Abstract
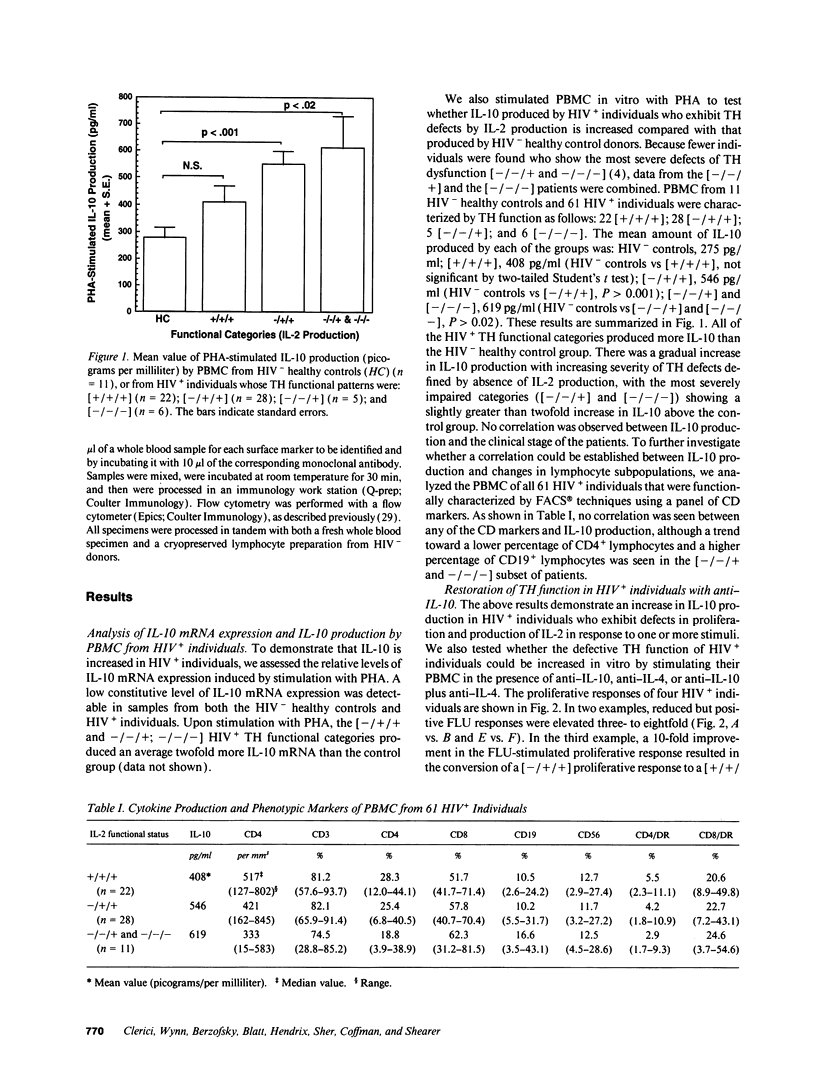
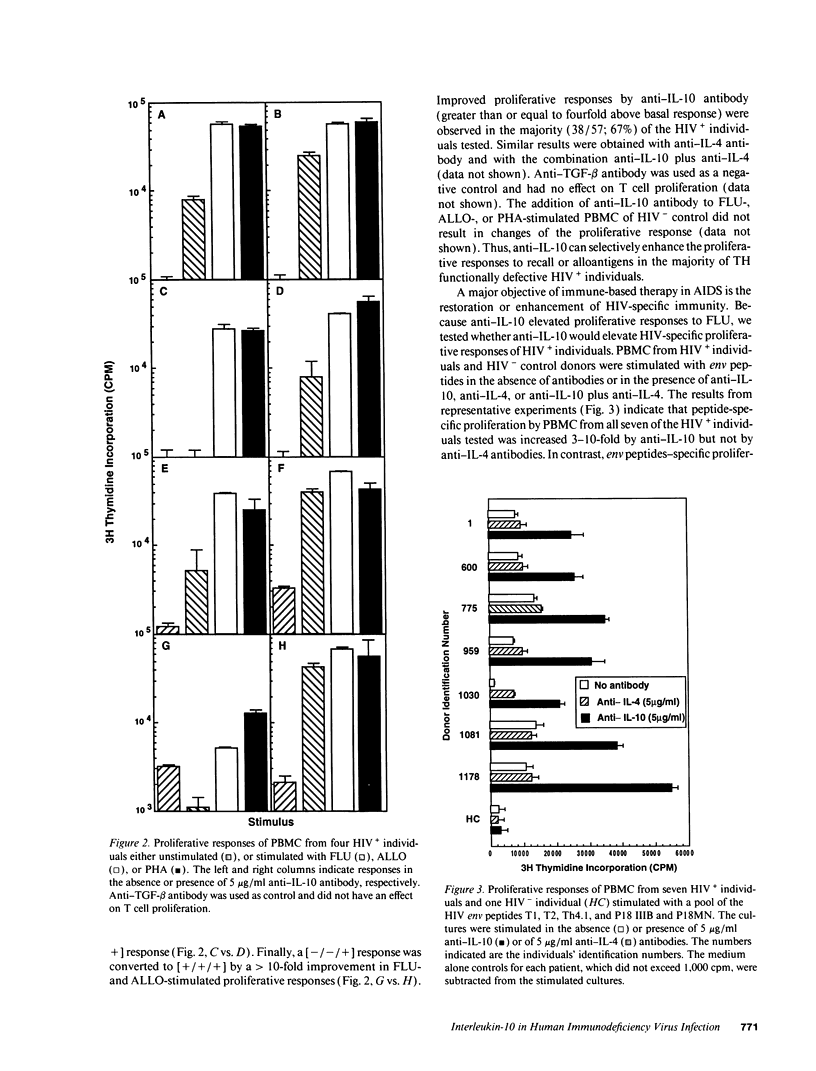
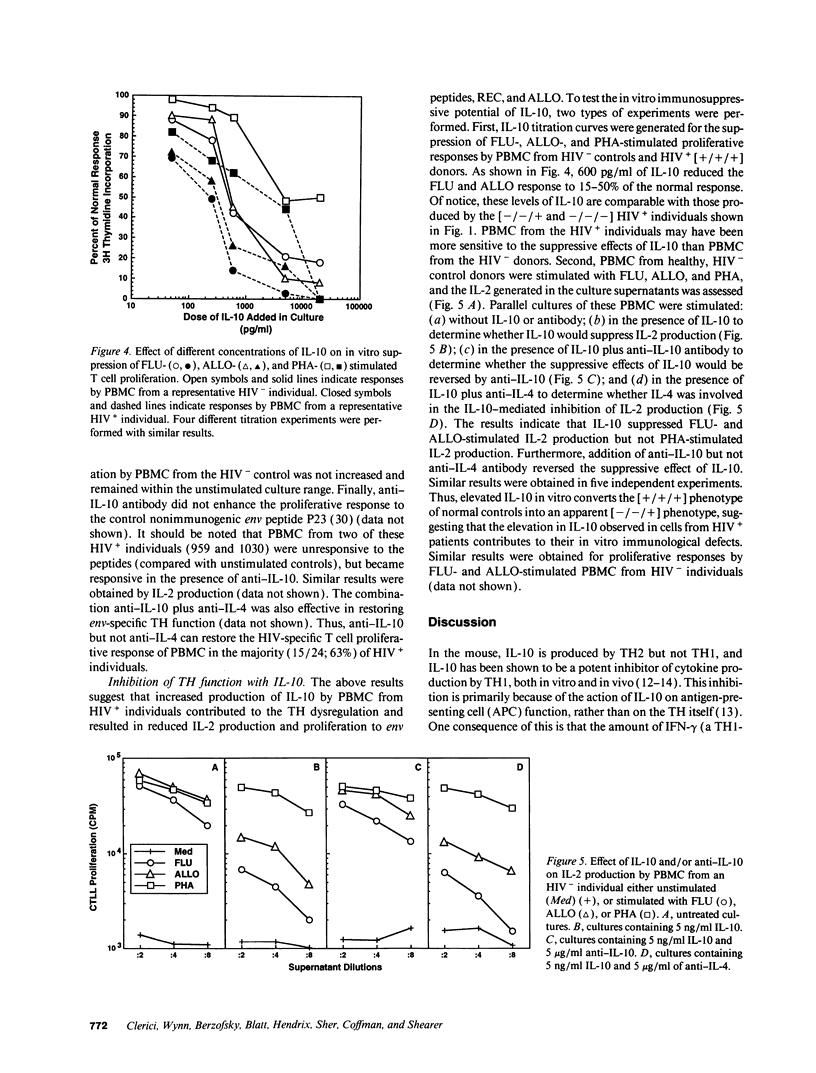
The loss of T helper cell (TH) function in asymptomatic HIV type 1-infected individuals occurs before the decline in CD4+ T cells. At least part of the loss in TH function results from changes in immunoregulatory cytokine profiles. To investigate the role of IL-10 in such dysregulation, we tested whether: (a) expression of IL-10-specific mRNA would be upregulated in PBMC from asymptomatic, HIV-infected (HIV+) individuals; (b) PBMC from these same individuals would produce increased levels of IL-10 when stimulated in vitro with phytohemagglutinin; and (c) defective antigen-specific TH function could be restored by anti-IL-10 antibody. We observed that IL-10-specific mRNA was marginally upregulated, and increased levels of IL-10 were produced by PBMC from HIV+ individuals compared with PBMC from uninfected individuals. Those individuals whose TH function was more severely compromised produced higher levels of IL-10. Additionally, defective antigen-specific TH function in vitro could be reversed by anti-IL-10 antibody, including the response to HIV envelope synthetic peptides. Furthermore, the antigen-specific TH responses of HIV-uninfected PBMC could be reduced with IL-10, a process reversed by anti-IL-10. These results confirm that the early loss of TH function in HIV+ individuals is due at least in part to cytokine-induced immune dysregulation, and support the hypothesis of a switch from a predominant type 1 state to a predominant type 2 condition in HIV infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin D., Knobloch T. J., Dayton M. A. Human B-cell interleukin-10: B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt's lymphoma constitutively secrete large quantities of interleukin-10. Blood. 1992 Sep 1;80(5):1289–1298. [PubMed] [Google Scholar]
- Bloom B. R. The power of negative thinking. J Clin Invest. 1993 Apr;91(4):1265–1266. doi: 10.1172/JCI116322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cease K. B., Margalit H., Cornette J. L., Putney S. D., Robey W. G., Ouyang C., Streicher H. Z., Fischinger P. J., Gallo R. C., DeLisi C. Helper T-cell antigenic site identification in the acquired immunodeficiency syndrome virus gp120 envelope protein and induction of immunity in mice to the native protein using a 16-residue synthetic peptide. