Abstract
Nitric oxide (NO) mediates host resistance to severe malaria and other infectious diseases. NO production and mononuclear cell expression of the NO producing enzyme-inducible nitric oxide synthase (NOS2) have been associated with protection from severe falciparum malaria. The purpose of this study was to identify single nucleotide polymorphisms (SNPs) and haplotypes in the NOS2 promoter, to identify associations of these haplotypes with malaria severity and to test the effects of these polymorphisms on promoter activity. We identified 34 SNPs in the proximal 7.3 kb region of the NOS2 promoter and inferred NOS2 promoter haplotypes based on genotyping 24 of these SNPs in a population of Tanzanian children with and without cerebral malaria. We identified 71 haplotypes; 24 of these haplotypes comprised 82% of the alleles. We determined whether NOS2 promoter haplotypes were associated with malaria severity in two groups of subjects from Dar es Salaam (N = 185 and N = 250) and in an inception cohort of children from Muheza-Tanga, Tanzania (N = 883). We did not find consistent associations of NOS2 promoter haplotypes with malaria severity or malarial anemia, although interpretation of these results was potentially limited by the sample size of each group. Furthermore, cytokine-induced NOS2 promoter activity determined using luciferase reporter constructs containing the proximal 7.3 kb region of the NOS2 promoter and the G-954C or C-1173T SNPs did not differ from NOS2 promoter constructs that lacked these polymorphisms. Taken together, these studies suggest that the relationship between NOS2 promoter polymorphisms and malaria severity is more complex than previously described.
Introduction
Nitric oxide (NO) is a lipid soluble free radical that mediates host resistance to infectious organisms including parasites (Clark and Rockett 1996; Fang 1997; Nathan 1997). NO is formed during enzymatic conversion of L-arginine to L-citrulline (Clancy and Abramson 1995; Moncada and Higgs 1993; Weinberg 1998) and is produced by three different NO synthases (NOS) in humans (Bredt and Snyder 1994). Inducible NOS (NOS2) is transcribed in response to several stimuli including pro-inflammatory cytokines and pathogens (Fang 1997; Nathan 1997; Weinberg 1998; Weinberg et al. 2008). Mononuclear cell NOS2 expression/activity and systemic, endothelial and pulmonary production of NO are all inversely associated with malaria severity in humans infected with Plasmodium falciparum (Anstey et al. 1996; Boutlis et al. 2003b;Perkins et al.1999; Yeo et al. 2007). Higher levels of the NO metabolites, nitrite and nitrate (NOx) and NOS2 are present in the peripheral blood of Tanzanian children and Indonesian adults with mild forms of malaria as compared to individuals with severe malaria (Anstey et al. 1996; Boutlis et al. 2003b). The importance of NO to malaria resistance is also supported by studies in a mouse model of malaria (Gramaglia et al. 2006). In this mouse model and in recent reports from studies in human subjects, NO appears to be protective in malaria because of its effects in maintaining endothelial function and microvascular perfusion, preventing endothelial activation, reducing red blood cell—endothelial cell adhesion and ameliorating deleterious cytokine production (Gramaglia et al. 2006; Weinberg et al. 2008; Yeo et al. 2007, 2008).
The association of increased NO production and NOS2 expression with milder forms of malaria may be due to NOS2 gene differences between individuals with milder malaria and individuals with more severe forms of malaria. Because NOS2-mediated NO production is primarily regulated at the transcriptional level, studies of genetic determinants of NO expression in malaria have focused on the 5′ promoter region of the NOS2 gene. The human NOS2 promoter contains multiple potential binding sites for transcription factors including binding sites for NFjB, activator protein 1 (AP-1), CCAAT/enhancer-binding protein (C/EBP β) and signal transducers and activators of transcription protein 1 (STAT-1) (Chu et al. 1998; Linn et al. 1997; Marks-Konczalik et al. 1998; Sakitani et al. 1998; Spitsin et al. 1997; Taylor et al. 1998). More detailed mapping of functional regions of the human NOS2 promoter has been performed using mutational analyses of predicted transcription factor binding sites (Marks-Konczalik et al. 1998; Taylor et al. 1998). Deletion studies of the human NOS2 promoter indicated that sequences up to 4.7 kb 5′ from the transcription start site conferred basal promoter activity in unstimulated cells, while sequences up to 7.2 and 16 kb 5′ from the NOS2 transcription start site resulted in an approximately four- and tenfold increase, respectively, in promoter activity with IL-1β, IFNγ and TNFα stimulation as compared to basal promoter activity (de Vera et al. 1996; Laubach et al. 1997; Linn et al. 1997; Marks-Konczalik et al. 1998; Taylor et al. 1998).
