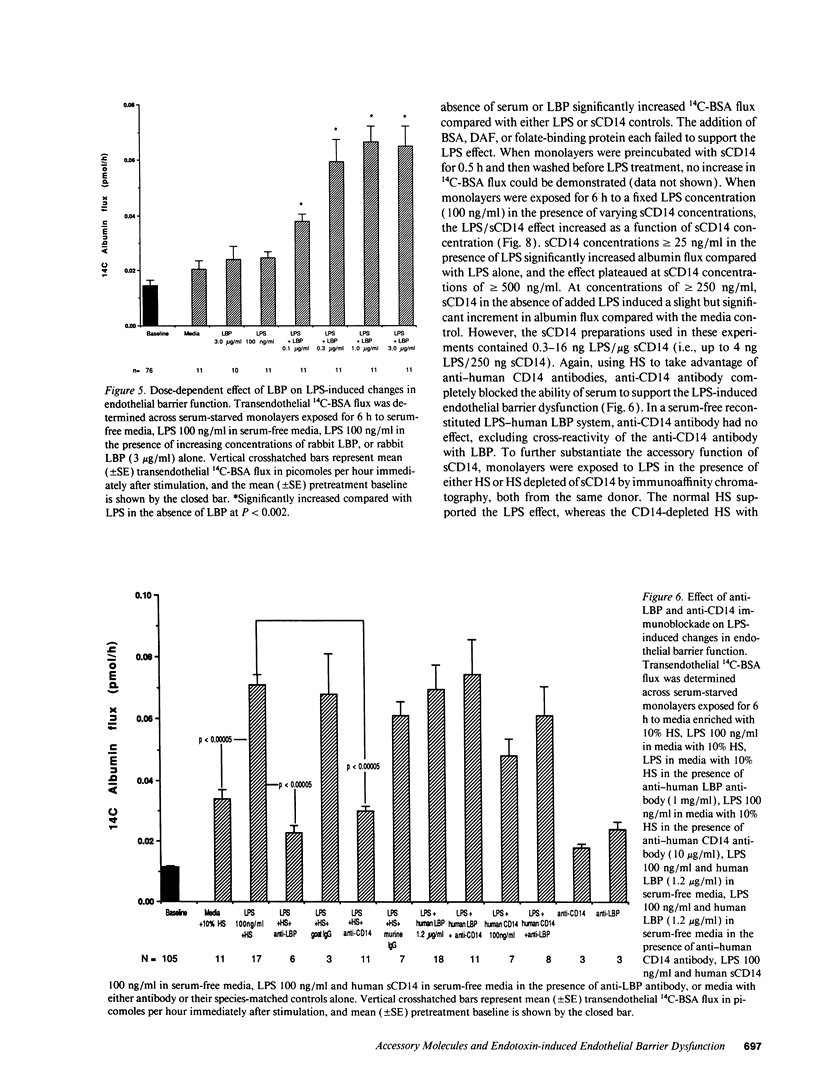
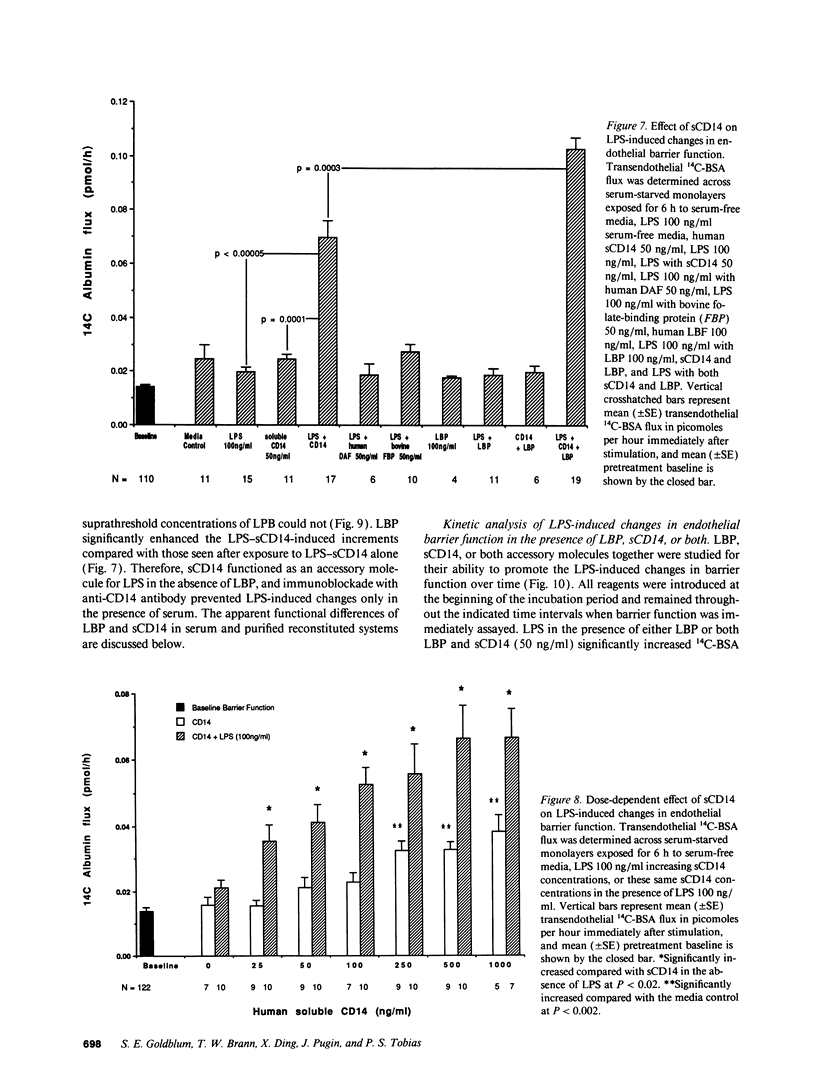
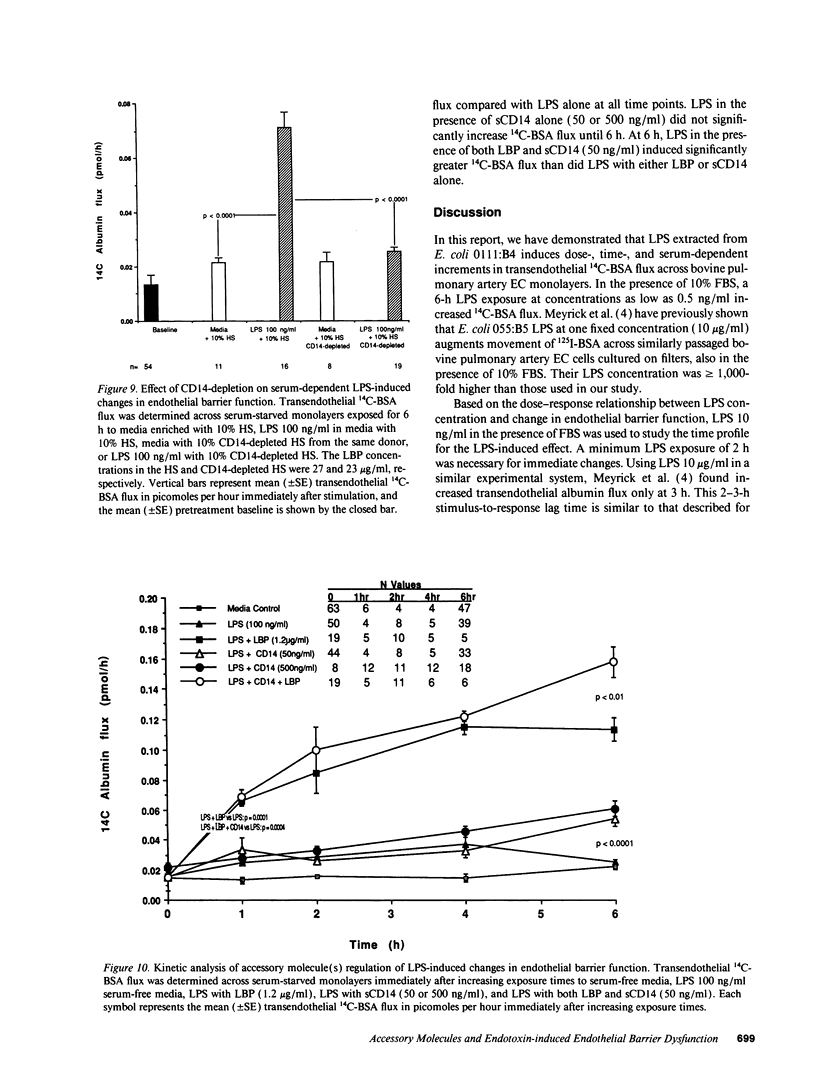
Bacterial LPS induces endothelial cell (EC) injury both in vivo and in vitro. We studied the effect of Escherichia coli 0111:B4 LPS on movement of 14C-BSA across bovine pulmonary artery EC monolayers. In the presence of serum, a 6-h LPS exposure augmented (P < 0.001) transendothelial 14C-BSA flux compared with the media control at concentrations > or = 0.5 ng/ml, and LPS (10 ng/ml) exposures of > or = 2-h increased (P < 0.005) the flux. In the absence of serum, LPS concentrations of up to 10 micrograms/ml failed to increase 14C-BSA flux at 6 h. The addition of 10% serum increased EC sensitivity to the LPS stimulus by > 10,000-fold. LPS (10 ng/ml, 6 h) failed to increase 14C-BSA flux at serum concentrations < 0.5%, and maximum LPS-induced increments could be generated in the presence of > or = 2.5%. LPS-binding protein (LBP) and soluble CD14 (sCD14) could each satisfy this serum requirement; either anti-LBP or anti-CD14 antibody each totally blocked (P < 0.00005) the LPS-induced changes in endothelial barrier function. LPS-LBP had a more rapid onset than did LPS-sCD14. The LPS effect in the presence of both LBP and sCD14 exceeded the effect in the presence of either protein alone. These data suggest that LBP and sCD14 each independently functions as an accessory molecule for LPS presentation to the non-CD14-bearing endothelial surface. However, in the presence of serum both molecules are required.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Elias J. A., Levine E. M., Kern J. A. Endotoxin stimulates platelet-derived growth factor production from cultured human pulmonary endothelial cells. Am J Physiol. 1989 Aug;257(2 Pt 1):L65–L70. doi: 10.1152/ajplung.1989.257.2.L65. [DOI] [PubMed] [Google Scholar]
