Abstract
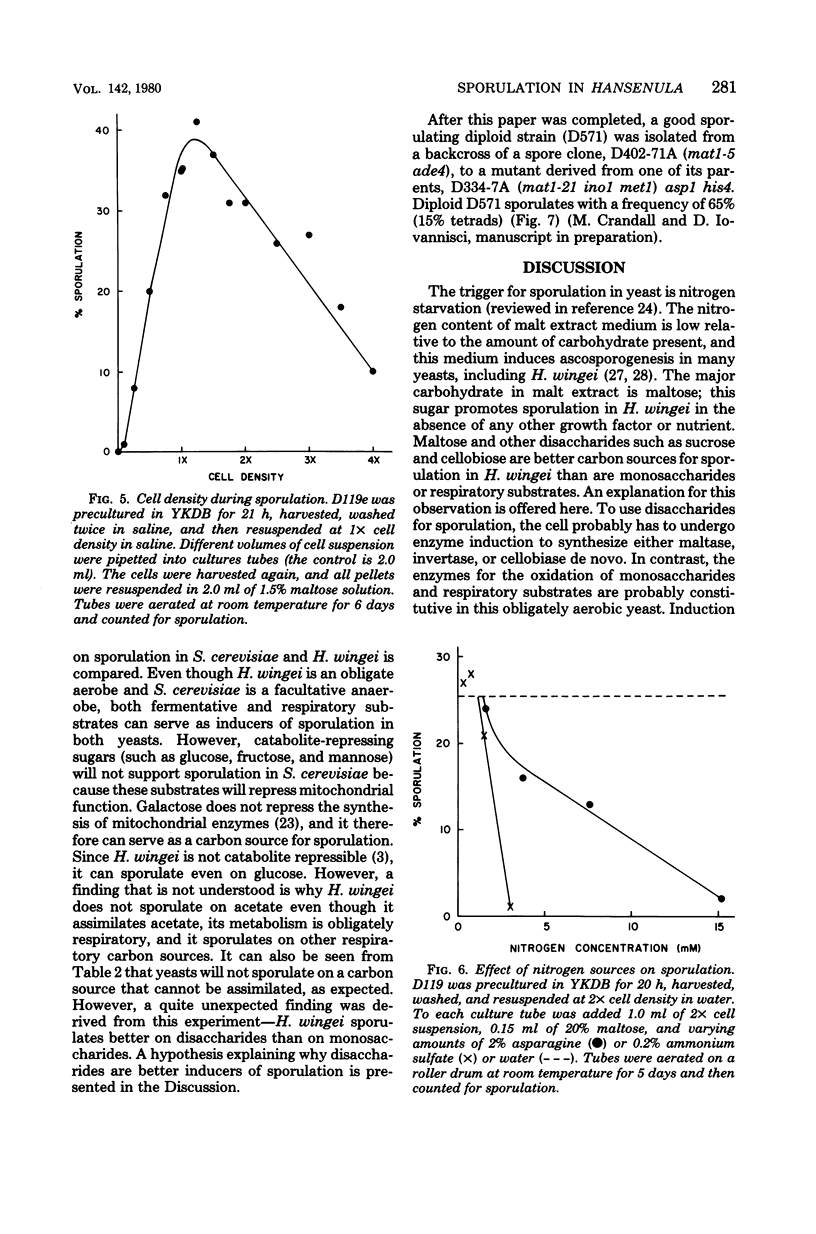
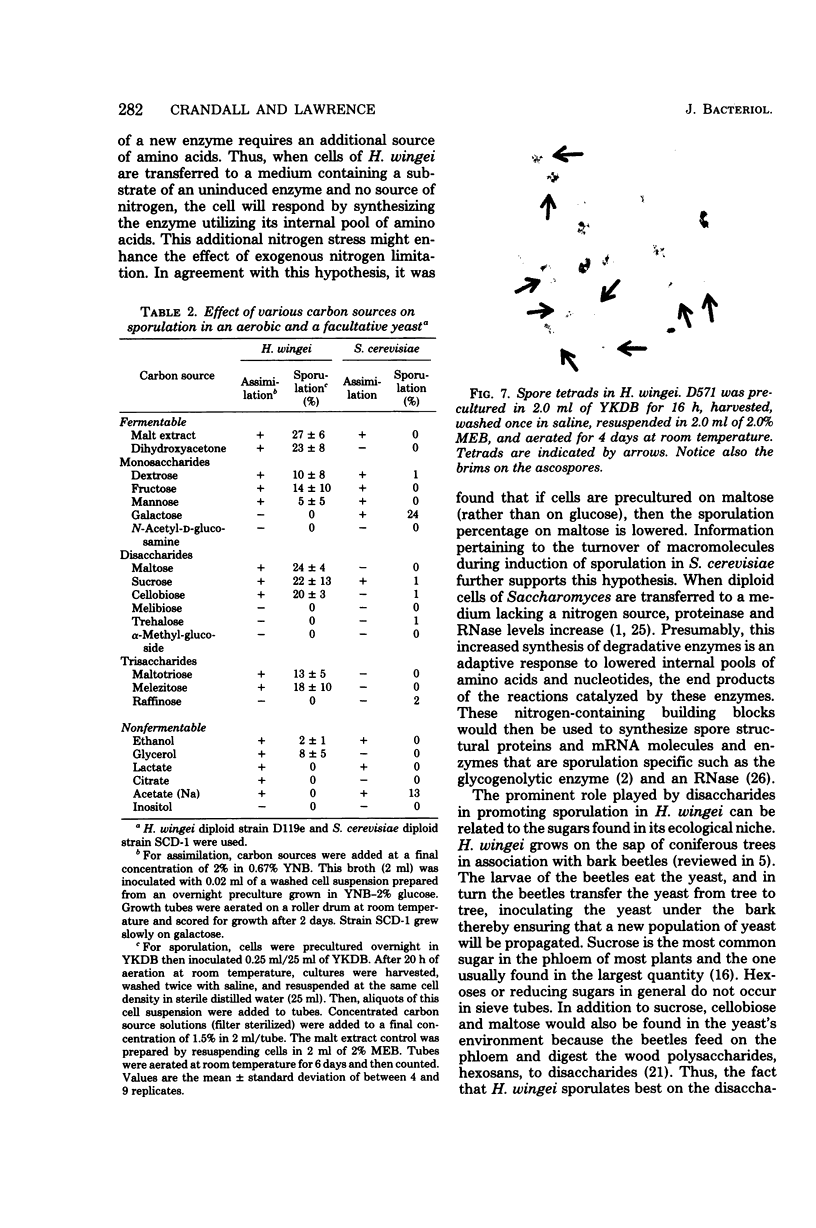
The sexually agglutinative yeast Hansenula wingei lives in association with bark beetles that inhabit coniferous trees. This yeast was induced to sporulate by malt extract, which contains a high percentage of maltose (50%) and a low percentage of nitrogen (0.5%). A solution of 1.5% maltose without any growth factors also induced ascosporogenesis in H. wingei. Thus, only a carbon source is required for sporulation as in Saccharomyces. However, potassium acetate did not induce sporulation in H. wingei as it does in S. cerevisiae. Instead, disaccharides (such as maltose, sucrose, or cellobiose) promote sporulation better than either monosaccharides (such as dextrose, fructose, or mannose) or respiratory substrates (such as ethanol or glycerol). The specificity of disaccharides in promoting sporulation in H. wingei may be considered an adaptation since these disaccharides are present in the natural environment of this yeast. In addition, the specificity of disaccharides may be related to the induction of the disaccharidase because cells precultured on dextrose sporulate well on maltose, but cells precultured on maltose sporulate poorly on maltose. When (NH4)2SO4 was added at a low concentration (3 mM) to synthetic sporulation medium (1.5% maltose solution), sporulation was abolished, whereas other salts and nitrogen sources inhibited to a lesser extent and vitamins and trace elements had no effect. Oxygen was required for sporulation, as expected for an obligate aerobe. Maximal sporulation was achieved in 2% malt extract broth at high cell density (109 cells per ml), pH 5, and 25°C. By using these optimal physiological conditions and hybrid strains selected from an extensive genetic breeding program, about 30% asci (10% tetrads) were obtained routinely. Thus, the genetics of cell recognition in this yeast can now be studied.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Weiser U. Protein degradation during yeast sporulation. Enzyme and cytochrome patterns. Eur J Biochem. 1976 Nov 15;70(2):385–395. doi: 10.1111/j.1432-1033.1976.tb11028.x. [DOI] [PubMed] [Google Scholar]