Several NOS2 promoter polymorphisms have been studied in the context of malaria severity. Kun et al. (1998) identified an association of a single nucleotide polymorphism (SNP) at position −954 in the NOS2 promoter region (G-954C) of patients from Gabon with less severe malaria. Healthy children with the NOS2 G-954C SNP as compared to those without the G-954C SNP expressed higher levels of NOS enzyme activity in freshly isolated and cultured peripheral blood mononuclear cells (Perkins et al.1999). Another group, working with a cohort of children from Gambia reported an association of fatal malaria with shorter forms of a CCTTT microsatellite repeat 2.5 kb 5′ to the NOS2 transcription start site (Burgner et al. 1998). However, the G-954C and CCTTT repeat NOS2 promoter polymorphisms were not associated with malaria severity in our analysis of a cross-sectional population of children from Tanzania (Levesque et al. 1999). This prompted our search for other NOS2 promoter SNPs associated with malaria severity and led to the subsequent identification of the C-1173T SNP (Hobbs et al. 2002). The C-1173T SNP was associated with protection from two syndromes of severe malaria, cerebral malaria in Tanzanian children and severe malarial anemia in a cohort of malaria patients from Kenya (Hobbs et al. 2002). The C-1173T SNP was associated with increased systemic NOx levels in healthy children.
Given the importance of NO production and NOS2 gene regulation to malaria severity, we undertook a systematic analysis of NOS2 promoter SNPs and haplotypes. In this report, we describe a haplotype analysis of NOS2 promoter SNPs in three separate study populations of Tanzanian children with malaria or exposed to malaria. We report associations of NOS2 promoter haplotypes with malaria severity and susceptibility and report results of functional promoter construct assays containing these SNPs.
Subjects and methods
Patients
Three different populations were studied. The first study population comprised healthy malaria-exposed children and children with uncomplicated and cerebral malaria (6 months to 9 years old) recruited at Muhimbili Medical Center (MMC) in Dar es Salaam, Tanzania for a study of the role of NO in malaria severity between 1994 and 1995 as previously described (Anstey et al. 1996). The protocol was approved by the College Research and Publications Committee at MMC and the Institutional Review Board at Duke University Medical Center. Informed consent was obtained in Kiswahili from all parents or guardians of study children. “Healthy controls” (HCs) (N = 47) were children admitted to the surgical wards for elective surgery for non-inflammatory conditions or for long bone fractures, with no fever within the preceding 2 weeks, a normal white cell count, no other acute illness and either the absence (N = 39) or presence (N = 8) of P. falciparum parasites on thick blood film. “Mild malaria” (MM) (N = 53) was defined as hospitalization with a febrile illness with P. falciparum parasitemia of C10,000 trophozoites/ll, no history of convulsions, no other evident cause of fever, full alertness, normoglycemia and without severe respiratory distress. “Cerebral malaria” (CM) (N = 85) was defined as unarousable coma with a Blantyre coma score (Molyneux et al. 1989) of ≤2 persisting for ≥30 min after the last convulsion, no other cause of coma evident from clinical or CSF analysis and any level of P. falciparum parasitemia on thick film examination. Bacterial meningitis was ruled out in subjects presenting with coma or seizure by lumbar puncture and spinal fluid microscopy.
The second population of HC subjects (N = 250) was recruited in Mikocheni, Dar es Salaam, Tanzania between 2004 and 2005 as part of an IRB-approved protocol; the enrollment criteria for this group of subjects was similar to the criteria used for the 1994–1995 study population. However, while the HC subjects from the 1994–1995 study (6 months to 9 years) were recruited from the surgery wards at MMC and were ethnically diverse and from various regions of Tanzania, the HC subjects in the 2004–2005 study population were school children from the Mikocheni Primary School of Kinondoni District in Dar es Salaam (age 3–12) and were predominantly (~70%) from the Makonde tribe. The children were screened for any previous history of: (1) hospitalization for malaria, (2) coma, (3) seizures and/or (4) unarousable state. If positive for any, they were excluded.