- Arditi M., Zhou J., Dorio R., Rong G. W., Goyert S. M., Kim K. S. Endotoxin-mediated endothelial cell injury and activation: role of soluble CD14. Infect Immun. 1993 Aug;61(8):3149–3156. doi: 10.1128/iai.61.8.3149-3156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmun R. A., Peiper S. C., Rebentisch M. B., Look A. T. Expression of the human monocyte membrane antigen gp55 by murine fibroblasts after DNA-mediated gene transfer. Blood. 1987 Mar;69(3):886–892. [PubMed] [Google Scholar]
- Bazil V., Horejsí V., Baudys M., Kristofová H., Strominger J. L., Kostka W., Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986 Dec;16(12):1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- Beekhuizen H., Blokland I., Corsèl-van Tilburg A. J., Koning F., van Furth R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J Immunol. 1991 Dec 1;147(11):3761–3767. [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986 May;133(5):913–927. [PubMed] [Google Scholar]
- Campbell W. N., Ding X., Goldblum S. E. Interleukin-1 alpha and -beta augment pulmonary artery transendothelial albumin flux in vitro. Am J Physiol. 1992 Jul;263(1 Pt 1):L128–L136. doi: 10.1152/ajplung.1992.263.1.L128. [DOI] [PubMed] [Google Scholar]
- Frey E. A., Miller D. S., Jahr T. G., Sundan A., Bazil V., Espevik T., Finlay B. B., Wright S. D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992 Dec 1;176(6):1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallay P., Carrel S., Glauser M. P., Barras C., Ulevitch R. J., Tobias P. S., Baumgartner J. D., Heumann D. Purification and characterization of murine lipopolysaccharide-binding protein. Infect Immun. 1993 Feb;61(2):378–383. doi: 10.1128/iai.61.2.378-383.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S. L., Sieckmann D. G., Kang Y. H., Watson L. P., Homer L. D. Effects of lipopolysaccharide, lipid A, lipid X, and phorbol ester on cultured bovine endothelial cells. Lab Invest. 1988 Aug;59(2):181–191. [PubMed] [Google Scholar]
