Abstract
Chronic hypoxia (CH)-induced pulmonary hypertension is associated with decreased basal pulmonary artery endothelial cell (EC) Ca2+, which correlates with reduced store-operated Ca2+ (SOC) entry. Protein kinase C (PKC) attenuates SOC entry in ECs. Therefore, we hypothesized that PKC has a greater inhibitory effect on EC SOC and receptor-operated Ca2+ entry after CH. To test this hypothesis, we assessed SOC in the presence or absence of the nonselective PKC inhibitor GF109203X [2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide] in freshly isolated, Fura-2-loaded ECs obtained from intrapulmonary arteries of control and CH rats (4 weeks at 0.5 atm). We found that SOC entry and 1-oleoyl-2-acetyl-sn-glycerol (OAG)- and ATP-induced Ca2+ influx were attenuated in ECs from CH rats versus controls, and GF109203X restored SOC and OAG responses to the level of controls. In contrast, nonselective PKC inhibition with GF109203X or the selective PKCε inhibitor myristoylated V1-2 attenuated ATP-induced Ca2+ entry in ECs from control but not CH pulmonary arteries. ATP-induced Ca2+ entry was also attenuated by the T-type voltage-gated Ca2+ channel (VGCC) inhibitor mibefradil in control cells. Consistent with the presence of endothelial T-type VGCC, we observed depolarization-induced Ca2+ influx in control cells that was inhibited by mibefradil. This response was largely absent in ECs from CH arteries. We conclude that CH enhances PKC-dependent inhibition of SOC- and OAG-induced Ca2+ entry. Furthermore, these data suggest that CH may reduce the ATP-dependent Ca2+ entry that is mediated, in part, by PKCε and mibefradil-sensitive Ca2+ channels in control cells.
Introduction
Conditions associated with chronic hypoxia (CH), such as chronic bronchitis and emphysema, often lead to pulmonary hypertension. The endothelium is an important regulator of pulmonary vascular tone that may be affected by CH. It has been recently observed that basal endothelial cell (EC) [Ca2+]i and store-operated Ca2+ (SOC) entry are reduced in pressurized intrapulmonary arteries from CH rats (Paffett et al., 2007). Because many vasodilatory pathways, such as the production of nitric oxide and prostacyclin, are Ca2+-dependent, diminution of [Ca2+]i could promote the vasoconstriction observed in this setting. However, the mechanism of reduced EC Ca2+ entry after CH has not been investigated. The present study examines the role of PKC in reduced Ca2+ entry.
Many families of ion channels in a variety of cell types are regulated by PKC. Some of the first reports of PKC modulating voltage-gated Ca2+ channels (VGCCs) were in neuronal (Ewald et al., 1988; Yang and Tsien, 1993) and vascular smooth muscle (VSM) preparations (Schuhmann and Groschner, 1994). These early investigations revealed that stimulating PKC with phorbol esters can either potentiate or inhibit VGCC activity depending on the cell type or concentration of PKC agonist. Later investigations reported that thrombin-induced activation of T-type VGCCs has been characterized in the pulmonary microvasculature (Wu et al., 2003) and shown to involve PKCε (Park et al., 2006). In addition to modulating VGCC, PKC can target transient receptor potential (TRP) channels in the vasculature. For example, Earley et al. (2007) observed PKC-dependent activation of the mechanosensitive TRPM4 isoform that contributes to control of cerebral vascular tone. PKC also regulates members of the canonical subfamily of TRP channels known to be expressed in vascular endothelium and smooth muscle. TRPC1/4/5 isoforms are activated by depletion of intracellular Ca2+ stores, resulting in extracellular Ca2+ influx or SOC entry, whereas TRPC3/6/7 are believed to be activated in a store-independent manner by a variety of second-messenger systems and are often referred to as receptor-operated Ca2+ (ROC) entry [for review see Pedersen and Nilius (2007))]. PKC has been identified as both an activator of SOC entry in portal vein (Albert and Large, 2002) and an inhibitor in pulmonary artery VSM cells (Horibe et al., 2001), but there is little information regarding the role of PKC in modulating SOC and ROC entry in the vascular endothelium.
PKC isoforms are classified into three categories determined by their NH2-terminal regulatory domain structure. Conventional PKCs (α, βI, βII, and γ) contain a C1 domain that binds diacylglycerol (DAG) and a C2 domain that binds anionic phospholipids in a Ca2+-dependent manner. Novel PKCs (δ, θ, ε, and η) are activated by DAG but not by changes in cytosolic Ca2+. Unlike conventional and novel PKCs, atypical PKCs (ζ and ι/λ) are characterized as DAG- and Ca2+-insensitive, but they are activated by phosphatidylinositol trisphosphate or ceramide [for review see Gallegos and Newton (2008)]. Whereas most PKC subfamilies and their isoforms are ubiquitously expressed throughout various tissues, their regulatory actions on SOC and ROC entry vary widely. For example, PKCα contributes to activation of SOC entry in cultured mesangial (Ma et al., 2002) and ECs (Ahmmed et al., 2004). Likewise, δ and β PKC isoforms are required for SOC entry in corneal epithelium (Zhang et al., 2006). Yang et al. (2008) found that nonspecific inhibition of PKC enhanced SOC entry in pulmonary artery VSM, suggesting an inhibitory rather than a potentiating role within the pulmonary vasculature. These reports highlight the diverse effects of PKC on SOC entry in the nonpathological setting; however, little is known about the role of PKC regulation of EC SOC and ROC entry after CH.
Therefore, we hypothesized that reduced EC SOC and ROC entry after CH are mediated by altered PKC-dependent regulation. We tested this hypothesis by examining the effect of different PKC inhibitors on SOC and ROC entry in freshly isolated endothelium from intrapulmonary arteries from control rats and pulmonary hypertensive animals exposed to 4 weeks of CH.
Materials and Methods
All protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center.
Exposure of Rats to Chronic Hypoxia.
