Abstract
BACKGROUND
The inadequacies of oocyte in vitro maturation (IVM) systems for both non-human primates and humans are evidenced by reduced fertilization and poor embryonic development, and may be partly explained by significantly lower glutathione (GSH) contents compared with in vivo matured (IVO) oocytes. As this influence has not been fully explored, this study investigated the effect of the GSH donor, glutathione ethyl ester (GSH-OEt), on the IVM and development of macaque oocytes as a model of human oocyte IVM.
METHODS
Macaque oocytes derived from unstimulated ovaries were cultured in mCMRL-1066 alone or supplemented with 3 or 5 mM GSH-OEt. In vitro matured oocytes were subjected to the GSH assay, fixed for the assessment of spindle morphology or prepared ICSI. Embryo development of zygotes cultured in mHECM-9 was assessed up to Day 9 post-ICSI.
RESULTS
Supplementation of the maturation medium with GSH-OEt significantly increased oocyte maturation and normal fertilization rates compared with control oocytes, but only 5 mM GSH-OEt significantly increased the oocyte and cumulus cell GSH content. Confocal microscopy revealed significant differences in the spindle morphology between IVO and control in vitro matured metaphase II oocytes. Oocytes matured with 5 mM GSH-OEt exhibited spindle area and spindle pole width similar to that seen in the IVO oocyte. While no significant differences were observed in blastocyst rates, addition of 3 mM GSH-OEt during IVM significantly increased the proportion of embryos developing to the 5–8 cell stage while 5 mM GSH-OEt significantly increased the proportion of morula-stage embryos compared with controls.
CONCLUSIONS
Supplementation of the IVM medium with GSH-OEt promotes better maturation and normal fertilization of macaque oocytes compared with non-supplemented medium. However, further improvement of the primate oocyte IVM culture system is required to support better blastocyst development of oocytes derived from unstimulated ovaries.
Keywords: male pronucleus formation, oocyte glutathione, primate oocyte maturation, oxidative stress, spindle morphology
Introduction
During mammalian oocyte maturation, the intracellular reduction–oxidation (redox) state is crucial for ensuring viable preimplantation embryo development (Dumollard et al., 2007; Agarwal et al., 2008). Oocyte cellular redox status regulates metabolic and enzyme activity and is defined by a number of parameters including thiol/disulfide ratios and the intracellular level of reactive oxygen species (ROS).
While physiological levels of ROS appear to have a regulatory role in oocyte maturation, abnormal levels of ROS result in oxidative stress (Guerin et al., 2001; Agarwal et al., 2008). The in vitro culture environment exposes the oocyte and embryo to supraphysiological oxygen tensions that generate damaging levels of ROS (Takahashi et al., 2000; Guerin et al., 2001; Kitagawa et al., 2004). In order to regulate the redox balance during maturation, the oocyte employs a number of enzymatic and non-enzymatic antioxidant systems (Cetica et al., 2001; Dumollard et al., 2007; Agarwal et al., 2008). As the oocyte is particularity susceptible to oxidative stress, excessive generation of ROS in culture media has the potential to deplete antioxidant stores and compromise the embryo developmental potential through DNA damage and ROS-mediated apoptotic events (Liu et al., 2000; Guerin et al., 2001).
Glutathione (γ-glutamyl-cysteinyl-glycine; GSH) is the cell's main non-enzymatic defense against oxidative stress (Dickenson and Forman, 2002a) and its synthesis during oocyte maturation is the focus of study in several non-primate mammalian species (de Matos and Furnus, 2000; de Matos et al., 2002; Whitaker and Knight, 2004; Luciano et al., 2006; Furnus et al., 2008). Synthesis of GSH is initiated with the resumption of meiosis and germinal vesicle (GV) breakdown and ceases at metaphase arrest (Perreault et al., 1988; Zuelke et al., 2003). GSH stores attained at the completion of oocyte maturation are essential for male pronucleus formation (MPN) during fertilization and preimplantation embryo development (Abeydeera et al., 1999; de Matos and Furnus, 2000; Maedomari et al., 2007). When depletion of the intracellular GSH content of the oocyte occurs, MPN formation fails (Sutovsky and Schatten, 1997), embryo development is compromised (de Matos and Furnus, 2000; Furnus et al., 2008) and embryonic cellular apoptosis is increased (Pierce et al., 1991; Van Soom et al., 2002).
GSH is also required for the assembly and preservation of spindle and cytoplasmic microtubules (Oliver et al., 1976). Both spindle formation and chromosome segregation are particularly sensitive to the physical and chemical environment (Albertini, 1992; Sanfins et al., 2003) with differences in spindle morphology evident between in vivo (IVO) and in vitro matured oocytes of several non-primate mammalian species (Sanfins et al., 2003; Ueno et al., 2005). The increased oxidative stress and lower oocyte GSH content associated with in vitro culture conditions may therefore influence the spindle morphology and the structural integrity of the in vitro matured oocyte (Tarín et al., 1996; Zuelke et al., 1997; Choi et al., 2007).
In the non-human primate (NHP), development of in vitro matured oocytes is inferior to that of their IVO counterparts (Schramm and Bavister, 1999a). A major limitation of oocyte IVM in both the human and NHP is the reduced rate of normal fertilization, as evidenced by two pronuclei (PN), and an increased frequency of interphase failure (Hewitson et al., 1996; Vanhoutte et al., 2009; Curnow et al., 2010a). The failure of MPN formation at fertilization and reduced embryo development of the macaque in vitro matured oocyte may in part be explained by a significantly lower GSH content compared with the macaque IVO oocyte (Curnow et al., 2010a). Cellular GSH loading approaches that rely on the γ-glutamyl cycle are energy dependent and limited by the regulation of GSH synthesis through feedback inhibition (Richman and Meister, 1975; Deneke and Fanburg, 1989). The cell-permeable GSH donor, glutathione ethyl ester (GSH-OEt), may provide an alternative for GSH loading of primate oocytes during IVM. In bovine animals, GSH-OEt has been used to manipulate oocyte GSH content, spindle morphology and blastocyst cell number (Curnow et al., 2008, 2010b) without an adverse effect on IVM outcome measures.
Although understood to be of importance, the relationship between GSH content of in vitro matured human and NHP oocytes and developmental outcome has not been fully investigated. As species differences are evident in the manipulation of oocyte GSH synthesis (Rodríguez-González et al., 2003; Luciano et al., 2006; Curnow et al., 2010a), studies of in vitro matured oocytes derived from unstimulated NHP ovaries provide a unique opportunity for investigating the acquisition of oocyte developmental competence in a preclinical model for human oocyte IVM.
In this study, IVM of M. nemestrina and M. fascicularis (macaque) oocytes supplemented with GSH-OEt was investigated. Maturation rate, oocyte GSH content and spindle morphology following IVM was assessed. The fertilization outcome following ICSI and embryo development is also described for oocytes matured in the presence of GSH-OEt.
Materials and Methods
Except where otherwise indicated chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA). All animal studies were approved by the University of Washington Institutional Animal Care and Use Committee (3387-01).