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4249–4253. doi: 10.1073/pnas.84.12.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Lagakos S. W., Schooley R. T., Volberding P. A. CD4+ lymphocytes are an incomplete surrogate marker for clinical progression in persons with asymptomatic HIV infection taking zidovudine. Ann Intern Med. 1993 May 1;118(9):674–680. doi: 10.7326/0003-4819-118-9-199305010-00003. [DOI] [PubMed] [Google Scholar]
- Clerici M., Giorgi J. V., Chou C. C., Gudeman V. K., Zack J. A., Gupta P., Ho H. N., Nishanian P. G., Berzofsky J. A., Shearer G. M. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J Infect Dis. 1992 Jun;165(6):1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- Clerici M., Hakim F. T., Venzon D. J., Blatt S., Hendrix C. W., Wynn T. A., Shearer G. M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993 Mar;91(3):759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Landay A. L., Kessler H. A., Phair J. P., Venzon D. J., Hendrix C. W., Lucey D. R., Shearer G. M. Reconstitution of long-term T helper cell function after zidovudine therapy in human immunodeficiency virus-infected patients. J Infect Dis. 1992 Oct;166(4):723–730. doi: 10.1093/infdis/166.4.723. [DOI] [PubMed] [Google Scholar]
- Clerici M., Roilides E., Butler K. M., DePalma L., Venzon D., Shearer G. M., Pizzo P. A. Changes in T-helper cell function in human immunodeficiency virus-infected children during didanosine therapy as a measure of antiretroviral activity. Blood. 1992 Nov 1;80(9):2196–2202. [PubMed] [Google Scholar]
- Clerici M., Roilides E., Via C. S., Pizzo P. A., Shearer G. M. A factor from CD8 cells of human immunodeficiency virus-infected patients suppresses HLA self-restricted T helper cell responses. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8424–8428. doi: 10.1073/pnas.89.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Bernstein D. C., Mann D. L., Shearer G. M., Berzofsky J. A. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature. 1989 Jun 1;339(6223):383–385. doi: 10.1038/339383a0. [DOI] [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Lucey D. R., Via C. S., Shearer G. M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989 Dec;84(6):1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Tacket C. O., Via C. S., Lucey D. R., Muluk S. C., Zajac R. A., Boswell R. N., Berzofsky J. A., Shearer G. M. Immunization with subunit human immunodeficiency virus vaccine generates stronger T helper cell immunity than natural infection. Eur J Immunol. 