The third study population was a cohort of malaria exposed children (N = 883) in the Mother–Offspring Malaria Study (MOMS) in Muheza-Tanga, Tanzania, recruited as previously described (Mutabingwa et al. 2005). The Muheza-Tanga region is approximately 350 km from Dar es Salaam. The Muheza-Tanga population is comprised of two dominant tribes and several smaller tribes, and the population is not significantly admixed; the Muheza-Tanga population is different from the Dar es Salaam populations; the latter are urban and periurban with a greater diversity of tribal background and admixture. The Muheza-Tanga cohort consisted of mother–infant pairs enrolled at the time of delivery during admission at the Muheza Designated District Hospital between September 2002 and October 2005; subjects were followed until 31 January 2006. For the MOMS project, mother–infant pairs were consented as part of a protocol approved by both Tanzanian and United States IRBs. The Muheza-Tanga cohort of infants was followed longitudinally from birth at 2-week intervals during infancy (<1 year) and 4-week intervals thereafter for clinical examination and blood sampling. Study participants were also evaluated by clinical examination and blood sample at the time of any illness. Information regarding hospitalizations, parasitemia (the presence of any parasites on blood film examination), malaria symptoms and other illnesses were recorded.
Whole genome amplification of DNA samples
For the samples from MMC in Dar es Salaam collected between 1994 and 1995 (HC, MM and CM patients; N = 185), whole genome amplification of DNA present in blood spotted on FTA paper was used to prepare DNA samples as previously described (Levesque et al. 1999).
For the HC samples from Mikocheni in Dar es Salaam collected between 2004 and 2005 (N = 250), blood spotted on FTA paper was used to prepare DNA. FTA paper punches were washed three times with Whatman FTA purification reagent (Florham Park, NJ) and three times with TE buffer (Fluka; Switzerland). The washed FTA paper punches were used to produce whole genome amplified DNA using a GenomiPhiTM kit (GE Healthcare Life Sciences; Piscataway, NJ) according to the manufacturer’s recommendations.
For the samples from the Muheza-Tanga cohort, DNA was extracted from frozen blood pellets using a Qiagen (Valencia, CA) DNA purification kit and amplified prior to analysis using a Qiagen Repli-gTM whole genome amplification kit.
NOS2 promoter sequence analysis and SNP discovery
Single-stranded conformational polymorphism (SSCP) assays of the proximal 7.3 kb region of the NOS2 promoter in 28 individuals from the 1994–1995 Tanzanian study population (16 HC patients and 12 CM patients) were performed as previously described (Hobbs et al. 2002). The sequence of the proximal 7.3 kb of the NOS2 promoter was analyzed by PCR amplification and sequencing as described in the Supplemental Online Material (N = 13, 7 HC patient samples and 6 CM patient samples from MMC collected between 1994 and 1995). The NOS2 promoter sequences between positions −700 and −4395 and between positions −1310 and −1786 were PCR amplified and sequenced in all 185 subjects enrolled at MMC between 1994 and 1995.
Assays for individual NOS2 promoter SNPs
For the MMC samples collected between 1994 and 1995, the analyses of the G-954C, G-5613A, T-5937C and T-5091C SNPs were performed using restriction enzyme assays as previously described (Levesque et al. 1999) and as described in the Supplemental Online Material. The analyses of the C-1173T, C-1657T, G-1026T, and A-718C SNPs were performed using SSCP assays as previously described (Hobbs et al. 2002). The analyses of the G-1459A, C-1503T, A-1641C, C-1657T, C-3780T, C-3824A, G-3880A, C-3975T, G-3978A, C-4044T, T-4058C, C-4072G and C-4276G SNPs were performed by direct sequencing. The analyses of the T-4414C, C-6316G, C-7122T and G-7164A SNPs were performed using TaqManTM assays (see Supplemental Online Material for details).
