Abstract
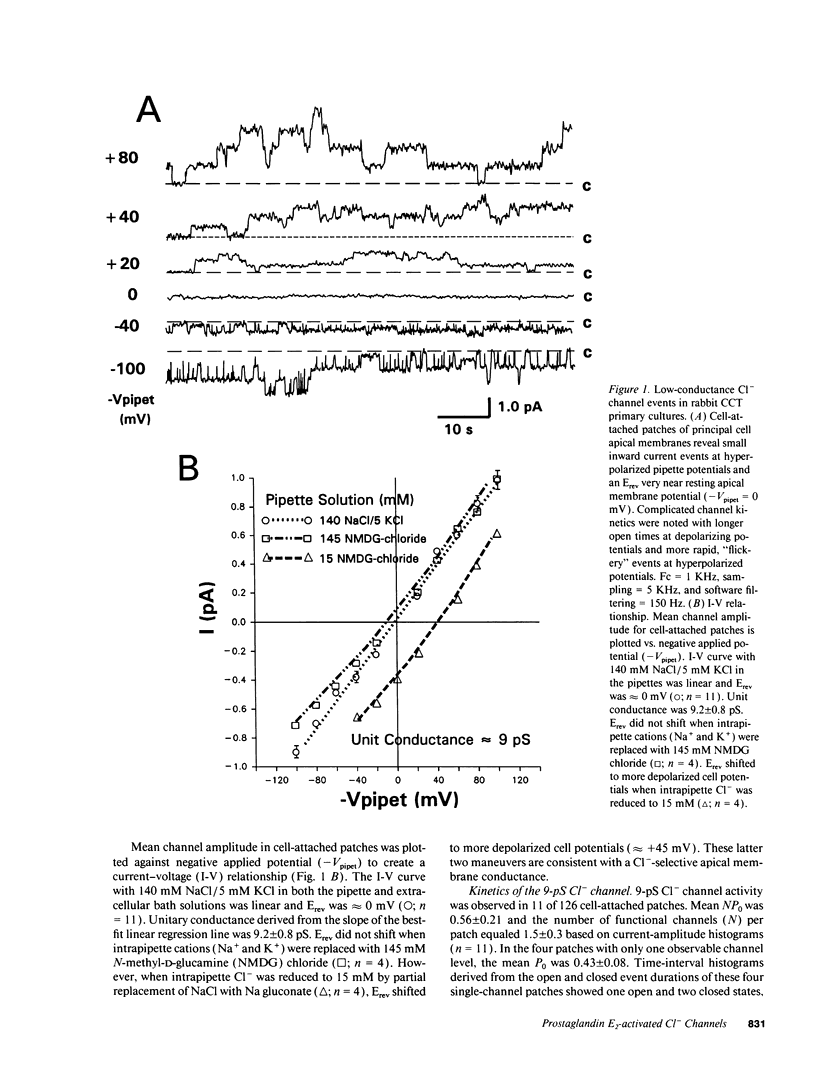
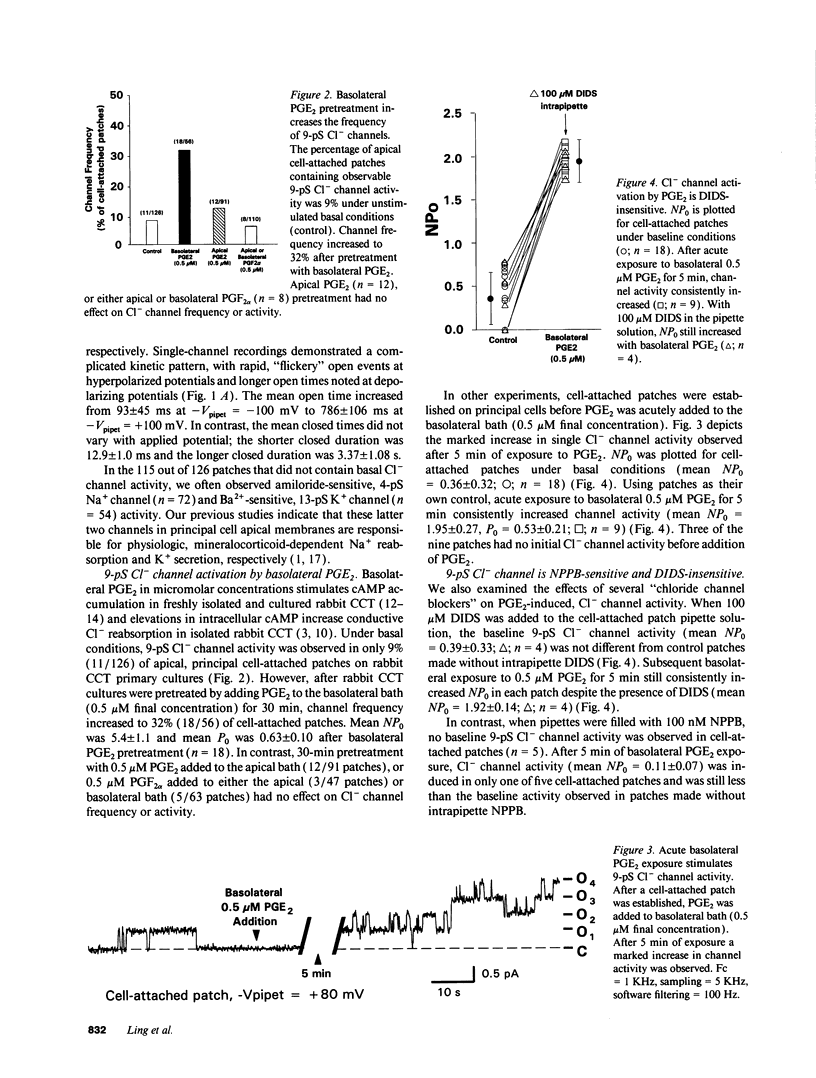
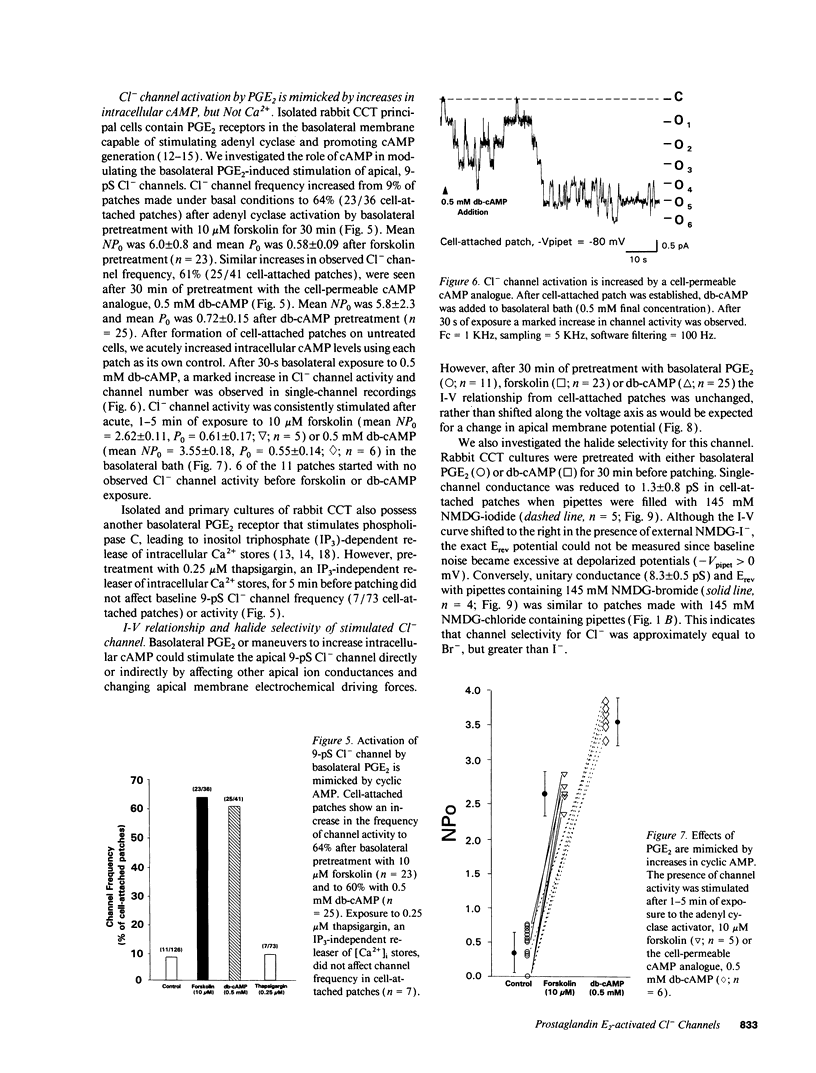
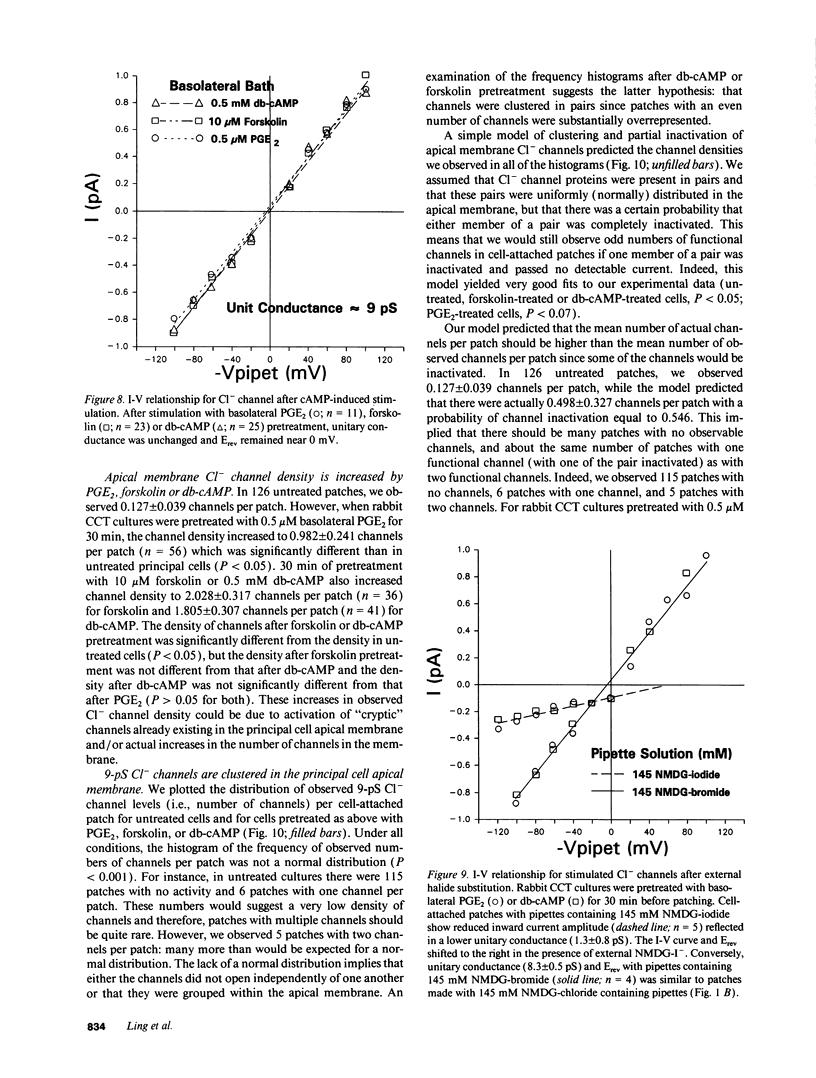
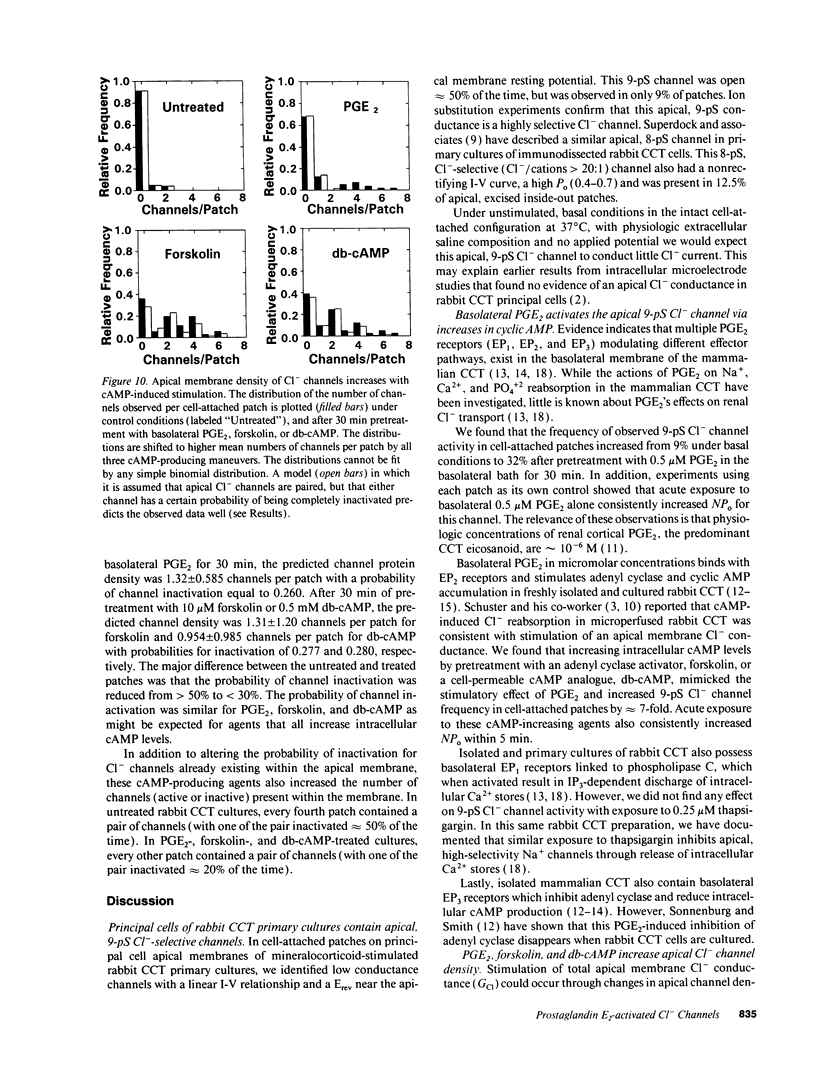
We examined cell-attached patches on principal cells of primary cultured, rabbit cortical collecting tubules. Under basal conditions, apical 9-pS Cl(-)-selective channels were observed in 9% of patches (11/126), and number of channels times open probability (NP0) was 0.56 +/- 0.21. The channel had a linear current-voltage relationship, reversal potential (Erev) near resting membrane potential, a P0 (0.30-0.70) that was independent of voltage, and complicated kinetics (i.e., bursting) at hyperpolarized potentials. NP0 and channel frequency were increased after 30 min of basolateral exposure to 0.5 microM PGE2 (18/56), 10 microM forskolin (23/36), or 0.5 mM dibutyryl cyclic adenosine monophosphate (cAMP) (25/41). Increases in NP0 appeared to be mediated primarily through an increase in the number of observed channels per patch (N), not changes in P0. After these cAMP-increasing maneuvers, N was inconsistent with a uniform distribution of channels in the apical membrane (P < 0.001), but rather the channels appeared to be clustered in pairs. Apical 0.5 microM PGE2 (12/91), apical or basolateral 0.5 microM PGF2 alpha (8/110), or 0.25 microM thapsigargin (releaser of intracellular Ca2+ stores) (7/73) did not increase NP0 or channel