1991 Jun;21(6):1345–1349. doi: 10.1002/eji.1830210603. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Diamond D. C., Sleckman B. P., Gregory T., Lasky L. A., Greenstein J. L., Burakoff S. J. Inhibition of CD4+ T cell function by the HIV envelope protein, gp120. J Immunol. 1988 Dec 1;141(11):3715–3717. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Giorgi J. V., Fahey J. L., Smith D. C., Hultin L. E., Cheng H. L., Mitsuyasu R. T., Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987 Jun 1;138(11):3725–3730. [PubMed] [Google Scholar]
- Golding H., Shearer G. M., Hillman K., Lucas P., Manischewitz J., Zajac R. A., Clerici M., Gress R. E., Boswell R. N., Golding B. Common epitope in human immunodeficiency virus (HIV) I-GP41 and HLA class II elicits immunosuppressive autoantibodies capable of contributing to immune dysfunction in HIV I-infected individuals. J Clin Invest. 1989 Apr;83(4):1430–1435. doi: 10.1172/JCI114034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale P. M., Cease K. B., Houghten R. A., Ouyang C., Putney S., Javaherian K., Margalit H., Cornette J. L., Spouge J. L., DeLisi C. T cell multideterminant regions in the human immunodeficiency virus envelope: toward overcoming the problem of major histocompatibility complex restriction. Int Immunol. 1989;1(4):409–415. doi: 10.1093/intimm/1.4.409. [DOI] [PubMed] [Google Scholar]
- Karp C. L., el-Safi S. H., Wynn T. A., Satti M. M., Kordofani A. M., Hashim F. A., Hag-Ali M., Neva F. A., Nutman T. B., Sacks D. L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993 Apr;91(4):1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekow J., Wachsman W., McCutchan J. A., Cronin M., Carson D. A., Lotz M. Transforming growth factor beta and noncytopathic mechanisms of immunodeficiency in human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8321–8325. doi: 10.1073/pnas.87.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Laurence J., Gottlieb A. B., Kunkel H. G. Soluble suppressor factors in patients with acquired immune deficiency syndrome and its prodrome. Elaboration in vitro by T lymphocyte-adherent cell interactions. J Clin Invest. 