For the Mikocheni samples collected between 2004 and 2005 and the Muheza-Tanga cohort samples, DNA was analyzed for the presence of the G-954C, C-1173T, G-1459A, A-1641C, G-3978A, T-5091C, T-5937C and G-7164A SNPs using TaqManTM assays (see Supplemental Online Material for details).
Nucleotide heterogeneity analyses
The heterogeneity measures π (nucleotide diversity) and θn (Watterson’s estimate of the mutation parameter) were calculated using the following equations as described (Li 1997; Sunyaev et al. 2000): θn = 1/L × K/Σn-1,i=11/i, where L is the length of the sequence, n the number of alleles and K is the number of polymorphisms; π = 1/L × 2(n/n − 1) × Σi,j xixj Πij, where L is the length of the sequence, n the number of alleles, xi and xj are the frequencies of variants i and j among n alleles and Πij is the number of nucleotide differences between alleles. The equation for calculation of π described by Li (1997) differs from the equation used in this manuscript by a factor of 2. Tajima’s D statistic (π − θn/SD of this difference) and the confidence interval for D were calculated as described (Tajima 1989).
Repeat region sequence analysis
The 7.3 kb region of the NOS2 promoter proximal to the NOS2 transcription start site was analyzed for the presence of repetitive nucleotide elements using the program CENSOR (Jurka 1998; Jurka et al. 1996). The CENSOR program is available at http://www.girinst.org/.
Haplotype construction and linkage disequilibrium analysis
Haplotype construction was performed using the method described by Stephens et al. (2001) (Stephens and Donnelly 2003) using the PHASE Program (v2.0.2 for Microsoft Windows). The PHASE program is available at http://www.stats.ox.ac.uk/mathgen/software.html. Haplotypes were estimated from ten runs (×10 option) of the PHASE algorithm using default settings for the number of iterations (100), thinning interval (1) and burn-in (100), except for the final run of each algorithm when the number of iterations, thinning interval and burn-in steps were ten times longer (×10 option). The BEST program was used to confirm empiric selection of SNPs for haplotype tags (Sebastiani et al. 2003). The four-gamete test and Rm were calculated as described by Hudson and Kaplan (1985). Pairwise linkage disequilibrium calculations were performed using D′ (Lewontin 1964). 2 × 2contingency tables and Fischer’s exact test were used to test the significance of each pairwise comparison (Lewontin 1995).
Recombination hot spot analysis
The PHASE program (v2.0.2) was used to calculate λ values for each SNP marker interval (Li and Stephens 2003; Stephens and Donnelly 2003). λ is the factor by which the recombination rate exceeds the background recombination rate. Median λ values are reported from ten runs (×10 option) of the PHASE algorithm using default settings for the number of iterations (100), thinning interval (1) and burn-in (100), except for the final run of each algorithm when the number of iterations, thinning interval and burn-in steps were ten times longer (×10 option). The program LALIGN (Huang and Miller 1991) was used to search for DNA elements associated with recombination hot spots.
DNA sequence comparisons
Pairwise BLAST searches were used to compare human (AF017634), bovine (AF333248) and murine (AL592185) DNA sequences corresponding to the NOS2 promoter (Tatusova and Madden 1999). The program LALIGN (Huang and Miller 1991) was used for comparisons of the Charcot–Marie–Tooth type 1A (CMT1A) distal (U41165) and proximal (U41166) repeats with the human NOS2 (AF017634) promoter.
NOS2 promoter luciferase reporter construct assays
A plasmid clone (L7) containing the proximal 7.3 kb of the NOS2 promoter was produced by BioEfficiency Solutions (Irvine, CA) and re-sequenced to confirm sequence comparability to reference sequence AF017634 (http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nuccore&id=3421050) between positions 1025 and 8340. The L7 NOS2 promoter clone was the same as the reference sequence except for a C to A base change at position −4193. Base changes at positions G-954C, C-1173T, G-1459A, T-5091C, C-7076T and C-7122T were introduced into the L7 construct with a QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The parent and mutated constructs were sub-cloned individually into pGL4.10[luc2] Luciferase Reporter Vectors (Promega, Madison, WI). Sub-cloned promoter constructs were entirely re-sequenced at each cloning step to confirm sequence fidelity.