- Goldblum S. E., Ding X., Brann T. W., Campbell-Washington J. Bacterial lipopolysaccharide induces actin reorganization, intercellular gap formation, and endothelial barrier dysfunction in pulmonary vascular endothelial cells: concurrent F-actin depolymerization and new actin synthesis. J Cell Physiol. 1993 Oct;157(1):13–23. doi: 10.1002/jcp.1041570103. [DOI] [PubMed] [Google Scholar]
- Goldblum S. E., Sun W. L. Tumor necrosis factor-alpha augments pulmonary arterial transendothelial albumin flux in vitro. Am J Physiol. 1990 Feb;258(2 Pt 1):L57–L67. doi: 10.1152/ajplung.1990.258.2.L57. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Striker G. E., Weaver L. J. Effects of lipopolysaccharide on human endothelial cells in culture. Thromb Res. 1983 Jan 1;29(1):15–26. doi: 10.1016/0049-3848(83)90121-4. [DOI] [PubMed] [Google Scholar]
- Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988 Jul 15;141(2):547–552. [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Kalter E. S., Daha M. R., ten Cate J. W., Verhoef J., Bouma B. N. Activation and inhibition of Hageman factor-dependent pathways and the complement system in uncomplicated bacteremia or bacterial shock. J Infect Dis. 1985 Jun;151(6):1019–1027. doi: 10.1093/infdis/151.6.1019. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Marks C. B., Luce J. M., Montgomery A. B., Turner J., Metz C. A., Murray J. F. Plasma tumor necrosis factor in patients with septic shock. Mortality rate, incidence of adult respiratory distress syndrome, and effects of methylprednisolone administration. Am Rev Respir Dis. 1990 Jan;141(1):94–97. doi: 10.1164/ajrccm/141.1.94. [DOI] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Hoover R., Jones M. R., Berry L. C., Jr, Brigham K. L. In vitro effects of endotoxin on bovine and sheep lung microvascular and pulmonary artery endothelial cells. J Cell Physiol. 1989 Jan;138(1):165–174. doi: 10.1002/jcp.1041380122. [DOI] [PubMed] [Google Scholar]