1983 Dec;72(6):2072–2081. doi: 10.1172/JCI111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey D. R., Melcher G. P., Hendrix C. W., Zajac R. A., Goetz D. W., Butzin C. A., Clerici M., Warner R. D., Abbadessa S., Hall K. Human immunodeficiency virus infection in the US Air Force: seroconversions, clinical staging, and assessment of a T helper cell functional assay to predict change in CD4+ T cell counts. J Infect Dis. 1991 Oct;164(4):631–637. doi: 10.1093/infdis/164.4.631. [DOI] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Meyaard L., Schuitemaker H., Miedema F. T-cell dysfunction in HIV infection: anergy due to defective antigen-presenting cell function? Immunol Today. 1993 Apr;14(4):161–164. doi: 10.1016/0167-5699(93)90279-T. [DOI] [PubMed] [Google Scholar]
- Miedema F., Petit A. J., Terpstra F. G., Schattenkerk J. K., de Wolf F., Al B. J., Roos M., Lange J. M., Danner S. A., Goudsmit J. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988 Dec;82(6):1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. S., Hoffmann M. K. Synergism between HIV gp120 and gp120-specific antibody in blocking human T cell activation. Science. 1989 Sep 22;245(4924):1380–1382. doi: 10.1126/science.2571187. [DOI] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Phair J. P. Estimating prognosis in HIV-1 infection. Ann Intern Med. 1993 May 1;118(9):742–744. doi: 10.7326/0003-4819-118-9-199305010-00015. [DOI] [PubMed] [Google Scholar]
- Pirmez C., Yamamura M., Uyemura K., Paes-Oliveira M., Conceiço-Silva F., Modlin R. L. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993 Apr;91(4):1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Greenhouse J., Justement J. S., Baseler M., Fauci A. S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Kaufmann S. H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991 Oct;12(10):346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Clerici M. Early T-helper cell defects in HIV infection. AIDS. 1991 Mar;5(3):245–253. doi: 10.1097/00002030-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Viscidi R. P., Mayur K., Lederman H. M., Frankel A. D. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science. 1989 Dec 22;246(4937):1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- Weinhold K. J., Lyerly H. K., Stanley S. D., Austin A. A., Matthews T. J., Bolognesi D. P. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J Immunol. 1989 May 1;142(9):3091–3097. [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]