Male Sprague-Dawley rats (200–250 g; Harlan, Indianapolis, IN) were used for all studies. CH exposure consisted of housing rats in a pressure-controlled environment (∼380 torr) for 4 weeks. Age-matched control rats were boarded in similar cages under ambient barometric pressure (∼630 torr). The hypobaric chamber was opened three times a week to provide fresh rat chow, water, and clean bedding.
Isolation and Preparation of Pulmonary Artery Endothelial Cells.
Rats were euthanized with sodium pentobarbital (200 mg/kg i.p.), and the left lung was rapidly excised and placed in HEPES-buffered saline solution (HBSS). The HBSS contained 150 mM NaCl, 6 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 10 mM HEPES, and 10 mM glucose, titrated to pH 7.4 with NaOH. Intrapulmonary arteries (third and fourth order; 200–400 μm i.d.) were dissected from the cranial most region of the left lung and carefully cleaned of surrounding lung parenchyma. Endothelial sheets were enzymatically dissociated and stored for up to 5 h at 4°C as described previously (Paffett et al., 2007). Freshly isolated rat pulmonary artery endothelial cells were then placed on a poly-l-lysine-coated glass-bottom 35-mm culture dish (BD Biosciences, San Jose, CA) with a small bore fire-polished Pasteur pipette and allowed to equilibrate for 30 min at room temperature before experimentation.
Fura-2 Loading of Freshly Isolated Endothelial Sheets.
Ca2+ entry was determined in freshly isolated endothelial sheets by using the ratiometric Ca2+-sensitive dye Fura-2 AM (Invitrogen). Endothelial sheets were loaded with 3 μM Fura-2 AM (0.05% pluronic acid) in HBSS for 5 min at ∼23°C and washed for 15 min at 37°C. Ratiometric changes in endothelial cell [Ca2+]i were acquired by alternating specimen excitation for 50 ms between 340- and 380-nm bandpass filters at 1 Hz (Hyperswitch; Ionoptix, Milton, MA) in which the interleaved Fura-2 emissions at 510 nm were detected with a photomultiplier tube.
Assessing the Role of PKC-Dependent Modulation of SOC and ROC Entry.
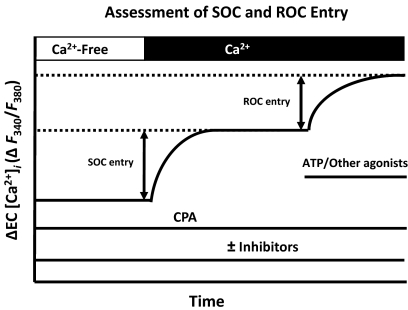
After a 30-min recovery period and Fura-2 loading, endothelial sheets were superfused with HBSS at 37°C and then switched to Ca2+-free HBSS (equimolar Mg2+ substitution) for 2 to 3 min. Passive depletion of intracellular Ca2+ stores by inhibition of the sarco/endoplasmic reticulum Ca2+ ATPase with 10 μM cyclopiazonic acid (CPA) was performed, and SOC entry was defined as the change in 340/380 fluorescence after repletion of extracellular Ca2+ (Fig. 1). After the SOC entry response, stabilized ROC entry was assessed by the addition of OAG (100 μM) or ATP (20 μM) in the continued presence of CPA. Any further increase in Fura-2 ratio was defined as ROC entry (Fig. 1) as described previously (Jernigan et al., 2006). In separate experiments, ROC entry was assessed in cells preincubated for 10 min with the nonspecific inhibitor of PKC [GF109203X (2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide)] (1 μM) before the reapplication of extracellular Ca2+ and ROC entry agonist. In parallel experiments, the cell-permeant PKCε peptide inhibitor (V1-2myr) (10 μM) or a concentration-specific PKCα/β inhibitor (Gö6976) [5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]py rrolo[3,4-c]carbazole-12-propanenitrile] (6 nM) was applied for period of 10 min before the addition of ATP.
Fig. 1.
Experimental protocol depicting assessment of endothelial SOC and ROC entry. SOC entry was defined by a change in F340/F380 (ΔR) in freshly isolated endothelial cells depleted of intracellular Ca2+ stores with 10 μM CPA before the readdition of extracellular Ca2+. Endothelial ROC entry was defined similarly and assessed by application of OAG (100 μM) or ATP (20 μM) after the stabilization of the SOC response. Depolarization-induced entry was assessed in a likewise fashion with high extracellular K+. PKC, PLC, and Ca2+ channel inhibitors were administered in separate protocols.
Effect of Ca2+ Channel Blockers on ATP-Induced Ca2+ Entry.
To determine whether ATP-induced ROC entry is mediated by voltage-dependent Ca2+ channels, we examined Ca2+ responses to ATP in store-depleted endothelial cells from control and CH arteries preincubated with the putative T-type Ca2+ channel inhibitor mibefradil (10 μM), the L-type Ca2+ channel inhibitor diltiazem (50 μM), the nonselective Ca2+ channel blocker SKF96365 [1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl]-1H-imidazole hydrochloride] (20 μM), or vehicle for 5 min before stimulation with ATP. These concentrations of diltiazem and mibefradil have been reported previously to selectively inhibit L-and T-type VGCCs, respectively (Wei et al., 2004; Zhou et al., 2007). Furthermore, we performed validation experiments using patch-clamp techniques to confirm the selective inhibitory actions of mibefradil and diltiazem in neonatal cardiomyocytes and pulmonary artery vascular smooth muscle cells, respectively (see Supplemental Figs. 1 and 2).
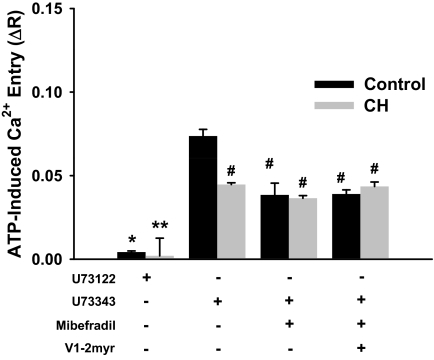
Role of PLC, Mibefradil-Sensitive Ca2+ Channels, and PKCε in ATP-Induced Ca2+ Entry.