- Colonna W. J., Magee P. T. Glycogenolytic enzymes in sporulating yeast. J Bacteriol. 1978 Jun;134(3):844–853. doi: 10.1128/jb.134.3.844-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M. A., Brock T. D. Molecular basis of mating in the yeast hansenula wingei. Bacteriol Rev. 1968 Sep;32(3):139–163. doi: 10.1128/br.32.3.139-163.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M., Caulton J. H. Rare-Cell Fusion Events between Diploid and Haploid Strains of the Sexually Agglutinative Yeast HANSENULA WINGEI. Genetics. 1979 Dec;93(4):903–916. doi: 10.1093/genetics/93.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M. Comparison of Hansenula wingei, a petite-negative, obligately aerobic yeast, to the petite-positive yeast Saccharomyces cerevisiae. J Gen Microbiol. 1973 Apr;75(2):363–375. doi: 10.1099/00221287-75-2-363. [DOI] [PubMed] [Google Scholar]
- Crandall M., Lawrence L. M., Saunders R. M. Molecular complementarity of yeast glycoprotein mating factors. Proc Natl Acad Sci U S A. 1974 Jan;71(1):26–29. doi: 10.1073/pnas.71.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Delavier-Klutchko C. Effect of ammonia and glutamine on macromolecule synthesis and breakdown during sporulation of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1977 Nov 21;79(2):438–442. doi: 10.1016/0006-291x(77)90177-2. [DOI] [PubMed] [Google Scholar]
- Ehrenberg M., Halbach-Keup G., Gerth H. Untersuchungen zur lichtinduzierten Hemmung der Ascoporenbildung bei Saccharomyces cerevisiae. Arch Mikrobiol. 1973;90(3):233–246. [PubMed] [Google Scholar]
- Fonzi W. A., Shanley M., Opheim D. J. Relationship of glycolytic intermediates, glycolytic enzymes, and ammonia to glycogen metabolism during sporulation in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):285–294. doi: 10.1128/jb.137.1.285-294.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling J. P., Duggan P. F. Activities of tricarboxylic acid cycle enzymes, glyoxylate cycle enzymes, and fructose diphosphatase in bakers' yeast during adaptation to acetate oxidation. J Bacteriol. 