frequency. Conclusions: (a) 9-pS Cl- channels provide a conductive pathway for apical membrane Cl- transport across principal cells. (b) Channel activation by basolateral PGE2 is mediated via a cAMP-, but not a Ca(2+)-dependent mechanism. (c) Apical channels are clustered in pairs. (d) With its low baseline frequency and Erev near resting membrane potential, this channel would not contribute significantly to transcellular Cl- flux under basal conditions. (e) However, cAMP-producing agonists (i.e., PGE2, arginine vasopressin) would increase apical Cl- transport with the direction determined by the apical membrane potential.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello-Reuss E., Weber M. R. Electrophysiological studies of primary cultures of rabbit distal tubule cells. Am J Physiol. 1987 May;252(5 Pt 2):F899–F909. doi: 10.1152/ajprenal.1987.252.5.F899. [DOI] [PubMed] [Google Scholar]
- Breyer M. D. Feedback inhibition of cyclic adenosine monophosphate-stimulated Na+ transport in the rabbit cortical collecting duct via Na(+)-dependent basolateral Ca++ entry. J Clin Invest. 1991 Nov;88(5):1502–1510. doi: 10.1172/JCI115460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier L. W., Yorio T. Eicosanoids: their function in renal epithelia ion transport. Proc Soc Exp Biol Med. 1992 Dec;201(3):229–243. doi: 10.3181/00379727-201-43503a. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Benos D. J. CFTR! Am J Physiol. 