Additional experiments were conducted to confirm that ATP-induced Ca2+ entry involves PLC-initiated signaling events. After the development of a stable SOC entry response, 20 μM ATP was added in the presence of the PLC inhibitor U73122 [1-[6-[[(17b)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione] (3 μM) or its inactive analog U73343 [1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-pyrrolidine-2,5-dione] (3 μM). To corroborate the involvement of T-type Ca2+ channels in ATP-induced entry, parallel experiments were performed in the presence of mibefradil. Furthermore, to determine whether PKCε and T-type Ca2+ channel activation were operating in parallel after the addition of ATP, we assessed ATP-induced Ca2+ influx in the presence of mibefradil and the myristoylated V1-2 peptide PKCε inhibitor.
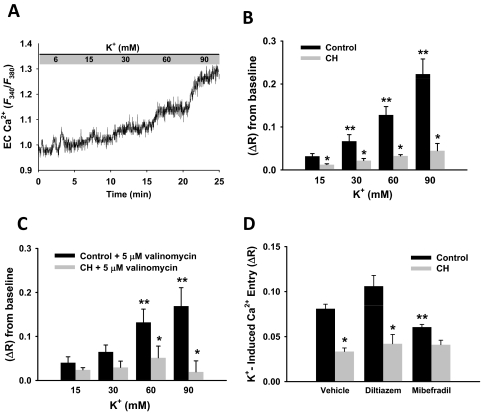
Endothelial Ca2+ Responses to Extracellular KCl.
Because results of the above studies suggested the presence of endothelial VGCCs, the response to depolarizing concentrations of KCl (15, 30, 60, and 90 mM) was assessed in cells from control and CH rats. Parallel experiments were performed in which the KCl-selective ionophore valinomycin (5 μM) was present to rule out the possibility of unequal K+ conductance differentially regulating Em between groups. To determine the potential involvement of L- and T-type voltage-sensitive Ca2+ channels, a 60-mM KCl depolarizing stimulus was applied in the presence or absence of the inhibitors diltiazem and mibefradil. Furthermore, to rule out any potential tonic influences of store-operated Ca2+ entry on the depolarizing effects of KCl, these experiments were conducted in the presence of CPA to inhibit sarco/endoplasmic reticulum Ca2+ ATPase.
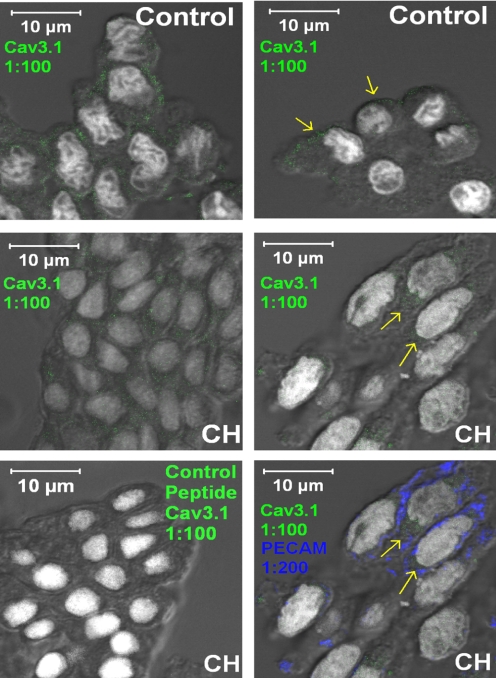
Qualitative Immunofluorescence of Cav3.1 (α1G) in the Pulmonary Endothelium.
Freshly isolated pulmonary arterial endothelium from control or CH animals were fixed in 4% paraformaldehyde at room temperature for 10 min. After fixation, all samples were permeabilized with 0.01% Triton X-100 and phosphate-buffered saline for 10 min and blocked with 3% donkey serum in phosphate-buffered saline for 1 h at room temperature. Fixed cells were incubated with primary antibodies for the Cav3.1 (α1G) T-type VGCC subunit (1:100; rabbit polyclonal) and PECAM-1 (1:200; mouse monoclonal) (Transduction Laboratories, Lexington, KY) overnight at 4°C. Primary antibodies were detected with Cy5-conjugated donkey anti-rabbit and Cy3-conjugated donkey anti-mouse secondary antibodies (1:500 dilution; Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Nuclei were stained with Sytox (1:10,000 dilution; Invitrogen, Carlsbad, CA) and applied to all samples. Specimens were visualized with a confocal laser microscope (LSM 510; Carl Zeiss Inc., Thornwood, NY) with a 63× oil immersion lens.
Calculations and Statistics.
All data are expressed as means ± S.E. Values of n refer to the number of endothelial sheets (40–100 cells/sheet) in which one to two sheets were studied from one rat. A one-way or two-way analysis of variance was used where appropriate for all comparisons between control and CH groups. If differences were detected by analysis of variance, individual groups were compared with the Student-Newman-Keuls test. Probability of ≤0.05 was accepted as statistically significant for all comparisons.
Results
Differential PKC Regulation of SOC and ROC Entry.