Oocyte IVM
Macaque ovaries (M. fascicularis and M. nemestrina; n = 30 pairs) from females aged 7.5–16 years and weighing from 2.5 to 12 kg were obtained from the Washington National Primate Research Center's (WaNPRC) tissue redistribution program and transported to the laboratory at 35°C within 1 h of collection. Each pair of ovaries from an individual female represents an experimental replicate. Cumulus–oocyte complexes (COCs) were retrieved by ovarian dissection and follicle puncture into HEPES-modified human tubal fluid (Irvine Scientific, Santa Ana, CA, USA) supplemented with 0.3% w/v bovine serum albumin (mHTF + BSA) at 37°C. Selected oocytes were cultured in 800 µl of maturation medium in an organ culture dish (BD Falcon, Franklin Lakes, NJ, USA) in a humidified atmosphere of 6% CO2 in air at 37°C. Maturation was performed in modified Connaught Medical Research Laboratories medium (mCMRL-1066; Invitrogen, Carlsbad, CA, USA) supplemented with l-glutamine (1.0 mM), sodium lactate (10 mM), pyruvate (0.2 mM), gentamycin sulfate (50 µg/ml), 1.0 IU of rhFSH (Serono Pty Ltd, Rockland, MA, USA), 1.0 IU of hLH and 0.4% w/v BSA. Following maturation, oocytes were treated with hyaluronidase (40 IU/ml) to remove cumulus cells and classified according to their nuclear maturation status as either immature (GV stage), metaphase I (MI) or metaphase II (MII).
Ovarian stimulation and oocyte retrieval
Adult female M. fascicularis (n = 2) with regular menstrual cycles, aged 6.5–12.0 years and weighing from 3.5 to 4.8 kg, were housed at the WaNPRC. Oocyte donors were subjected to ovarian stimulation as described previously (Curnow et al., 2010a). Oocyte recovery, sperm collection, insemination and embryo culture have been described previously (Curnow et al., 2010a). Follicular aspirates were collected into mHTF + BSA. Harvested oocytes were treated with hyaluronidase (40 IU/ml) to remove cumulus cells 2 h after collection, and MII oocytes were identified and prepared for the GSH assay or immunocytochemistry for spindle assessment.
ICSI
Epididymal spermatozoa, frozen in commercial Test-Yolk Buffer freezing medium (Cat. No. 90 128; Irvine Scientific), were thawed in a 33°C water bath for 1 min. Frozen/thawed epididymal spermatozoa were prepared by centrifugation (500g) on a single 80% Puresperm® density gradient (NidaCom laboratories, Gothenburg, Sweden) for 10 min at room temperature. The sperm were retrieved and re-pelleted by centrifugation at 400g for 5 min in mHTF + BSA. Final sperm preparations were held in mHTF + BSA at room temperature for 30 min prior to 1:4 dilution with 10% (w/v) polyvinylpyrrolidone (Irvine Scientific) and used for ICSI.
Following ICSI, injected oocytes were transferred to individual 20 µl drops of HTF + BSA under mineral oil in a humidified atmosphere of 6% CO2 in air at 37°C. Fertilization was assessed visually at 12–15 h post-injection by the presence of PN and polar bodies (PBs) and recorded as 2PN; 1PN; >2PN (polyspermy) or FF (failed fertilization; no PN, 1 PB).
In vitro embryo culture
Zygotes displaying 2PN following ICSI (Day 1) were transferred to individual 20 µl drops of Hamster Embryo Culture Medium-9 supplemented with 0.4% w/v BSA (HECM-9 + BSA) and 0.5 mM glucose under mineral oil in a humidified atmosphere of 6% CO2, 7% O2, 87% N2 at 37°C. On Day 3 embryos were assessed for cleavage and transferred to 20 µl drops of HECM-9 + BSA supplemented with 3 mM glucose, then cultured up to Day 9 after ICSI. Embryos at the 2–4 cell, 5–8 cell, 9–16 cell, morula and blastocyst stages were recorded as the number of embryos from total cleaved 2PN zygotes.
GSH sample preparation and analysis
Preparation of oocytes for GSH analysis has been described previously (Curnow et al., 2010a). Cumulus cells derived from treated oocytes were washed twice by centrifugation (700g) for 5 min in 0.2 ml of phosphate-buffered saline (PBS) + polyvinyl alcohol (PVA) and subjected to the GSH assay. The supernatant was removed and pellets were resuspended in 50 µl of PBS + PVA. A 10 µl aliquot of cell suspension was used for the cell count and the remainder was prepared for the GSH assay. The total GSH content of oocytes (1–4 oocytes/tube) and cumulus cell suspensions (range; 2–7 × 106 cells/ml) was determined using a commercial 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB)-GSH reductase recycling assay kit (Northwest Life Science, Vancouver, WA, USA). Samples and standards were analyzed at 405 nm with repeated reads at 2 min intervals for 30 min and the rate of change of absorbance was determined by linear regression analysis for each sample and blank. Concentrations of total oocyte GSH (pmol/oocyte) or total cumulus cell GSH (pmol/10 000 cells) were calculated from the standard curve. Inter-assay CV was 8.8% across 21 replicate plates.
Immunocytochemistry
MII oocytes were washed in PBS + PVA (1 mg/ml) at 37°C and fixed in 2% paraformaldehyde containing 0.1% Triton X-100 for 30 min. Fixed oocytes were then washed in PBS + PVA and stored in PBS + BSA at 4°C until analysis. For spindle and chromatin staining, oocytes were first blocked in PBS supplemented with 4% goat serum (GS) for 30 min at room temperature and then incubated in mouse monoclonal anti-α-tubulin (diluted 1:1000 in PBS + 1.5% GS) at 37°C for 1 h. After two washes in PBS + 1.5% GS (15 min each), oocytes were incubated in Alexa Fluor 488-labeled goat anti-mouse secondary antibody (diluted 1:100, Invitrogen) at 37°C for 1 h. Oocytes were then incubated in 5 µg/ml diamidino-2-phenylindole (DAPI; Invitrogen) for 20 min, washed in PBS + 1.5% GS and transferred to 50% glycerol solution in a chambered coverglass slide (NUNC, Rochester, NY, USA) in order to retain the spherical structure and avoid flattening of the oocyte during confocal imaging.
Three-dimensional analysis of MII spindle morphology
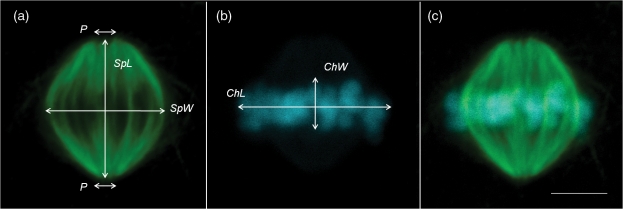
Stained oocytes were examined using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss Inc., Thornwood, NY, USA) equipped with a ×63 water objective and a ×1.2 numerical aperture. The DAPI was excited with a 405 nm laser and emission detected in the range 420–480 nm. The Alexa Fluor 488 was separately excited with a 488 nm laser line and emission detected using a 505 nm long-pass filter. The spindle, labeled with Alexa Fluor 488, and the chromosomes, labeled with DAPI, were identified, and isolated (×6 Zoom), and sequential confocal sections (z-series) at 0.40–0.44 µm intervals were recorded for the entire spindle structure (n = 21–39 sections/oocyte). A three-dimensional image was then rendered using Imaris software (v. 5.7, Bitplane Inc., MN, USA). MII spindles were assessed for morphology and measured as described in Fig. 1 using the criteria described by Roberts et al. (2005). Briefly, spindles were classified as normal if they were barrel shaped with two poles. Spindles were classified as abnormal if they were non-barrel shaped (asymmetric) or had more than two poles (multipolar). Chromosomes were considered normal if they were aligned on the metaphase plate or abnormal if they were displaced or disorganized. Measurements of the spindle dimensions were made using Imaris software. Spindle poles (Pole A and Pole B) were identified and the width of each recorded. Both spindle length (pole-to-pole) and width (at the spindle equator) were measured. Chromosome alignment was assessed by producing isosurfaces in the Imaris software and rotating the three-dimensional image. The width and length of chromosomes aligned on the metaphase plate were then measured using the z-series stacked image.