- Patrick D., Betts J., Frey E. A., Prameya R., Dorovini-Zis K., Finlay B. B. Haemophilus influenzae lipopolysaccharide disrupts confluent monolayers of bovine brain endothelial cells via a serum-dependent cytotoxic pathway. J Infect Dis. 1992 May;165(5):865–872. doi: 10.1093/infdis/165.5.865. [DOI] [PubMed] [Google Scholar]
- Paulsen D. B., Mosier D. A., Clinkenbeard K. D., Confer A. W. Direct effects of Pasteurella haemolytica lipopolysaccharide on bovine pulmonary endothelial cells in vitro. Am J Vet Res. 1989 Sep;50(9):1633–1637. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Pugin J., Schürer-Maly C. C., Leturcq D., Moriarty A., Ulevitch R. J., Tobias P. S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M., Podor T. J., Loskutoff D. J. Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. Induction by transforming growth factor-beta, lipopolysaccharide, and tumor necrosis factor-alpha. J Biol Chem. 1989 Jun 25;264(18):10396–10401. [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J. J., Pohl D. F., Bell R. C., Harris G. D., Johanson W. G., Jr Incidence, site, and outcome of infections in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1986 Jul;134(1):12–16. doi: 10.1164/arrd.1986.134.1.12. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Tan S., Tenen D. G., Nicholson-Weller A., Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989 Jan;73(1):284–289. [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989 Jun 25;264(18):10867–10871. [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Haberland M. E., Edwards P. A. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2704–2708. doi: 10.1073/pnas.83.8.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosbeck K., Tobias P., Mueller H., Allen R. A., Arfors K. E., Ulevitch R. J., Sklar L. A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J Leukoc Biol. 1990 Feb;47(2):97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Tobias P. S., Ulevitch R. J., Ramos R. A. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989 Oct 1;170(4):1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]