A549 cells were co-transfected for 4 h with 1 μg per well of each pGL4.10[luc2] NOS2 promoter construct plus the pGL4.74[hRluc/TK] vector (Promega) as an internal transfection control using Lipofectamine Reagent (Invitrogen, Carlsbad, CA). Transfected A549 cells were cultured at 37°C, 5% CO2 for 24 h and cytokines were added to the cultures [rh IL-1β (10 ng/ml), rh TNF-α (18 ng/ml) and rh IFN-c (24 ng/ml); final concentrations] and incubated at 37°C, 5% CO2 for 16 h.
The Dual-Luciferase Reporter Assay System (Promega) was used to lyse transfected cells and assay luciferase activity using a Veritas Microplate Luminometer (Turner BioSystems, Sunnyvale, CA) equipped with two automatic reagent injectors. The raw data were expressed as the ratios of firefly luciferase activity to Renilla luciferase activity. Raw data ratios of cytokine-stimulated luciferase activity to basal (no cytokine) luciferase activity are reported.
Statistical analyses
Fisher’s exact tests (two-tailed) were used to test the statistical significance of 2 × 2contingency tables of malaria severity and NOS2 promoter haplotypes and to test the significance of D′ calculations. To assess the effect of the haplotypes containing the G-954C, C-1173T, G-1459A and G-7164A SNPs on the probability of hospitalization outcomes, generalized estimating equations (GEE) logistic regression models were constructed with a first order autoregressive working correlation structure (the analysis with exchangeable and m-dependent correlation structure produced nearly identical results for the data) and model based standard errors (Liang and Zeger 1986). This method allowed full use of the longitudinal follow-up data while accounting for the correlation of repeated measurements over time. Univariate GEE models were constructed considering the main effects of each haplotype. Multivariate GEE models incorporated the main haplotype effects, as well as confounding factors including gravidity (primigravid, secundigravid, or multigravid), placental malaria (positive or negative), residence location (four geographical areas), bed net usage (treated, untreated, none, or unknown), birth weight (low or normal) season at birth and at clinic visit (high or low) and age at clinic visit. Models were also adjusted for HbS (AA or AS) and thalassemia as well as their interaction. ANOVA and Tukey–Kramer pairwise analyses were used to test the statistical significance of differences in luciferase activity between NOS2 promoter constructs with different polymorphisms. An alpha of 0.05 was used to determine the significance of statistical analyses. Where appropriate, P values were corrected for multiple testing using Sidak’s method (Ludbrook 1998). The programs PRISMTM (GraphPad Software, San Diego, CA) and JMPTM (version 5.0.1a; SAS, Cary, NC) were used for statistical analyses.
Results
Identification of SNPs in the 7.3 kb region of the NOS2 promoter proximal to the NOS2 transcription start site
For SNP discovery, we sequenced the 7.3 kb region of the NOS2 promoter proximal to the NOS2 transcription start site from 7 HC patients and 6 CM patients. We also performed SSCP analysis of the same 7.3 kb region of the NOS2 promoter in 28 individuals from the 1994–1995 Tanzanian study population (16 HC patients and 12 CM patients). Five (2 HC patients and 3 CM patients) of these 28 individuals were also part of the group of 13 individuals that were analyzed by direct sequencing. SNPs identified by SSCP were confirmed by either direct sequencing or by TaqMan assays. Finally, we also sequenced the regions between −1310 and −1786 and between −3700 and −4395 of the NOS2 promoter in all 185 subjects in the 1994–1995 Tanzanian study population. Table 1 lists the 34 SNPs identified in the sequences from these analyses. We found three SNPs that were only present in a single patient sample during direct sequencing and were not identified in a separate DNA sample from the same patient (A-206T, C-6131T, and A-6657G). These three SNPs are not included in the analysis of SNPs described below or in Table 1.
Table 1.