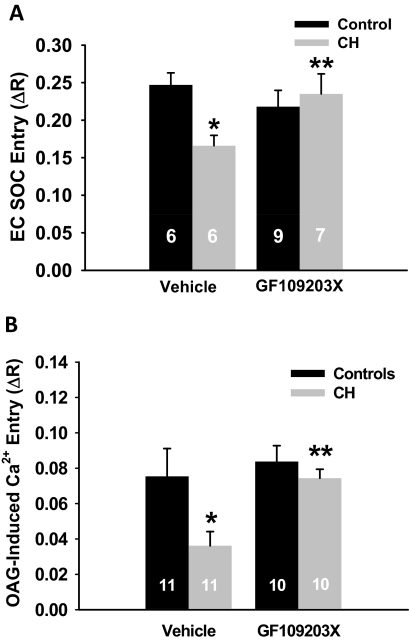
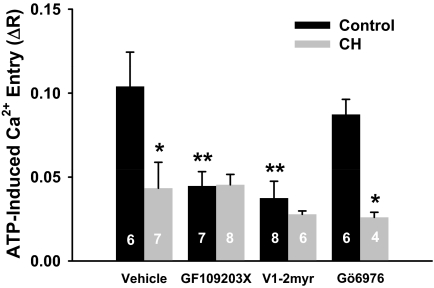
Both SOC and ROC entry were diminished in cells from CH compared with control arteries (Fig. 2). Diminished ROC entry was seen in experiments using OAG (Fig. 2) and those using ATP (Fig. 3) as an agonist. Nonselective PKC inhibition with GF109203X restored both SOC and OAG-induced Ca2+ entry in endothelial cells isolated from CH arteries to the level of controls without affecting the control group (Fig. 2). In contrast, GF109203X reduced ATP-induced Ca2+ responses in endothelial cells from control arteries (Fig. 3). Likewise, PKCε inhibition with V1-2myr effectively blunted ATP-induced Ca2+ entry in control endothelium, whereas PKCα/β inhibition with Gö6976 had no effect. In contrast to control cells, pan-specific inhibition of PKCs or selective PKCα/β or PKCε inhibition did not affect the blunted ATP-induced Ca2+ response in the CH group. These results demonstrate that CH exposure results in a generalized reduction in Ca2+ entry; however, there seems to be differential regulation by various PKC isoforms depending on the mode of activation.
Fig. 2.
PKC inhibition restores endothelial SOC and OAG-induced ROC entry in endothelial cells from CH rats. A, endothelial SOC entry was measured as a change in F340/F380 (ΔR) fluorescence upon repletion of extracellular Ca2+ (1.8 mM) in the presence or absence of the nonselective PKC inhibitor GF109203X. B, serial assessment of endothelial ROC entry was performed by evaluating OAG-induced Ca2+ entry (ΔR) after the SOC response in the presence or absence of GF109203X. Values are mean ± S.E. n is the number of endothelial sheets (40–100 cells/sheet) and is indicated within the data bars. P ≤ 0.05: *, versus control vehicle; **, versus CH vehicle.
Fig. 3.
PKC inhibition blunts ATP-induced Ca2+ influx in endothelium from controls but not from CH pulmonary arteries. ATP-induced Ca2+ entry (ΔR) was assessed after the SOC response in the presence of vehicle, the nonselective PKC inhibitor GF109203X (1 μM), the PKCε inhibitor V1-2myr (10 μM), or the PKCα/β inhibitor Gö6976 (6 nM). Values are mean ± S.E. n is the number of endothelial sheets (40–100 cells/sheet) and is indicated within the data bars. P ≤ 0.05: *, versus control vehicle; **, versus control vehicle.
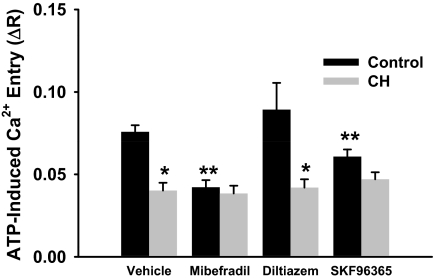
Effect of Ca2+ Channel Blockers on ATP-Induced Ca2+ Entry.
Inhibition of T-type Ca2+ channels with mibefradil blunted ATP-induced Ca2+ entry in endothelial cells from control rats compared with vehicle but was without effect in cells from CH rats (Fig. 4). Likewise, the nonselective inhibitor of voltage-dependent Ca2+ channels SKF96365 reduced entry only in control cells. In contrast, L-type Ca2+ channel inhibition was ineffective at blocking ROC entry in either group.
Fig. 4.
Receptor-mediated (ATP) Ca2+ influx involves T-type VGCCs in endothelium from controls but not CH pulmonary arteries. Experiments were conducted after the SOC response in the presence of VGCC inhibitors: 10 μM mibefradil, 50 μM diltiazem, or 20 μM SKF96365. Values are mean ± S.E. (n = 5/group). P ≤ 0.05: *, versus control; **, versus control vehicle.
Role of PLC, Mibefradil-Sensitive Ca2+ Channels, and PKCε in ATP-Induced Ca2+ Entry.
Experiments were performed with U73122 to verify that ATP-induced responses involved PLC-initiated events. PLC inhibition with U73122 abolished ATP-induced Ca2+ responses in endothelial cells from both control and CH arteries, whereas the inactive analog U73343 of this inhibitor had no effect (Fig. 5). Furthermore, as seen in previous protocols, mibefradil reduced ROC entry in only the control group. When mibefradil was combined with the PKCε inhibitor, V1-2myr, no additive effect was observed. It is noteworthy that neither of these inhibitors had an effect on ATP-induced Ca2+ entry in endothelial cells from CH arteries.
Fig. 5.
Receptor-mediated (ATP) Ca2+ influx operates through a PLC-dependent mechanism that potentially requires PKCε to activate T-type VGCCs in endothelium from controls but not CH pulmonary arteries. PLC-dependent signaling through PKCε, and T-type VGCCs in ATP-induced Ca2+ entry was examined in endothelium from control and CH pulmonary arteries. Experiments were conducted after the SOC entry response in the presence of U73122 (3 μM), U73343 (3 μM), mibefradil (10 μM), and V1-2myr (10 μM). Values are expressed as means ± S.E. (n = 5/group). P ≤ 0.05: *, versus inactive analog control; **, versus inactive analog CH; #, versus U73343 control.
Endothelial Ca2+ Responses to Extracellular KCl.
Application of increasing concentrations of extracellular KCl increased endothelial cell Ca2+ in control cells (Fig. 6A); however, this response was greatly attenuated in ECs from CH arteries (Fig. 6B). These differences persisted when endothelial K+ permeability and hence Em was equivalently clamped with valinomycin across all KCl concentrations (Fig. 6C), demonstrating that unequal K+ permeability does not account for the observed differences between groups. Additional experiments showed that the Ca2+ response to 60 mM KCl was inhibited by the T-type antagonist mibefradil in control cells, but had no effect in cells from CH rats. The L-type channel inhibitor diltiazem did not affect either group. These data suggest that mibefradil-sensitive T-type VGCCs account for depolarization-induced Ca2+ entry in control cells and that this response is lost after CH.
Fig. 6.