1992 Aug;263(2 Pt 1):C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- Gross P., Minuth W. W., Kriz W., Frömter E. Electrical properties of renal collecting duct principal cell epithelium in tissue culture. Pflugers Arch. 1986 Apr;406(4):380–386. doi: 10.1007/BF00590940. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Kokko J. P., Gross J. B., Jacobson H. R. Electrophysiologic study of the cortical collecting tubule of the rabbit. Kidney Int. 1980 Jan;17(1):74–81. doi: 10.1038/ki.1980.9. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Skach W., Baker O., Calayag M. C., Lingappa V., Verkman A. S. A multifunctional aqueous channel formed by CFTR. Science. 1992 Nov 27;258(5087):1477–1479. doi: 10.1126/science.1279809. [DOI] [PubMed] [Google Scholar]
- Hébert R. L., Jacobson H. R., Breyer M. D. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest. 1991 Jun;87(6):1992–1998. doi: 10.1172/JCI115227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P. Effect of prostaglandins on renal epithelial electrolyte transport. Kidney Int. 1981 Jun;19(6):791–796. doi: 10.1038/ki.1981.82. [DOI] [PubMed] [Google Scholar]
- Larsen E. H. Chloride transport by high-resistance heterocellular epithelia. Physiol Rev. 1991 Jan;71(1):235–283. doi: 10.1152/physrev.1991.71.1.235. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Eaton D. C. Cyclosporin A inhibits apical secretory K+ channels in rabbit cortical collecting tubule principal cells. Kidney Int. 1993 Nov;44(5):974–984. doi: 10.1038/ki.1993.339. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Eaton D. C. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol. 1989 Jun;256(6 Pt 2):F1094–F1103. doi: 10.1152/ajprenal.1989.256.6.F1094. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Hinton C. F., Eaton D. C. Potassium permeable channels in primary cultures of rabbit cortical collecting tubule. Kidney Int. 1991 Sep;40(3):441–452. doi: 10.1038/ki.1991.231. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Kokko K. E., Eaton D. C. Inhibition of apical Na+ channels in rabbit cortical collecting tubules by basolateral prostaglandin E2 is modulated by protein kinase C. J Clin Invest. 1992 Oct;90(4):1328–1334. doi: 10.1172/JCI115998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Imai M., Asano Y. Interaction of Cl- and other halogens with Cl- transport systems in rabbit cortical collecting duct. Am J Physiol. 1992 Nov;263(5 Pt 2):F870–F877. doi: 10.1152/ajprenal.1992.263.5.F870. [DOI] [PubMed] [Google Scholar]
- Noland T. D., Carter C. E., Jacobson H. R., Breyer M. D. PGE2 regulates cAMP production in cultured rabbit CCD cells: evidence for dual inhibitory mechanisms. Am J Physiol. 1992 Dec;263(6 Pt 1):C1208–C1215. doi: 10.1152/ajpcell.1992.263.6.C1208. [DOI] [PubMed] [Google Scholar]
- Reeves W. B., Andreoli T. E. Renal epithelial chloride channels. Annu Rev Physiol. 1992;54:29–50. doi: 10.1146/annurev.ph.54.030192.000333. [DOI] [PubMed] [Google Scholar]