NOS2 promoter SNPs, positions and allelic frequencies among the 1994–1995 study population of children from Dar es Salaam, Tanzania
| C-248T | 8049 | – | 1 | 26 | 3.8 |
| A-277Gb | 8020 | rs2779248 | 10 | 28 | 36 |
| A-718C | 7579 | rs28973255 | 22 | 350 | 6.3 |
| A-940G | 7357 | – | 1 | 26 | 3.8 |
| G-954C | 7343 | rs1800482 | 25 | 366 | 6.8 |
| G-1026Tb | 7271 | rs2779249 | 137 | 328 | 42 |
| C-1173T | 7124 | rs9282799 | 14 | 360 | 3.9 |
| G-1459A | 6838 | rs28998790 | 13 | 362 | 3.6 |
| C-1503T | 6794 | – | 3 | 362 | 0.8 |
| A-1641C | 6656 | rs57161411 | 10 | 362 | 2.8 |
| C-1657T | 6640 | rs8078340 | 86 | 362 | 24 |
| C-2441Gb | 5856 | rs2301369 | 9 | 26 | 35 |
| C-3051T | 5246 | rs11080358 | 6 | 66 | 9.1 |
| C-3780Tb | 4517 | rs2779251 | 41 | 368 | 11 |
| C-3824A | 4473 | rs2779252 | 2 | 368 | 0.5 |
| G-3880A | 4417 | – | 53 | 368 | 14 |
| C-3975T | 4322 | – | 6 | 368 | 1.6 |
| G-3978A | 4319 | rs61550278 | 36 | 368 | 9.8 |
| C-4044T | 4253 | – | 4 | 368 | 1.1 |
| T-4058C | 4239 | – | 8 | 368 | 2.2 |
| C-4072G | 4225 | – | 1 | 368 | 0.3 |
| C-4276G | 4021 | – | 22 | 368 | 6.0 |
| T-4414C | 3883 | – | 16 | 368 | 4.3 |
| T-5091C | 3206 | rs57234985 | 31 | 366 | 8.5 |
| T-5346C | 2951 | – | 1 | 64 | 1.6 |
| G-5631A | 2666 | rs34913965 | 27 | 364 | 7.4 |
| G-5792C | 2505 | – | 1 | 68 | 1.5 |
| T-5937C | 2360 | rs61589465 | 19 | 350 | 5.4 |
| C-6316G | 1981 | – | 12 | 368 | 3.3 |
| A-6561G | 1736 | rs9892226 | 12 | 64 | 19 |
| G-6689A | 1608 | rs9902311 | 4 | 26 | 15 |
| C-6885T | 1412 | rs12150211 | 4 | 26 | 15 |
| C-7122T | 1175 | rs2531860 | 165 | 368 | 45 |
| G-7164A | 1133 | rs8073782 | 65 | 368 | 18 |
Italic SNPs were selected empirically as htSNPs following initial analysis of NOS2 promoter sequencing data and haplotype analysis using PHASE (v 2.0.2) (Stephens and Donnelly 2003) of 13 Tanzanian children (7 HC and 6 CM patients)
From Chu et al. (1998)
The minor allele for each of these SNPs in the Tanzanian study population is the allele found in Chu et al. (1998)
We estimate that an analysis of 72 alleles (the 16 alleles analyzed by direct sequencing, the 46 alleles analyzed by SSCP, and the 10 alleles analyzed by both direct sequencing and SSCP) identified>99% of the SNPs with an allelic frequency greater than 5%. This analysis also identified 92% of SNPs with an allelic frequency>1% in the 7.3 kb region of the NOS2 promoter proximal to theNOS2 transcription start site (Ewens 1979). The sequence analysis between positions −1310 and −1786 and between positions −3700 and −4395 of all 185 DNA samples in our study population identified six new SNPs (C-1503T, C-3824A, C-3975T, C-4044T, T-4058C and C-4072G) that were not identified in our original discovery set. However, these six SNPs were all present at an allelic frequency below 5%in our population. This further emphasizes that our discovery strategy likely identified nearly all SNPs in the 7.3 kb region of the NOS2 promoter with an allelic frequency > 5%.
Burgner et al. (2003b) reported the positions of ten novel SNPs and confirmed the previously reported G-954C SNP (Kun et al. 1998) in the region between positions −1 and −2447 of the NOS2 promoter. We identified 8 of these 11 SNPs in our analysis of the region of the NOS2 promoter between positions −1 and −2447. We also identified four other SNPs in the region between position −1 and −2447 of the NOS2 promoter and 23 other upstream (between positions −2448 and −7164) SNPs. The four SNPs identified in our analysis but not in that of Burgner et al. (2003b) and the three SNPs identified by Burgner but not identified in our analysis, all had allelic frequencies that were less than 5% (range 0.8–3.8%).