Endothelial cell Ca2+ increases in response to increasing K+ concentrations. A, representative trace illustrating endothelial cell Ca2+ response to incremental K+ concentrations measured by Fura-2 in control cells. B, summary data illustrating K+-dependent Ca2+ responses in endothelium from control and CH pulmonary arteries. KCl-induced Ca2+ responses were less at all K+ concentrations in cells from CH rats compared with controls. C, K+-induced Ca2+ influx was also performed in the presence of the K+-selective ionophore valinomycin (5 μM). Values are expressed as means ± S.E. (n = 4/group). P ≤ 0.05: *, versus control; **, versus 15 mM K+ concentration. D, summary data illustrating reduced Ca2+ entry after CH in response to 60 mM K+ and the effects of VGCC channel inhibition in control endothelial cells. Diltiazem (50 μM) and 10 μM mibefradil were used to selectively inhibit L-type and T-type VGCCs, respectively. ΔR is defined by change in F340/F380. Values are expressed as means ± S.E. (n = 4/group). P ≤ 0.05: *, versus control; **, versus control vehicle and control diltiazem.
Qualitative Immunofluorescence of Cav3.1 (α1G) in the Pulmonary Endothelium.
Cav3.1 immunofluorescence was detected in the endothelium from control rats and seemed to be peripherally located (Fig. 7, top). Immunofluorescence was also detected in endothelium from CH vessels; however, Cav3.1 fluorescence seemed to be less abundant at the cell periphery (Fig. 7, middle). Primary antibody specificity was confirmed with the blocking antigen, and endothelial cells were positively identified by a PECAM-1 label (Fig. 7, bottom).
Fig. 7.
Immunofluorescence of Cav3.1 (α1G) T-type VGCC subunit (green) in freshly isolated endothelial cells from small pulmonary arteries harvested from control (top) and CH (middle and bottom) rats (magnification: ×630). Top and middle, images for control (top) and CH (middle) show detectable Cav3.1 channel subunit fluorescence (indicated by yellow arrows). Bottom left, coincubation with the blocking peptide prevented Cav3.1 immunofluorescence. Bottom right, positive PECAM-1 immunofluorescence is shown in blue. Cav3.1 and Sytox nuclear stain is in white in all cases.
Discussion
The present study illustrates the differential regulation of endothelial SOC and ROC entry pathways by PKC after CH-induced pulmonary hypertension. The major findings of this study are: 1) SOC entry and OAG- and ATP-induced Ca2+ influx pathways are attenuated in freshly dissociated endothelium from CH pulmonary arteries compared with controls; 2) nonselective inhibition of PKC restores SOC and OAG responses in endothelium from CH rats to the level of controls; 3) PKCε inhibition attenuates ATP-induced Ca2+ entry in endothelium from control but not CH pulmonary arteries; 4) ATP-induced Ca2+ entry was inhibited by mibefradil in control but not CH endothelia; and 5) CH attenuates high K+-induced Ca2+ entry, whereas this response was present in control ECs and blocked by mibefradil. Taken together, these findings suggest that CH up-regulates PKC-dependent inhibition of SOC- and OAG-induced Ca2+ entry. Furthermore, these data also suggest that CH reduces PLC-dependent Ca2+ entry that seems to be mediated, in part, by PKCε and mibefradil-sensitive Ca2+ channels in control cells. Impaired Ca2+ entry after CH could significantly diminish production and release of important vasodilatory mediators, thereby exacerbating the severity of pulmonary hypertension.
In most cells, receptor-dependent activation of PLC stimulates the production of IP3 and subsequent release of Ca2+ from intracellular stores, which leads to plasmalemmal Ca2+ influx. The store-dependent arm of this signaling pathway is activated by IP3 binding to IP3 receptors, depleting endoplasmic reticulum Ca2+ and stimulating SOC entry. However, there is considerable evidence that PLC-dependent DAG production mediates store-independent Ca2+ influx (Cheng et al., 2006; Leung et al., 2006). The present study demonstrates that endothelial cells from small pulmonary arteries possess store-independent Ca2+ entry elicited by either OAG or ATP application after CPA-induced store depletion (Fig. 2B). Furthermore, our results are consistent with studies that suggest DAG directly activates TRPC channels in endothelial cells (Pocock et al., 2004). In addition to exogenous DAG analogs, endogenous DAG has been shown to stimulate Ca2+ influx independent of PKC activation (Gamberucci et al., 2002; Trebak et al., 2003). The current understanding from those reports and others is that DAG stimulates TRPC3/6/7 isoforms, leading to Ca2+ influx, but that TRPC1/4/5 isoforms are not involved in DAG-dependent Ca2+ influx [reviewed in (Pedersen and Nilius, 2007)]. It is noteworthy that our results show reduced OAG- and ATP-dependent Ca2+ entry is reduced in CH-induced pulmonary hypertension.
The role of DAG-activated PKC in regulating SOC/ROC entry is controversial. Broad-spectrum PKC activators inhibit SOC entry in human neutrophils (Montero et al., 1993) and SOC entry-mediated photoreceptor activation (Hardie et al., 1993) in Drosophila. Furthermore, Venkatachalam et al. (2003) demonstrated that PLCγ2-dependent activation of TRPC3/4/5 Ca2+ influx is negatively regulated by PKC secondary to cytosolic Ca2+ and/or DAG accumulation after receptor activation. Likewise, our findings suggest that PKC inhibits SOC- and OAG-induced Ca2+ entry after CH (Fig. 2); however, this mechanism was not evident in endothelial cells from control rats.