Figure 1.
Representation of normal metaphase II spindle measurements on a confocal projection of an in vitro matured macaque oocyte; (a) Measurement of spindle dimensions (tubulin; green): SpL, spindle length; SpW, spindle width; P, pole width. (b) Measurement of chromatin dimensions (blue): ChL, chromatin length; ChW, chromatin width. (c) Merged image of spindle and chromatin. Bar = 5µm.
Experimental design
Experiment 1: effect of GSH-OEt supplementation on maturation rate, GSH content, fertilization and embryo development of macaque oocytes in vitro matured in mCMRL-1066 + BSA
Following oocyte isolation, cumulus-enclosed GV oocytes were randomly assigned to the GSH assay or cultured in mCMRL-1066+ BSA (control) or mCMRL-1066+ BSA supplemented with 3 mM or 5 mM GSH-OEt (20–30 replicates). Stock solutions of GSH-OEt (150 mM; Sigma# G-1404) were prepared in water and frozen (−20°C) until required and serial dilutions were added to mCMRL-1066 for final concentrations of 3 and 5 mM. As a negative control, COCs were exposed to 5 mM buthionine sulfoximine (BSO; 15 replicates) in order to block GSH synthesis during IVM. For comparison with the in vitro matured oocyte GSH content, a representative sample of IVO-derived MII oocytes was prepared for the GSH assay.
Following IVM, oocytes were denuded and assessed as GV, MI or MII. MII oocytes were either processed for the GSH assay (5–15 replicates) or prepared for ICSI (4–16 replicates) and assessed for fertilization as described above. Normally fertilized zygotes (2PN) were assessed for cleavage and embryo development up to Day 9 post-ICSI, as described above (9–11 replicates).
To determine the effects of GSH-OEt and BSO on follicular support cells, the GSH of cumulus cells collected from experimental oocytes was processed and prepared for the GSH assay as described above (4 replicates).
Experiment 2: effect of GSH content on MII oocyte spindle morphology
Given the known differences between the IVO and IVM oocyte spindle structure in non-primate mammalian species, we tested the effect of GSH-OEt supplementation on M. fascicularis in vitro matured oocyte MII spindle morphology using confocal microscopy.
COCs were matured in the presence of 5 mM GSH-OEt, 5 mM BSO or under control conditions (mCMRL + BSA). Following maturation, oocytes were denuded and MII oocytes were fixed for spindle analysis as described above (three replicates). A representative sample of in vitro matured MII oocytes (two replicates) was collected from M. fascicularis females subjected to gonadotrophin stimulation of their ovaries to enable a comparison of the IVO and in vitro matured spindle morphology. M. nemestrina in vitro matured oocytes were not included in the spindle analysis as IVO M. nemestrina oocytes were not available as a comparator group.
Statistical analysis
Numbers of MII oocytes, fertilization outcome and embryo development in all treatment groups were compared using the χ2 or Fisher exact test for proportional analysis. GSH content and spindle measurement outliers were excluded by applying Chauvenet's criteria (mean ± 1.645 × SD) before comparison by analysis of variance (ANOVA) with post-hoc Tukey test where appropriate. Oocyte and replicate numbers in tables represent the final data set after application of the exclusion of outliers as indicated above. Non-proportional data are expressed as mean ± SEM. Significant differences were determined at P < 0.05.
Results
Experiment 1: effect of GSH-OEt supplementation on maturation rate, GSH content, fertilization outcome and embryo development of macaque oocytes in vitro matured in mCMRL-1066 + BSA
Supplementation of the maturation medium with 3 or 5 mM GSH-OEt significantly increased the maturation rate compared with control oocytes (Table I). There was no significant difference in the maturation rate between BSO-treated and control oocytes (Table I).
Table I.
Maturation rate and GSH content of macaque oocytes derived from unstimulated ovaries and in vitro matured in mCMRL-1066 + BSA in the absence or presence of GSH-OEt or 5 mM BSO.
| Treatment | Maturation rate n (%) | GSH content pmol/oocyte (n) |
|---|---|---|
| mCMRL-1066 | 174/548 (31.8)a | 4.78 ± 0.24 (43)a |
| GSH-OEt 3 mM | 149/363 (41.0)b | 4.52 ± 0.08 (26)a |
| GSH-OEt 5 mM | 220/566 (38.9)b | 5.56 ± 0.22 (57)b,# |
| BSO | 62/218 (28.4)a | 1.04 ± 0.13 (15)c,# |
Different letters within columns indicate significant differences (P < 0.05). #, significant difference compared with GSH content of immature GV oocytes obtained at collection (4.37 ± 0.12, n = 22 oocytes). Maturation data from 15–30 replicate experiments are expressed as a proportion of total oocytes. GSH data from 4–11 replicate experiments are expressed as mean ± SEM.
Supplementation of the maturation medium with 5 mM GSH-OEt significantly increased the GSH content of the in vitro matured MII oocyte compared with control, 3 mM GSH-OEt and BSO groups (Table I). Maturation of GV oocytes in the presence of 5 mM BSO significantly lowered the GSH content of the in vitro matured MII oocyte compared with all other treatment groups (Table I). The GSH content of IVO-derived MII oocytes was significantly greater than the GSH content of in vitro matured MII oocytes irrespective of in vitro matured treatment (7.52 ± 0.34 versus 1.04 ± 0.13 to 5.56 ± 0.22, n = 15–57, P < 0.0001).
Oocytes matured in the presence of 3 or 5 mM GSH-OEt exhibited a significantly greater proportion of 2PN zygotes (Table II) and a higher overall oocyte activation rate compared with control in vitro matured MII oocytes (63/80 (78.8%) and 82/105 (78.1%) versus 63/99 (63.6%), respectively; χ2 = 7.16, df = 2, P = 0.03). Oocytes matured in the presence of BSO had a significantly lower proportion of 2PN zygotes and a higher rate of FF compared with the IVM control group (Table II).
Table II.