- Sauer M., Dörge A., Thurau K., Beck F. X. Effect of ouabain on electrolyte concentrations in principal and intercalated cells of the isolated perfused cortical collecting duct. Pflugers Arch. 1989 Apr;413(6):651–655. doi: 10.1007/BF00581816. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Hawk C. T. Regulation of Na+ channels in the cortical collecting duct by AVP and mineralocorticoids. Kidney Int. 1992 Feb;41(2):255–268. doi: 10.1038/ki.1992.37. [DOI] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated anion transport in rabbit cortical collecting duct. Kinetics, stoichiometry, and conductive pathways. J Clin Invest. 1986 Dec;78(6):1621–1630. doi: 10.1172/JCI112755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V. L., Stokes J. B. Chloride transport by the cortical and outer medullary collecting duct. Am J Physiol. 1987 Aug;253(2 Pt 2):F203–F212. doi: 10.1152/ajprenal.1987.253.2.F203. [DOI] [PubMed] [Google Scholar]
- Simmons N. L. Renal epithelial Cl- secretion. Exp Physiol. 1993 Mar;78(2):117–137. doi: 10.1113/expphysiol.1993.sp003674. [DOI] [PubMed] [Google Scholar]
- Smith W. L. Prostanoid biosynthesis and mechanisms of action. Am J Physiol. 1992 Aug;263(2 Pt 2):F181–F191. doi: 10.1152/ajprenal.1992.263.2.F181. [DOI] [PubMed] [Google Scholar]
- Sonnenburg W. K., Smith W. L. Regulation of cyclic AMP metabolism in rabbit cortical collecting tubule cells by prostaglandins. J Biol Chem. 1988 May 5;263(13):6155–6160. [PubMed] [Google Scholar]