To further characterize the effects of CH on ROC entry and how PKC may be regulating Ca2+ influx, we examined purinergic receptor-stimulated Ca2+ influx. Consistent with effects on SOC entry and OAG-induced Ca2+ influx, we found ATP-induced Ca2+ influx was decreased in CH compared with control endothelia. Although we observed a similar decrease in Ca2+ influx to SOC- and OAG-induced Ca2+ entry after CH, it seems that purinergic receptor activation may lead to a distinct signaling cascade requiring PKC activation to stimulate ROC entry in only control endothelial cells (Fig. 3). Similar findings by Lee et al. (1997) found that 30 μM ATP promoted PKC-dependent activation of ROC entry, whereas 300 μM ATP evoked PKC-dependent inhibition of this response. That earlier report suggests PKC activates Ca2+ influx at concentrations of ATP similar to those used in the current study. The apparently opposing roles for PKC in regulating endothelial cell ROC entry depending on the mode of activation (i.e., OAG versus ATP) suggests that different signaling cascades are activated by these approaches. Pharmacological characterization of ATP-induced Ca2+ entry revealed that PKCε seems to be a key regulator in control cells but that this mode of activation may be lost after CH. Although this possibility is likely, the use of submaximal concentrations of Ca2+ channel and/or PKC inhibitors could influence our conclusion that CH impairs Ca2+ influx. However, the concentrations of mibefradil and diltiazem used were effective at abolishing Ca2+ currents in cells known to express the targeted channels (see Supplemental Figs. 1 and 2). Thus, the residual Ca2+ influx mediated by KCl and ATP may represent diltiazem- and mibefradil-insensitive Ca2+ entry pathways that are still intact in endothelial cells from either experimental group. This interpretation of residual Ca2+ entry is further supported by the finding that PLC blockade abrogates ATP-induced Ca2+ influx in control and CH endothelial cells (Fig. 5), suggesting either PKC inhibitor concentrations were submaximal (particularly PKCα/β inhibition with Gö6976) or intact Ca2+ entry pathways were not regulated by PKCs, accounting for this residual Ca2+ influx.
CH could decrease PLC activity or PKCε activity, thereby limiting downstream activation of ROC entry. Information concerning altered PLC and PKCε activities in the pulmonary endothelium after CH is limited. However, attenuated PLC-dependent Ca2+ mobilization in myometrial smooth muscle exposed to hypobaric hypoxia has been reported (Arakawa et al., 2004). In addition, acute hypoxic exposure decreases phosphoinositide synthesis in carotid bodies (Rigual et al., 1999). Later studies demonstrated increased PKCα and PKCδ expression but reductions in PKCβII, PKCγ, and PKCε in hypertrophied right ventricles from CH rats (Uenoyama et al., 2010), suggesting differential effects on PKC expression by CH. Although this finding supports the differential regulation of various PKC isoforms by CH, further investigation into the effects of CH on pulmonary endothelial PKC and the disparate roles they play in regulating Ca2+ influx is warranted.
Until recently, there has been limited support for the existence and/or role for T-type VGCCs in the pulmonary endothelium. However, molecular (De Proost, et al., 2007) and biophysical and pharmacological (Wu et al., 2003) evidence of Cav3.1 T-type VGCCs in the pulmonary microcirculation supports our observation that endothelial cells freshly dissociated from small pulmonary arteries express functional T-type VGCCs. This conclusion was corroborated by demonstration of Cav3.1 T-type VGCC expression by immunofluorescence (Fig. 7). However, our findings that KCl-induced Ca2+ entry is reduced (Fig. 6, B and C) and insensitive to the T-type channel inhibitor mibefradil (Fig. 6D) in endothelial cells from CH-hypertensive arteries indicate a functional and/or expressional sensitivity of this Ca2+ channel to CH.
A potential caveat of this interpretation is nonspecific actions of mibefradil on L-type VGCCs. However, there was no effect of diltiazem on KCl-induced Ca2+ influx in endothelial cells from pulmonary normotensive rats (Fig. 6), indicating a benzothiazepine (diltiazem) insensitivity to depolarization-induced Ca2+ entry. Furthermore, the specific inhibitory actions of mibefradil and diltiazem were documented in neonatal cardiomyocytes and pulmonary artery vascular smooth muscle cells, respectively (see Supporting Text). Similar patch-clamp experiments were attempted in freshly dispersed endothelial sheets (data not shown), but space-clamping prevented precise control of membrane potential because these cells seem to have intact intercellular communication, leading to a very large capacitance proportional to the number of cells in a given sheet. Furthermore, we were unable to observe an inward rectifying Ca2+ current with the classic biophysical (rapid activation and inactivation) signature of T-type VGCCs in electrically isolated single endothelial cells (data not shown). It is possible that the elusive nature of identifying T-type VGCCs in the single-cell preparation is caused by a small subpopulation of endothelial cells that actually express T-type VGCCs. Unfortunately, these technical limitations prevented the complete dissection of the biophysical nature and pharmacology properties of the observed Ca2+ channels and the involved PKC isoforms.
In addition, our data suggest that depolarizing stimuli (high K+) promote endothelial Ca2+ entry from control but not CH rats. This finding was somewhat surprising, because there is a lack of consensus that VGCCs exist in the pulmonary endothelium. Similar to the relatively absent KCl-induced Ca2+ influx after CH, we found that receptor-mediated (ATP) Ca2+ influx was also reduced after CH. These parallel observations of absent Ca2+ influx pathways led us to hypothesize that VGCCs are activated by purinoceptor stimulation. Therefore, it is possible that T-type VGCCs represent another mode of ROC entry that is sensitive to PKC activation. Consistent with this hypothesis are multiple findings (Park et al., 2003, 2006; Chemin et al., 2007; Kim et al., 2007) illustrating that PKC activation stimulates Cav3.1 and Cav3.2 Ca2+ currents. Although the specific PKC isoforms modulating Ca2+ influx through T-type VGCCs were not determined in those prior reports, our data suggest that PKCε plays a role in stimulating Ca2+ influx in the pulmonary endothelium. Moreover, the lack of sensitivity to both V1-2myr and mibefradil and the inability of 60 mM KCl to elicit significant changes in Ca2+ influx in cells from CH rats compared with controls suggests that T-type VGCCs may be down-regulated at the expressional level or possibly not appropriately localized on the plasma membrane. Although this study did not examine ion channel trafficking or expression because of protein sample limitations, further experiments are needed to support these speculations.