Fertilization outcome of macaque oocytes derived from unstimulated ovaries and in vitro matured in mCMRL-1066 + BSA in the absence or presence of GSH-OEt or 5 mM BSO.
| Treatment | Fertilization outcome n (%) |
||||
|---|---|---|---|---|---|
| Total oocytes injected | 2PN | 1PN | >2PN | FF | |
| mCMRL-1066 | 99 | 48 (48.5)a | 14 (14.1)bc | 1 (1.0)e | 36 (36.4)a |
| GSH-OEt 3 mM | 80 | 55 (68.8)b | 6 (7.5)c | 2 (2.5)e | 17 (21.3)f |
| GSH-OEt 5 mM | 105 | 71 (67.6)b | 8 (7.6)c | 3 (2.9)ce | 23 (21.9)af |
| BSO | 26 | 2 (7.7)c | 7 (26.9)b | 0 (0)e | 17 (65.4)d |
Different letters within rows and within columns indicate significant differences (P < 0.05). 2PN, 2 pronuclei, ≥1 polar bodies; 1PN, 1 pronuclei, ≥1 polar bodies; >2PN, >2 pronuclei, ≥1 polar body; FF, failed fertilization, no PN, 1 polar body. Fertilization data from 4–16 replicate experiments are expressed as a proportion (percentage) of the total number of ICSI oocytes.
Following assessment of the fertilization outcome, 2PN zygotes from each treatment group were cultured further and cleavage and embryo development were assessed. Embryo development data for the BSO treatment group are not reported as insufficient numbers of 2PN zygotes were obtained following ICSI (n = 2). Although no significant difference in the overall cleavage rate was observed across treatment groups, significantly more zygotes reached the 5–8 cell stage in the 3-mM GSH-OEt group compared with the mCMRL-1066 IVM control (Table III). A significantly greater proportion of zygotes reached the morula stage in the 5-mM GSH-OEt group compared with the mCMRL-1066 IVM control group. Blastocyst development was not significantly different between treatment groups (Table III).
Table III.
Embryo development of macaque oocytes derived from unstimulated ovaries and in vitro matured in mCMRL-1066 + BSA in the absence or presence of GSH-OEt.
| Treatment | Total cleaved 2PN/Total 2PN n (%) | Total number of cleaved 2PN zygotes progressing to or beyond each developmental stage n (%) |
||||
|---|---|---|---|---|---|---|
| 2–4 cells | 5–8 cells | 9–16 cells | Morula | Blastocyst | ||
| mCMRL-1066 | 33/41 (80.5) | 33 (100.0) | 25 (75.8)a | 17 (51.5) | 3 (9.1)a | 2 (6.1) |
| GSH-OEt 3 mM | 27/31 (87.1) | 27 (100.0) | 26 (96.3)b | 15 (55.6) | 4 (14.8)ab | 0 (0.0) |
| GSH-OEt 5 mM | 40/52 (76.9) | 40 (100.0) | 36 (90.0)ab | 27 (67.5) | 12 (30.0)b | 0 (0.0) |
Different letters within columns are significantly different (P < 0.05). Embryo development data are from 9–11 replicate experiments.
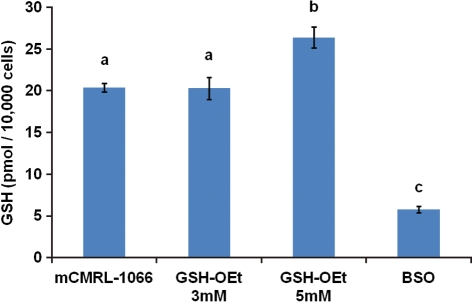
The GSH content of cumulus cells derived from cumulus-enclosed in vitro matured oocytes exposed to 5 mM GSH-OEt was significantly higher compared with that in cumulus cells from all other treatment groups (Fig. 2). Cumulus cells derived from in vitro matured oocytes matured in the presence of 5 mM BSO had a significantly lower GSH content compared with cumulus cells from all other treatment groups (Fig. 2).
Figure 2.
GSH content of cumulus cells collected from in vitro matured macaque oocytes. Bars with different letters are significantly different (P < 0.05). GSH data are expressed as mean ± SEM from 3–4 replicate experiments. GSH-Oet, mCMRL-1066 medium supplemented with GSH-OEt at the indicated concentration; BSO, mCMRL-1066 medium supplemented with BSO (5 mM).
Experiment 2: effect of oocyte GSH content on MII oocyte spindle morphology
Assessment of IVO and in vitro matured oocytes revealed no significant difference in the proportion of oocytes from each treatment group displaying both normal spindle and chromatin morphology (Table IV). The proportion of oocytes at anaphase/telophase was also not significantly different between the IVO and IVM treatment groups (range: 1/20 (5.0%) to 4/18 (22.2%); n = 11–20 oocytes; χ2 = 2.72, df = 3, P = 0.44). As these oocytes had incomplete PB extrusion and metaphase spindle formation, they were excluded from further data analysis. There was no significant difference between the IVO and IVM treatment groups for combined spindle and chromosome abnormalities (range 2/9 (22.2%) to 5/14 (35.7%); n = 9–18 oocytes; χ2 = 0.59, df = 3, P = 0.90). While there were no significant differences in the proportion of abnormal oocytes with displaced chromosome alignment (Table IV and Fig. 3a), the incidence of tripolar spindles (Fig. 3b) among abnormal oocytes was significantly higher in in vitro matured oocytes in the presence of 5 mM GSH-OEt compared with control in vitro matured and IVO oocytes (Table IV).
Table IV.
Comparison of MII spindle and chromosome morphology of in vitro and in vivo matured Macaca fascicularis oocytes.
| Treatment | Normal* n (%) | Abnormal n (%) |
||
|---|---|---|---|---|
| Spindle |
Chromosome | |||
| Asymmetric | Tripolar | |||
| mCMRL-1066 | 10/15 (66.7) | 2 (13.3) | 0 (0.0)a | 3 (20.0) |
| GSH-OEt | 9/14 (64.3) | 0 (0.0) | 4 (28.6)b | 1 (7.1) |
| BSO | 7/9 (77.8) | 0 (0.0) | 2 (22.2)ab | 0 (0.0) |
| IVO | 13/18 (72.2) | 3 (16.7) | 0 (0.0)a | 2 (11.1) |
*Symmetrical bipolar barrel-shaped spindle with centrally aligned chromosomes. Different letters within columns are significantly different (P < 0.05). Data are expressed as a proportion (percentage) of assessed oocytes from 2–3 replicate experiments. GSH-OEt, mCMRL-1066 medium supplemented with glutathione ethyl ester (5 mM); BSO, mCMRL-1066 medium supplemented with buthionine sulfoximine (5 mM).
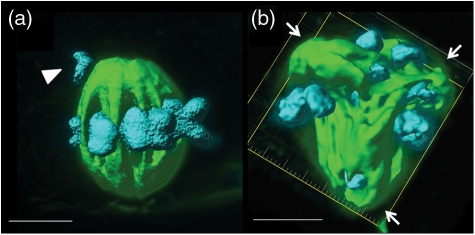
Figure 3.
Three-dimensional isosurfaces produced from the confocal z-series of abnormal MII spindles in in vitro matured macaque oocytes. Spindle tubulin (green) and chromatin (blue) (a) Abnormal chromatin, displaced chromosome(s) indicated by an arrowhead (▸); (b) Tripolar spindle, spindle poles indicated by arrows (→). Bar = 5 µm.