The frequency of transitions (C ↔ T or A ↔ G base pair changes) was 24/34 (71%) and the frequency of transversions (C ↔ A, C ↔ G, T ↔ A or T ↔ G base pair changes) was 10/34 (29%) among the 34 NOS2 promoter SNPs identified in our discovery analysis. 13/34 (38%) of these SNPs occurred at a CpG nucleotide pair. The percentages of SNP transitions and transversions are comparable to percentages reported for other genes (Halushka et al. 1999).
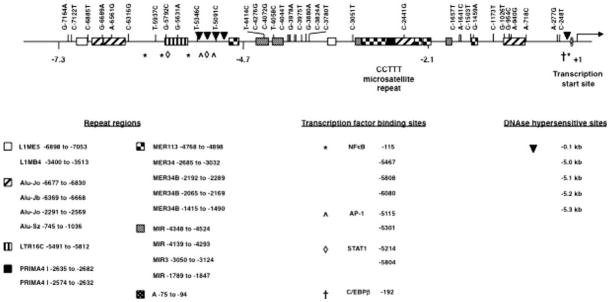
Figure 1 is a summary map of the relative positions of the 34 SNPs reported in Table 1. Figure 1 depicts these 34 SNPs in relation to other relevant NOS2 promoter features including the transcription start site (Nunokawa et al. 1994), the CCTTT microsatellite (Xu et al. 1997), the G-954C polymorphism (Kun et al. 1998; Levesque et al. 1999), functional transcription factor binding sites (Ganster et al. 2001; Marks-Konczalik et al. 1998; Sakitani et al. 1998; Taylor et al. 1998), DNase hypersensitive sites (Mellott et al. 2001) and repetitive sequence regions (Jurka 1998; Jurka et al. 1996). The positions of the functional transcription factor binding sites, DNase hypersensitive sites and repetitive sequence regions are indicated in Fig. 1.
Fig. 1.
Summary map of the relative positions of 34 NOS2 promoter SNPs. The 34 SNPs are depicted in relation to other relevant NOS2 promoter features including the transcription start site, the CCTTT region, functional transcription factor binding sites, DNase hypersensitive sites and repetitive sequence regions. The positions of the functional transcription factor binding sites, DNase hypersensitive sites and repetitive sequence regions are listed
NOS2 promoter SNP heterogeneity analysis and test of neutral mutation hypothesis
Selective pressures are important determinants of nucleotide diversity. The overall nucleotide diversity values (θn and π) in our study population (Table 2) (range from 9.74 × 10−4 to 17.1 × 10−4 for θn and 8.92 × 10−4 to 11.5 × 10−4 for π) are within the range of θn values reported for 5′ UTRs in 75 candidate genes for blood pressure homeostasis (θn = 6.8 × 10−4) (Halushka et al. 1999), and θn and π reported for the lipoprotein lipase (LPL) gene (θn = 16 × 10−4 and π = 20 × 10−4) by Clark et al. (1998). Our estimates of θn (9.74 ×10−4) for the proximal 7.3 kb portion of the NOS2 promoter are comparable to the nucleotide diversity (θn = 8.63 × 10−4) reported by Burgner et al. (2003b) for the NOS2 promoter between positions +60 and −2541. In our study population, p (8.92 × 10−4; Table 2) was similar to π (9.16 × 10−4) calculated from the data reported by Burgner et al. (2003b).
Table 2.