1971 Jun;106(3):908–914. doi: 10.1128/jb.106.3.908-914.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H. O., SPIEGELMAN S. The effect of free amino acid pool levels on the induced synthesis of enzymes. J Bacteriol. 1953 May;65(5):496–504. doi: 10.1128/jb.65.5.496-504.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Halvorson H. O. Proteinase activities of Saccharomyces cerevisiae during sporulation. J Bacteriol. 1975 Nov;124(2):863–869. doi: 10.1128/jb.124.2.863-869.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. J. The metabolism of yeast sporulation. II. Stimulation and inhibition by monosaccharides. Can J Microbiol. 1957 Feb;3(1):81–90. doi: 10.1139/m57-010. [DOI] [PubMed] [Google Scholar]
- Opheim D. J. Effect of ammonium ions on activity of hydrolytic enzymes during sporulation of yeast. J Bacteriol. 1979 Jun;138(3):1022–1025. doi: 10.1128/jb.138.3.1022-1025.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñon R. Effects of ammonium ions on sporulation of Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 15;105(2):367–378. doi: 10.1016/0014-4827(77)90134-3. [DOI] [PubMed] [Google Scholar]
- STRITTMATTER C. F. Adaptive variation in the level of oxidative activity in Saccharomyces cerevisiae. J Gen Microbiol. 1957 Feb;16(1):169–183. doi: 10.1099/00221287-16-1-169. [DOI] [PubMed] [Google Scholar]
- Stock D. A., Black S. H. Meiosis in Hansenula holstii and H. wingei. J Gen Microbiol. 1970 Dec;64(3):365–372. doi: 10.1099/00221287-64-3-365. [DOI] [PubMed] [Google Scholar]
- Tsuboi M. Correlation among turnover of nucleic acids, ribonuclease activity and sporulation ability of Saccharomyces cerevisiae. Arch Microbiol. 1976 Dec 1;111(1-2):13–19. doi: 10.1007/BF00446544. [DOI] [PubMed] [Google Scholar]
- Tsuboi M., Yanagishima N. Promotion of sporulation by caffeine pretreatment in Saccharomyces cerevisiae. II. Changes in ribonuclease activity during sporulation. Arch Microbiol. 1976 Jun;108(2):149–152. doi: 10.1007/BF00428944. [DOI] [PubMed] [Google Scholar]