Measurement of normal MII spindles revealed no significant difference in the spindle width between in vitro matured and IVO oocytes (Table V). However, the spindle length of the in vitro matured MII oocytes from each of the treatment groups was significantly shorter than the spindles observed in IVO MII oocytes (Table V and Fig. 4a–d). The average spindle pole width in oocytes matured in 5 mM GSH-OEt was significantly less compared with both control and BSO-treated MII oocytes and equivalent to the IVO MII oocyte (Table V). GSH-OEt tended to decrease the spindle area in the in vitro matured oocyte, while in vitro matured MII oocytes exposed to 5 mM BSO had a significantly greater spindle area compared with IVO- and IVM-derived MII oocytes treated with 5 mM GSH-OEt (Table V). Three-dimensional isosurface imaging allowed for assessment of chromatin and indicated that there were no significant changes in the chromatin length or width with IVM treatment and that the chromatin dimensions were comparable to IVO-derived MII oocytes.
Table V.
Dimensions of bipolar MII spindles of in vitro and in vivo matured Macaca fascicularis oocytes.
| Treatment | Spindle |
Pole | Chromatin |
|||
|---|---|---|---|---|---|---|
| Length (μm) | Width (μm) | Area (μm2) | Width (μm) | Length (μm) | Width (μm) | |
| mCMRL-1066 | 10.80 ± 0.17a | 11.71 ± 0.09 | 21.18 ± 0.31ab | 3.65 ± 0.20a | 13.07 ± 0.21 | 4.62 ± 0.04 |
| GSH-OEt | 10.20 ± 0.27a | 11.07 ± 0.58 | 18.85 ± 0.64b | 2.51 ± 0.13b | 12.39 ± 0.54 | 4.12 ± 0.11 |
| BSO | 10.39 ± 0.07a | 11.78 ± 0.60 | 21.84 ± 0.89a | 3.39 ± 0.08a | 13.63 ± 0.18 | 4.33 ± 0.26 |
| IVO | 12.51 ± 0.15b | 11.10 ± 0.16 | 19.68 ± 0.27b | 2.71 ± 0.12b | 13.27 ± 0.22 | 4.15 ± 0.17 |
Different letters within columns are significantly different (P < 0.05). Pole width data represent the average of pooled pole width for Pole A and B. Spindle and chromatin data are expressed as mean ± SEM for 7–13 oocytes from 2–3 replicate experiments. GSH-OEt, mCMRL-1066 medium supplemented with glutathione ethyl ester (5 mM); BSO, mCMRL-1066 medium supplemented with buthionine sulfoximine (5 mM).
Figure 4.
Three-dimensional isosurfaces produced from the confocal z-series of normal MII spindles of in vitro and in vivo matured macaque oocytes. Spindle tubulin (green) and chromatin (blue) (a) in vitro matured control; (b) GSH-OEt-treated (5 mM); (c) BSO-treated (5 mM); (d) in vivo matured. Bar = 5 µm.
Discussion
Under control IVM conditions, the GSH content of the MII oocyte did not differ from that of the immature oocyte at collection, suggesting that during IVM of macaque oocytes GSH synthesis and/or accumulation is perturbed. Supplementation of the IVM medium with 5 mM GSH-OEt significantly increased the MII oocyte GSH content, and the addition of GSH-OEt at both 3 and 5 mM significantly increased the MII rate independent of the oocyte GSH level. As a decrease in the oocyte GSH content following BSO treatment did not significantly alter the maturation rate compared with the control group, it is unlikely that the oocyte GSH content per se influences meiotic maturation. Instead, the positive influence of GSH-OEt on oocyte maturation may be related to the method of GSH loading. In several species, including the macaque, traditional GSH loading techniques, which have relied on improving the availability of substrates, such as cysteine and cystine, for de novo synthesis of GSH via the γ-glutamyl cycle, do not effect the maturation rate but do increase the oocyte GSH content (Grupen et al., 1995; Bing et al., 2002; Urdaneta et al., 2004; Gasparrini et al., 2006; Curnow et al., 2010a). Several processes involved in oocyte maturation are ATP dependent, including de novo GSH synthesis (Dickenson and Forman, 2002b). As a reduction in oocyte ATP is associated with reduced MII rates (Hashimoto et al., 2000), provision of GSH to the maturing oocyte independent of both the γ-glutamyl cycle and ATP may indirectly benefit other metabolic processes involved in meiotic maturation. It would be interesting to determine if and to what extent de novo oocyte GSH synthesis is affected by exogenous GSH-OEt exposure.
IVM of macaque oocytes in the presence of GSH-OEt significantly increased the overall oocyte activation and normal fertilization rates (2PN) to levels previously observed in macaque IVO oocytes (Curnow et al., 2010a). Consistent with previous studies in non-primate species, a reduction of GSH synthesis by BSO during macaque oocyte IVM produced a significant reduction in the incidence of 2PN formation following insemination. GSH has been identified in a number of species as a critical component of fertilization and supports both sperm head decondensation, through disulfide bond reduction in the sperm nucleus, and pronuclear apposition, while depletion of oocyte GSH results in the failure of MPN (Perreault et al., 1988; Sutovsky and Schatten, 1997). In the 5-mM GSH-OEt group, increased 2PN formation in the fertilized oocyte may be attributed to the increase in the oocyte GSH content. However, a similar effect on the fertilization outcome was observed in the 3 mM GSH-OEt group, which did not significantly increase the oocyte GSH content relative to control oocytes. In the pig, GSH-OEt infusion into the heart significantly increases the ratio of reduced to oxidized GSH (GSH:GSSG) compared with controls (Guarnieri et al., 1993). In the present study, 3 mM GSH-OEt may have led to a similar shift in the GSH:GSSG ratio toward a higher ratio of intracellular reduced GSH sufficient to support higher rates of 2PN fertilization. Alternatively, under control IVM conditions the impact of oxidative stress on the oocyte may have caused a significant elevation in the GSSG content through increased hydrogen peroxide production and GSH peroxidase activity (Dickenson and Forman, 2002b), thereby limiting GSH availability during fertilization. The GSH assay used in this study measured the total GSH (GSH and GSSG) and did not enable the determination of GSH:GSSG ratios. Although it is unclear what is the specific effect of 3 mM GSH-OEt on the oocyte GSH:GSSG ratio, the results do suggest that the maturation rate alone may be a less sensitive biomarker of the GSH content compared with the fertilization outcome and that our understanding of primate oocyte GSH requirements and utilization during oocyte maturation is incomplete.
The GSH profile observed in the mature oocyte was similar to that observed in the cumulus cells retrieved from oocytes in each treatment group following IVM. Previous studies in the cow and pig have shown a positive correlation between cumulus cell and oocyte GSH content (Furnus et al., 1998; Ozawa et al., 2010). However, it has yet to be determined if the observed positive effect of an intact cumulus vestment on the oocyte GSH content (Luciano et al., 2005; Maedomari et al., 2007; Zhou et al., 2010) is mediated through (i) indirect promotion of cysteine uptake and/or (ii) the direct transfer of GSH or small-molecule signals for GSH synthesis from the cumulus cell to the oocyte via gap junction communication (Mori et al., 2000; Ozawa et al., 2010). Further study is required to ascertain the importance of cumulus cell GSH content and its relationship to primate oocyte nuclear and cytoplasmic maturation, but it may provide a non-invasive means by which to assess the oocyte GSH content in a clinical setting.