- Strange K. Ouabain-induced cell swelling in rabbit cortical collecting tubule: NaCl transport by principal cells. J Membr Biol. 1989 Mar;107(3):249–261. doi: 10.1007/BF01871940. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., Winter M. C., Ostedgaard L. S., O'Riordan C., Smith A. E., Welsh M. J. Cyclic AMP-dependent protein kinase activation of cystic fibrosis transmembrane conductance regulator chloride channels in planar lipid bilayers. J Biol Chem. 1992 May 15;267(14):9470–9473. [PubMed] [Google Scholar]
- Turnheim K. Intrinsic regulation of apical sodium entry in epithelia. Physiol Rev. 1991 Apr;71(2):429–445. doi: 10.1152/physrev.1991.71.2.429. [DOI] [PubMed] [Google Scholar]
- Vasuvattakul S., Quaggin S. E., Scheich A. M., Bayoumi A., Goguen J. M., Cheema-Dhadli S., Halperin M. L. Kaliuretic response to aldosterone: influence of the content of potassium in the diet. Am J Kidney Dis. 1993 Feb;21(2):152–160. doi: 10.1016/s0272-6386(12)81086-9. [DOI] [PubMed] [Google Scholar]
- Wang W., Sackin H., Giebisch G. Renal potassium channels and their regulation. Annu Rev Physiol. 1992;54:81–96. doi: 10.1146/annurev.ph.54.030192.000501. [DOI] [PubMed] [Google Scholar]
- Wingo C. S. Active and passive chloride transport by the rabbit cortical collecting duct. Am J Physiol. 1990 May;258(5 Pt 2):F1388–F1393. doi: 10.1152/ajprenal.1990.258.5.F1388. [DOI] [PubMed] [Google Scholar]