It is also possible that ATP binds to P2X receptors, leading to membrane depolarization through nonselective cation influx and activation of T-type Ca2+ channels. It is generally accepted that ionotropic P2X receptors are expressed in smooth muscle and contribute to vasoconstriction (Matsuura et al., 2004); however, more recent evidence shows a novel role for P2X activation in endothelium-dependent vasodilation (Harrington et al., 2007). Although there are no known reports, it is possible that CH leads to a decrease in purinergic receptor expression in the pulmonary endothelium. We are unable to completely rule out the possibility that P2X activation leads to membrane depolarization and subsequent Ca2+ influx or whether purinergic receptor expression is down-regulated after CH. PLC inhibition did, however, abolish residual Ca2+ response to ATP in both groups, indicating that P2X receptor activation does not play a role in this response. PKCε inhibition diminished Ca2+ influx similar to T-type channel blockade, suggesting that PKCε and T-type VGCCs are serially activated, assuming maximal PKCε inhibition was achieved. Endothelial cells from CH arteries were also insensitive to either of these antagonists, and the Ca2+ response was strikingly similar to that in control endothelial cells when PKCε and T-type VGCCs were inhibited, indicating that residual DAG-dependent Ca2+ influx pathways may not differ between groups.
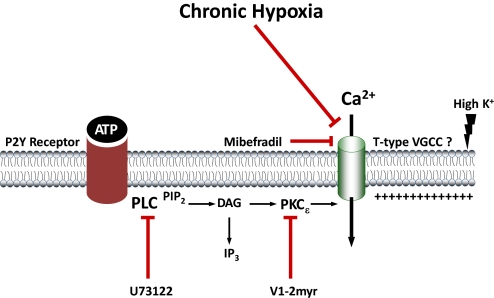
Although the effects of CH on Ca2+ entry have been examined in pulmonary VSM (Jernigan et al., 2006), little is known regarding effects of this stimulus on the endothelium. It is possible that CH decreases endothelial expression of TRP channels to mediate decreased SOC and ROC entry, but our findings suggest that differential PKC regulation of these pathways more likely contributes to the impaired endothelial Ca2+ influx observed in pulmonary hypertension. In conclusion, the present study establishes that there is a generalized decrease in endothelial Ca2+ entry in the pulmonary hypertensive vasculature involving PKC that could significantly impair production of endothelium-derived vasodilators. In addition, we provide evidence of a novel PKC-dependent regulation of agonist-induced Ca2+ entry that may involve a mibefradil-sensitive Ca2+ entry pathway and is impaired after CH (Fig. 8).
Fig. 8.
Diagram depicting the hypothesized effects of CH on purinergic stimulation of a mibefradil-sensitive Ca2+ entry pathway via PKCε or high extracellular K+ in pulmonary endothelium from intrapulmonary arteries. Mibefradil (T-type channel blocker), U73122 (PLC inhibitor), and V1-2myr (PKCε inhibitor) are shown.
Supplementary Material
Acknowledgments
We thank Minerva Murphy for maintaining the hypobaric chambers used in this study.
This work is supported by the American Heart Association [Grant 0615551Z]; and the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL58124, HL63207, HL77876, HL88192].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.165563.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- CH
- chronic hypoxia
- PKC
- protein kinase C
- [Ca2+]i
- intracellular calcium concentration
- EC
- endothelial cell
- SOC
- store-operated Ca2+
- CPA
- cyclopiazonic acid
- OAG
- 1-oleoyl-2-acetyl-sn-glycerol
- ROC
- receptor-operated Ca2+
- VGCC
- voltage-gated Ca2+ channel
- VSM
- vascular smooth muscle
- TRP
- transient receptor potential
- DAG
- diacylglycerol
- HBSS
- HEPES-buffered saline solution
- PECAM-1
- platelet/endothelial cell adhesion molecule-1
- IP3
- inositol trisphosphate
- V1-2myr
- myristoylated V1-2
- GF109203X
- 2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide
- Gö6976
- 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]py rrolo[3,4-c]carbazole-12-propanenitrile
- SKF96365
- 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl]-1H-imidazole hydrochloride
- U73122
- 1-[6-[[(17b)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione
- U73343
- 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-pyrrolidine-2,5-dione.
References
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. (2004) Protein kinase Cα phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem 279:20941–20949 [DOI] [PubMed] [Google Scholar]
- Albert AP, Large WA. (2002) Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J Physiol 544:113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa TK, Mlynarczyk M, Kaushal KM, Zhang L, Ducsay CA. (2004) Long-term hypoxia alters calcium regulation in near-term ovine myometrium. Biol Reprod 71:156–162 [DOI] [PubMed] [Google Scholar]
- Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, Barrère C, Nargeot J, Lory P. (2007) Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem 282:32710–32718 [DOI] [PubMed] [Google Scholar]
- Cheng HW, James AF, Foster RR, Hancox JC, Bates DO. (2006) VEGF activates receptor-operated cation channels in human microvascular endothelial cells. Arterioscler Thromb Vasc Biol 26:1768–1776 [DOI] [PubMed] [Google Scholar]
- De Proost I, Brouns I, Pintelon I, Timmermans JP, Adriaensen D. (2007) Pulmonary expression of voltage-gated calcium channels: special reference to sensory airway receptors. Histochem Cell Biol 128:301–316 [DOI] [PubMed] [Google Scholar]
- Earley S, Straub SV, Brayden JE. (2007) Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292:H2613–H2622 [DOI] [PubMed] [Google Scholar]