The developmental potential of in vitro matured NHP oocytes from unstimulated ovaries is considerably lower compared with their IVO-derived counterparts (Schramm and Bavister, 1999a). The developmental failure of NHP in vitro matured oocytes tends to occur at the 5–8 cell stage coinciding with the transition from maternal to embryonic genome activation (Schramm and Bavister, 1999b; Schramm et al., 2003). In several non-primate species, increased oocyte GSH content following IVM has been associated with improved embryo development (de Matos et al., 2002; Gasparrini et al., 2003; Kitagawa et al., 2004; Furnus et al., 2008; Zhou et al., 2008). While the addition of GSH-OEt improved embryo development to the 5–8 cell stage and supported higher rates of morula formation at concentrations of 3 and 5 mM further development of the morula to the blastocyst stage was not improved compared with control in vitro matured oocytes. It is possible that the switch from maternal to embryonic transcription in the in vitro matured oocyte was either delayed or impaired (Tesařík, 1989). However, in this study the in vitro matured oocyte GSH content did not reach levels observed in macaque IVO oocytes (Curnow et al., 2010a) and it remains to be determined if an improvement in blastocyst development may be achieved with promotion of higher intracellular GSH in the in vitro matured oocyte.
Confocal imaging of the metaphase spindle identified several differences between IVO- and IVM-derived macaque oocytes. In this study, the IVO oocyte displayed a similar spindle length (11–13 µm) to that observed in the human IVO oocyte (11–14 µm; Wang et al., 2001; Coticchio et al., 2010), while the average spindle lengths for in vitro matured oocytes from each of the treatment groups were significantly shorter than spindles in the IVO oocyte. In addition to the spindle length, differences in the spindle area and the pole width were also observed. While GSH depletion with BSO tended to increase the spindle pole width, GSH loading with GSH-OEt decreased both the spindle area and the pole width compared with control in vitro matured oocytes resulting in a spindle morphology similar to that observed in the IVO oocyte. In Drosophila S2 cells and mouse oocytes, spindle formation is particularly sensitive to oxidative stress-mediated shortening (Tarín et al., 1996; Goshima et al., 2005; Zhang et al., 2006), while an increased pole width has been associated with altered γ-tubulin accumulation and the recruitment of multiple microtubule-organizing centers in the in vitro matured mouse oocyte (Sanfins et al., 2003). These results suggest that further optimization of the culture environment with respect to redox balance may be necessary to ensure spindle integrity of the in vitro matured NHP oocyte.
In the human oocyte, abnormal spindle and chromosome alignment is often associated with maternal age or the underlying cause of infertility (Battaglia et al., 1996; Li et al., 2006), making it difficult to ascertain the specific effects of clinical oocyte IVM on spindle formation. In the present study, there were no differences in the combined rate of spindle and chromosome abnormalities in IVO or in vitro matured oocytes, with <36% of MII oocytes exhibiting abnormal morphology. The observed rates were similar to those reported previously for the M. mulatta (Nichols et al., 2010). However, treatment-related effects on the type of abnormal spindle morphology were observed. Abnormal oocytes from the 5-mM GSH-OEt group had higher rates of tripolar spindle formation compared with abnormal control IVM and IVO oocytes. Given that GSH-OEt did not raise the oocyte GSH content to the levels observed in macaque IVO oocytes, it is unlikely that tripolar spindle formation was directly related to the GSH content and the resultant increase in reducing power.
GSH-OEt undergoes intracellular de-esterification and conversion to GSH and ethanol (Anderson et al., 1985; Levy et al., 1993). Although intra-oocyte ethanol concentrations were not measured in this study, the detrimental effects of ethanol on spindle morphology have been described in the mouse. Short-term exposure of mouse oocytes to ethanol (7%) during parthenogenic activation is associated with the formation of multipolar spindles (O'Neill et al., 1989), possibly through altered regulation of calcium concentration (Ohnishi et al., 1984) and changes to actin filament dynamics (Schliwa, 1981; Allansson et al., 2001). Alternatively, modulation of the redox state of oocytes with GSH-OEt may have altered the dynamics of tubulin polymerization and aster formation. GSH is involved in the maintenance of tubulin sulfhydryl groups in their reduced form, which is crucial for tubulin polymerization and spindle formation (Landino et al., 2004). The possibility that GSH-OEt induced the formation of multiple tubulin foci exists but, as the proportion of oocytes with abnormal spindles was not higher following GSH-OEt treatment compared with control oocytes, the data suggest that this effect was confined to oocytes that were perhaps already compromised.
While not significantly different from in vitro matured and IVO control oocytes, a similar increase in oocytes with tripolar spindles was observed in BSO-treated oocytes, possibly as a result of disrupted microtubule function and subsequent polymerization of microtubules at multiple focal points following GSH depletion (Zuelke et al., 1997). In a bovine oocyte IVM model, tripolar spindle formation was not significantly increased in GSH-OEt- or BSO-treated oocytes (EC Curnow, unpublished data), suggesting that the spindle apparatus of the primate oocyte may be more sensitive to elevated cellular ethanol levels and/or changes in the cellular redox status compared with bovine oocytes.
The results of this study suggest that the reduced rate of normal fertilization and poor embryo development observed for in vitro matured macaque oocytes derived from unstimulated ovaries may in part be related to inadequate levels of oocyte GSH. In this study, the maturation and fertilization outcome of macaque oocytes were improved following supplementation of GSH-OEt to the IVM medium. Although GSH-OEt also improved morula development, it was unable to improve blastocyst development relative to controls. Taken together the results of this study indicate that exogenous GSH supplementation with GSH-OEt is beneficial to the primate oocyte and that further studies will be required to ascertain if the benefits of GSH-OEt supplementation may be useful for clinical application of human oocyte IVM.
Authors' roles
E.C.C. and E.S.H. contributed to the study design, acquisition and analysis of data and manuscript preparation and review; J.P.R. and D.M.S. contributed to the study design and the manuscript review.
Funding
This work was supported by the National Institutes of Health (P51 grant RR00166).
Acknowledgements
The authors would like to thank the WaNPRC tissue distribution program for their assistance with the collection of reproductive tissues, C. Astley and J. Aherns for surgery support and C. Ferrier for animal care.
References
- Abeydeera LR, Wang W-H, Cantley TC, Prather RS, Day BN. Glutathione content and embryo development after in vitro fertilization of pig oocytes matured in the presence of a thiol compound and various concentrations of cysteine. Zygote. 1999;7:203–210. doi: 10.1017/s0967199499000581. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- Albertini DF. Cytoplasmic microtubular dynamics and chromatin organization during mammalian oogenesis and oocyte maturation. Mutat Res. 1992;296:57–68. doi: 10.1016/0165-1110(92)90032-5. [DOI] [PubMed] [Google Scholar]
- Allansson L, Khatibi S, Olsson T, Hansson E. Acute ethanol exposure induces [Ca2+]i transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. J Neurochem. 2001;76:472–479. doi: 10.1046/j.1471-4159.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Powrie F, Puri RN, Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985;239:538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Klein NA, Soules MR. Changes in centrosomal domains during meiotic maturation in the human oocyte. Mol Hum Reprod. 1996;2:845–851. doi: 10.1093/molehr/2.11.845. doi:10.1093/molehr/2.11.845. [DOI] [PubMed] [Google Scholar]
- Bing YZ, Hirao Y, Iga K, Che LM, Takenouchi N, Kuwayama M, Fuchimoto D, Rodriguez-Martinez H, Nagai T. In vitro maturation and glutathione synthesis of porcine oocytes in the presence or absence of cysteamine under different oxygen tensions: role of cumulus cells. Reprod Fertil Dev. 2002;14:125–131. doi: 10.1071/rd01127. [DOI] [PubMed] [Google Scholar]
- Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51:57–64. doi: 10.1080/15216540119253. [DOI] [PubMed] [Google Scholar]
- Choi WJ, Banerjee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factor-alpha-induced alterations in metaphase II mouse oocyte spindle structure. Fertil Steril. 2007;88:1220–1231. doi: 10.1016/j.fertnstert.2007.02.067. [DOI] [PubMed] [Google Scholar]
- Coticchio G, Sciajno R, Hutt K, Bromfield J, Borini A, Albertini DF. Comparative analysis of the metaphse II spindle of human oocytes through polarized light and high-performance confocal microscopy. Fertil Steril. 2010;93:2056–2064. doi: 10.1016/j.fertnstert.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Curnow EC, Ryan J, Saunders D, Hayes ES. Bovine in vitro oocytes maturation as a model for manipulation of the γ-glutamyl cycle and intraoocyte glutathione. Reprod Fertil Dev. 2008;20:579–588. doi: 10.1071/rd08041. [DOI] [PubMed] [Google Scholar]
- Curnow EC, Ryan JR, Saunders DM, Hayes ES. Oocyte glutathione and fertilization outcome of Macaca nemestrina and Macaca fascicularis in vivo and in vitro matured oocytes. Reprod Fertil Dev. 2010a;22:1032–1040. doi: 10.1071/RD09308. doi:10.1071/RD09308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnow EC, Ryan JR, Saunders DM, Hayes ES. Developmental potential of bovine oocytes following in vitro maturation in the presence of glutathione ethyl ester. Reprod Fertil Dev. 2010b;22:597–605. doi: 10.1071/RD09228. doi:10.1071/RD09228. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Furnus CC. The importance of having high glutathione (GSH) levels after bovine in vitro maturation on embryo development: effect of β-mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Gasparrini B, Pasqualini SR, Thompson JG. Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology. 2002;57:811–821. doi: 10.1016/s0093-691x(02)00643-x. [DOI] [PubMed] [Google Scholar]
- Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- Dickenson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002a;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Dickenson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002b;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Ward Z, Carroll J, Duchen MR. Regulation of redox metabolism in the mouse oocyte and embryo. Development. 2007;134:455–465. doi: 10.1242/dev.02744. [DOI] [PubMed] [Google Scholar]
- Furnus CC, de Matos DG, Moses DF. Cumulus expansion during in vitro maturation of bovine oocytes: relationship with intracellular glutathione level and its role on subsequent embryo development. Mol Reprod Dev. 1998;51:76–83. doi: 10.1002/(SICI)1098-2795(199809)51:1<76::AID-MRD9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Furnus CC, de Matos DG, Picco S, Peral García P, Inda AM, Mattioli G, Errecalde AL. Metabolic requirements associated with GSH synthesis during in vitro maturation of cattle oocytes. Anim Reprod Sci. 2008;109:88–99. doi: 10.1016/j.anireprosci.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Gasparrini B, Sayoud H, Neglia G, Matos DG, Donnay I, Zicarelli L. Glutathione synthesis during in vitro maturation of buffalo (Bubalus bubalis) oocytes: effects of cysteamine on embryo development. Theriogenology. 2003;60:943–952. doi: 10.1016/s0093-691x(03)00098-0. doi:10.1016/S0093-691X(03)00098-0. [DOI] [PubMed] [Google Scholar]
- Gasparrini B, Boccia L, Marchandise J, Di Palo R, George F, Donnay I, Zicarelli L. Enrichment of in vitro maturation medium for buffalo (Bubalus bubalis) oocytes with thiol compounds: effects of cystine on glutathione synthesis and embryo development. Theriogenology. 2006;65:275–287. doi: 10.1016/j.theriogenology.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Goshima G, Wollman R, Stuurman N, Scholey JM, Vale RD. Length control of the metaphase spindle. Curr Biol. 2005;15:1979–1988. doi: 10.1016/j.cub.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Grupen CG, Nagashima H, Nottle MB. Cysteamine enhances in vitro development of porcine oocytes matured and fertilized in vitro. Biol Reprod. 1995;53:173–178. doi: 10.1095/biolreprod53.1.173. [DOI] [PubMed] [Google Scholar]
- Guarnieri C, Turinetto B, Colí G, Muscari C, Cattabriga I, Vaona I, Finelli C, Pigini F, Caldarera CM. Effect of glutathione monoethyl ester on glutathione level and cardiac energetic in reperfused pig heart. Res Commun Chem Pathol Pharmacol. 1993;81:33–44. [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Minami N, Takakura R, Yamada M, Imai H, Kashima N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Mol Reprod Dev. 2000;57:353–360. doi: 10.1002/1098-2795(200012)57:4<353::AID-MRD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hewitson LC, Simerly CR, Tengowski MW, Sutovsky P, Navara CS, Haavisto AJ, Schatten G. Microtubule and chromatin configurations during rhesus intracytoplasmic sperm injection: successes and failures. Biol Reprod. 1996;55:271–280. doi: 10.1095/biolreprod55.2.271. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62:1186–1197. doi: 10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Landino LM, Moynihan KL, Todd JV, Kennett KL. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun. 2004;314:555–560. doi: 10.1016/j.bbrc.2003.12.126. [DOI] [PubMed] [Google Scholar]
- Levy EJ, Anderson ME, Meister A. Transport of glutathione diethyl ester into human cells. Proc Natl Acad Sci USA. 1993;90:9171–9175. doi: 10.1073/pnas.90.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, Critser JK, Chen ZJ. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85:827–832. doi: 10.1016/j.fertnstert.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod. 2000;62:1745–1753. doi: 10.1095/biolreprod62.6.1745. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Lodde V, Beretta MS, Colleoni S, Lauria A, Modina S. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3′,5′-monophosphate, and glutathione. Mol Reprod Dev. 2005;71:389–397. doi: 10.1002/mrd.20304. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Goudet G, Perazzoli F, Lahuec C, Gerard N. Glutathione content and glutathione peroxidase expression in in vivo and in vitro matured equine oocytes. Mol Reprod Dev. 2006;73:658–666. doi: 10.1002/mrd.20469. [DOI] [PubMed] [Google Scholar]