- Ewald DA, Matthies HJ, Perney TM, Walker MW, Miller RJ. (1988) The effect of down-regulation of protein kinase C on the inhibitory modulation of dorsal root ganglion neuron Ca2+ currents by neuropeptide Y. J Neurosci 8:2447–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos LL, Newton AC. (2008) Spatiotemporal dynamics of lipid signaling: protein kinase C as a paradigm. IUBMB Life 60:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberucci A, Giurisato E, Pizzo P, Tassi M, Giunti R, McIntosh DP, Benedetti A. (2002) Diacylglycerol activates the influx of extracellular cations in T-lymphocytes independently of intracellular calcium-store depletion and possibly involving endogenous TRP6 gene products. Biochem J 364:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Peretz A, Suss-Toby E, Rom-Glas A, Bishop SA, Selinger Z, Minke B. (1993) Protein kinase C is required for light adaptation in Drosophila photoreceptors. Nature 363:634–637 [DOI] [PubMed] [Google Scholar]
- Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, Ali F, Carrier MJ, Mitchell JA. (2007) Purinergic 2X1 receptors mediate endothelial-dependent vasodilation to ATP. Mol Pharmacol 72:1132–1136 [DOI] [PubMed] [Google Scholar]
- Horibe M, Kondo I, Damron DS, Murray PA. (2001) Propofol attenuates capacitative calcium entry in pulmonary artery smooth muscle cells. Anesthesiology 95:681–688 [DOI] [PubMed] [Google Scholar]
- Jernigan NL, Broughton BR, Walker BR, Resta TC. (2006) Impaired NO-dependent inhibition of store- and receptor-operated calcium entry in pulmonary vascular smooth muscle after chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 290:L517–L525 [DOI] [PubMed] [Google Scholar]
- Kim Y, Park MK, Uhm DY, Chung S. (2007) Modulation of T-type Ca2+ channels by corticotropin-releasing factor through protein kinase C pathway in MN9D dopaminergic cells. Biochem Biophys Res Commun 358:796–801 [DOI] [PubMed] [Google Scholar]
- Lee H, Suh BC, Kim KT. (1997) Feedback regulation of ATP-induced Ca2+ signaling in HL-60 cells is mediated by protein kinase A- and C-mediated changes in capacitative Ca2+ entry. J Biol Chem 272:21831–21838 [DOI] [PubMed] [Google Scholar]
- Leung PC, Cheng KT, Liu C, Cheung WT, Kwan HY, Lau KL, Huang Y, Yao X. (2006) Mechanism of non-capacitative Ca2+ influx in response to bradykinin in vascular endothelial cells. J Vasc Res 43:367–376 [DOI] [PubMed] [Google Scholar]
- Ma R, Kudlacek PE, Sansom SC. (2002) Protein kinase Cα participates in activation of store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol Cell Physiol 283:C1390–C1398 [DOI] [PubMed] [Google Scholar]
- Matsuura M, Saino T, Satoh Y. (2004) Response to ATP is accompanied by a Ca2+ influx via P2X purinoceptors in the coronary arterioles of golden hamsters. Arch Histol Cytol 67:95–105 [DOI] [PubMed] [Google Scholar]
- Montero M, Garcia-Sancho J, Alvarez J. (1993) Inhibition of the calcium store-operated calcium entry pathway by chemotactic peptide and by phorbol ester develops gradually and independently along differentiation of HL60 cells. J Biol Chem 268:26911–26919 [PubMed] [Google Scholar]
- Paffett ML, Naik JS, Resta TC, Walker BR. (2007) Reduced store-operated Ca2+ entry in pulmonary endothelial cells from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 293:L1135–L1142 [DOI] [PubMed] [Google Scholar]
- Park JY, Jeong SW, Perez-Reyes E, Lee JH. (2003) Modulation of Ca(v)3.2 T-type Ca2+ channels by protein kinase C. FEBS Lett 547:37–42 [DOI] [PubMed] [Google Scholar]
- Park JY, Kang HW, Moon HJ, Huh SU, Jeong SW, Soldatov NM, Lee JH. (2006) Activation of protein kinase C augments T-type Ca2+ channel activity without changing channel surface density. J Physiol 577:513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Nilius B. (2007) Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol 428:183–207 [DOI] [PubMed] [Google Scholar]
- Pocock TM, Foster RR, Bates DO. (2004) Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ Physiol 286:H1015–H1026 [DOI] [PubMed] [Google Scholar]
- Rigual R, Cachero MT, Rocher A, González C. (1999) Hypoxia inhibits the synthesis of phosphoinositides in the rabbit carotid body. Pflugers Arch 437:839–845 [DOI] [PubMed] [Google Scholar]
- Schuhmann K, Groschner K. (1994) Protein kinase C mediates dual modulation of L-type Ca2+ channels in human vascular smooth muscle. FEBS Lett 341:208–212 [DOI] [PubMed] [Google Scholar]
- Trebak M, St J, Bird G, McKay RR, Birnbaumer L, Putney JW., Jr (2003) Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem 278:16244–16252 [DOI] [PubMed] [Google Scholar]
- Uenoyama M, Ogata S, Nakanishi K, Kanazawa F, Hiroi S, Tominaga S, Seo A, Matsui T, Kawai T, Suzuki S. (2010) Protein kinase C mRNA and protein expressions in hypobaric hypoxia-induced cardiac hypertrophy in rats. Acta Physiol (Oxf) 198:431–440 [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Zheng F, Gill DL. (2003) Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem 278:29031–29040 [DOI] [PubMed] [Google Scholar]
- Wei Z, Manevich Y, Al-Mehdi AB, Chatterjee S, Fisher AB. (2004) Ca2+ flux through voltage-gated channels with flow cessation in pulmonary microvascular endothelial cells. Microcirculation 11:517–526 [DOI] [PubMed] [Google Scholar]
- Wu S, Haynes J, Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. (2003) Cav3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res 93:346–353 [DOI] [PubMed] [Google Scholar]
- Yang J, Tsien RW. (1993) Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron 10:127–136 [DOI] [PubMed] [Google Scholar]
- Yang M, Ding X, Murray PA. (2008) Differential effects of intravenous anesthetics on capacitative calcium entry in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 294:L1007–L1012 [DOI] [PubMed] [Google Scholar]
- Zhang F, Wen Q, Mergler S, Yang H, Wang Z, Bildin VN, Reinach PS. (2006) PKC isoform-specific enhancement of capacitative calcium entry in human corneal epithelial cells. Invest Ophthalmol Vis Sci 47:3989–4000 [DOI] [PubMed] [Google Scholar]
- Zhou C, Chen H, Lu F, Sellak H, Daigle JA, Alexeyev MF, Xi Y, Ju J, van Mourik JA, Wu S. (2007) Cav3.1 (α1G) controls von Willebrand factor secretion in rat pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 292:L833–L844 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.