- Maedomari N, Kikuchi K, Ozawa M, Noguchi M, Kaneko H, Ohnuma K, Nakai M, Shino M, Nagai T, Kashiwazaki N. Cytoplasmic glutathione regulated by cumulus cells during porcine oocytes maturation affects fertilization and embryonic development in vitro. Theriogenology. 2007;67:983–993. doi: 10.1016/j.theriogenology.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Mori T, Amano T, Shimizu H. Roles of gap junctional communication of cumulus cells in cytoplasmic maturation of porcine oocytes cultured in vitro. Biol Reprod. 2000;62:913–919. doi: 10.1095/biolreprod62.4.913. [DOI] [PubMed] [Google Scholar]
- Nichols SM, Gierbolini L, Gonzalez-Martinez JA, Bavister BD. Effects of in vitro maturation and age on oocyte quality in the rhesus macaque Macaca mulatta. Fertil Steril. 2010;93:1591–1600. doi: 10.1016/j.fertnstert.2008.12.141. doi:10.1016/j.fertnstert.2008.12.141. [DOI] [PubMed] [Google Scholar]
- Ohnishi ST, Flick JL, Rubin E. Ethanol increases calcium permeability of heavy sarcoplasmic reticulum of skeletal muscle. Arch Biochem Biophys. 1984;233:588–594. doi: 10.1016/0003-9861(84)90483-1. [DOI] [PubMed] [Google Scholar]
- Oliver JM, Albertini DF, Berlin RD. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976;71:921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill GT, McDougall RD, Kaufman MH. Ultrastructural analysis of abnormalities in the morphology of the second meiotic spindle in ethanol-induced parthenogenones. Gamete Res. 1989;22:285–299. doi: 10.1002/mrd.1120220306. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Nagai T, Somfai TS, Nakai M, Maedomari N, Miyazaki H, Kaneko H, Noguchi J, Kikuchi K. Cumulus cell-enclosed oocytes acquire a capacity to synthesize GSH by FSH stimulation during in vitro maturation in pigs. J Cell Physiol. 2010;222:294–301. doi: 10.1002/jcp.21949. [DOI] [PubMed] [Google Scholar]
- Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol. 1988;125:181–186. doi: 10.1016/0012-1606(88)90070-x. [DOI] [PubMed] [Google Scholar]
- Pierce GB, Parchment RE, Lewellyn AL. Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation. 1991;46:181–186. doi: 10.1111/j.1432-0436.1991.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Richman PG, Meister A. Regulation of γ-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker D, Franks S, Hardy K. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod. 2005;72:107–118. doi: 10.1095/biolreprod.104.032003. [DOI] [PubMed] [Google Scholar]
- Rodríguez-González E, López-Bejar M, Mertens M-J, Paramio M-T. Effects on in vitro embryo development and intracellular glutathione content of the presence of thiol compounds during maturation of prepubertal goat oocytes. Mol Reprod Dev. 2003;65:446–453. doi: 10.1002/mrd.10316. [DOI] [PubMed] [Google Scholar]
- Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes. Biol Reprod. 2003;69:2059–2067. doi: 10.1095/biolreprod.103.020537. [DOI] [PubMed] [Google Scholar]
- Schliwa M. Proteins associated with cytoplasmic actin. Cell. 1981;25:587–590. doi: 10.1016/0092-8674(81)90166-5. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. A macaque model for studying mechanisms controlling oocyte development and maturation in human and non-human primates. Hum Reprod. 1999a;14:2544–2555. doi: 10.1093/humrep/14.10.2544. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. Onset of nucleolar and extranucleolar transcription and expression of fibrillarin in macaque embryos developing in vitro. Biol Reprod. 1999b;60:721–728. doi: 10.1095/biolreprod60.3.721. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM, VandeVoort CA. Causes of development failure of in-vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Hum Reprod. 2003;18:826–833. doi: 10.1093/humrep/deg144. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Schatten G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biol Reprod. 1997;56:1503–1512. doi: 10.1095/biolreprod56.6.1503. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Keicho K, Takahashi H, Ogawa H, Schultz RM, Okano A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by COMET assay. Theriogenology. 2000;54:137–145. doi: 10.1016/s0093-691x(00)00332-0. [DOI] [PubMed] [Google Scholar]
- Tarín JJ, Vendrell FJ, Ten J, Blanes R, van Blerkom J, Cano A. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Mol Hum Reprod. 1996;2:895–901. doi: 10.1093/molehr/2.12.895. [DOI] [PubMed] [Google Scholar]
- Tesařík J. Involvement of oocyte-coded message in cell differentiation control of early human embryos. Development. 1989;105:317–322. doi: 10.1242/dev.105.2.317. [DOI] [PubMed] [Google Scholar]
- Ueno S, Kurome M, Ueda H, Tomii R, Hiruma K, Nagashima H. Effects of maturation conditions on spindle morphology in porcine MII oocytes. J Reprod Dev. 2005;51:405–410. doi: 10.1262/jrd.16091. [DOI] [PubMed] [Google Scholar]
- Urdaneta A, Jiménez AR, Paramio M-T, Izquierdo D. Cysteamine, glutathione and ionomycin treatments improve in vitro fertilization of prepubertal goat oocytes. Zygote. 2004;12:277–284. doi: 10.1017/s0967199404002874. [DOI] [PubMed] [Google Scholar]
- Vanhoutte L, Nogueira D, Dumortier F, De Sutter P. Assessment of a new in vitro maturation system for mouse and human cumulus-enclosed oocytes: three-dimensional pre-maturation culture in the presence of a phosphodiesterase 3-inhibitor. Hum Reprod. 2009;24:1946–1959. doi: 10.1093/humrep/dep104. [DOI] [PubMed] [Google Scholar]
- Van Soom A, Yuan YQ, Peelman LJ, de Matos DG, Dewulf J, Laevens H, de Kruif A. Prevalence of apoptosis and inner cell allocation in bovine embryos cultured under different oxygen tensions with or without cysteine addition. Theriogenology. 2002;57:1453–1465. doi: 10.1016/s0093-691x(01)00726-9. [DOI] [PubMed] [Google Scholar]
- Wang WH, Meng L, Hacket RJ, Odenburg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001;75:348–353. doi: 10.1016/s0015-0282(00)01692-7. doi:10.1016/S0015-0282(00)01692-7. [DOI] [PubMed] [Google Scholar]
- Whitaker BD, Knight JW. Exogenous γ-glutamyl cycle compounds supplemented to in vitro maturation medium influence in vitro fertilization, culture, and viability parameters of porcine oocytes and embryos. Theriogenology. 2004;62:311–322. doi: 10.1016/j.theriogenology.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16:841–850. doi: 10.1038/sj.cr.7310095. [DOI] [PubMed] [Google Scholar]
- Zhou P, Wu YG, Li Q, Lan GC, Wang G, Gao D, Tan JH. The interactions between cysteamine, cystine and cumulus cells increase the intracellular glutathione level and developmental capacity of goat cumulus-denuded oocytes. Reproduction. 2008;135:605–611. doi: 10.1530/REP-08-0003. doi:10.1530/REP-08-0003. [DOI] [PubMed] [Google Scholar]
- Zhou P, Wu Y-G, Wei D-L, Li Q, Wang G, Zhang J, Luo M-J, Tan J-H. Mouse cumulus-denuded oocytes restore developmental capacity completely when matured with optimal supplementation of cysteamine, cystine and cumulus cells. Biol Reprod. 2010;82:759–768. doi: 10.1095/biolreprod.109.082206. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod. 1997;57:1413–1419. doi: 10.1095/biolreprod57.6.1413. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev. 2003;64:106–112. doi: 10.1002/mrd.10214. [DOI] [PubMed] [Google Scholar]