Estimates of NOS2 promoter nucleotide diversity and statistical test of the neutral mutation hypothesis proposed by Tajima (D) based on allele groupings and region of NOS2 promoter analyzed
| Genotype method | Promoter region | Number of alleles | θn | π | Tajima’s D |
|---|---|---|---|---|---|
| Direct seq | −1 to −7300 | 26 | 9.74 × 10−4 | 8.92 × 10−4 | −0.310 |
| SSCP direct seq | −1 to −7300 | 370 | 7.21 × 10−4 | 7.71 × 10−4 | 0.284 |
| Direct seq | −1310 to 1786 and −3700 to −4395 | 370a | 17.1 × 10−4 | 11.5 × 10−4 | −0.959 |
For 10/34 SNPs, not all alleles were tested for the presence or absence of the SNP. As such, the value for π is an estimate
Calculation of Tajima’s D permits comparison of π and θn and forms the basis for a test of the neutral mutation hypothesis (Tajima 1989). θn is highly dependent on the number of sequences analyzed, while the nucleotide diversity measure, π, is dependent on the number of sequences analyzed and the frequency of SNPs in the population. Depending on how the samples were grouped, Tajima’s D ranged from −0.310 to −0.959 (Table 2). These values for Tajima’s D were within the 95% confidence intervals for a DNA sequence meeting Tajima’s expectation for neutral mutation. This suggests that selective pressures, such as malaria severity, may not have been important determinants of nucleotide diversity in the NOS2 promoter.
Haplotype analysis of SNPs in the 7.3 kb region of the NOS2 promoter proximal to the NOS2 transcription start site
The 27 NOS2 promoter SNPs present in the 13 patients (26 alleles) originally analyzed by direct sequencing of the proximal 7.3 kb region of the NOS2 promoter were analyzed to infer haplotypes using the Phase program (v2.0.2) (Stephens and Donnelly 2003; Stephens et al. 2001). Based on the haplotype analysis of these 13 patient samples, we selected 17 SNPs to tag haplotypes (htSNPs) in the entire study population of 185 malaria patients. These 17 htSNPs are highlighted in Table 1 and were selected empirically. The C-248T, A-940G, T-5346C and G-5792T SNPs were identified in the 13 patient samples analyzed by direct sequencing but were not included for further analysis because of their low frequency in the initial analysis of 72 alleles. We included one other htSNP, T-4414C, for analysis in the entire study population of 185 Tanzanian subjects; this SNP was identified by SSCP analysis of 23 patients (46 alleles) and was not identified in the direct sequencing analysis. Later, we also identified htSNPs by analyzing the haplotypes from the 13 patient samples using the program BEST (Sebastiani et al. 2003). Compared to our empiric selection of htSNPs, the BEST program selected fewer htSNPs, and the htSNPs selected by the BEST program were either present in or in linkage disequilibrium with the set of 17 htSNPs that we selected empirically (data not shown). The BEST program also identified the T-5346C and G-5792T SNPs as htSNPs; as stated previously, these SNPs were not included in the set of 17 htSNPs because of their low frequency. The T-5346C SNP together with the C-248T and A-940G SNPs formed a haplotype and the G-5792T SNP together with the C-4276G SNP formed another haplotype (data not shown).
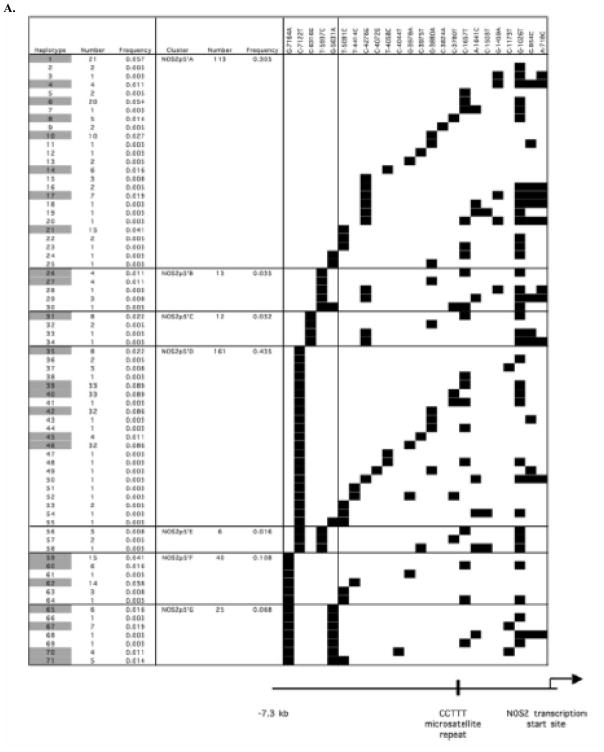
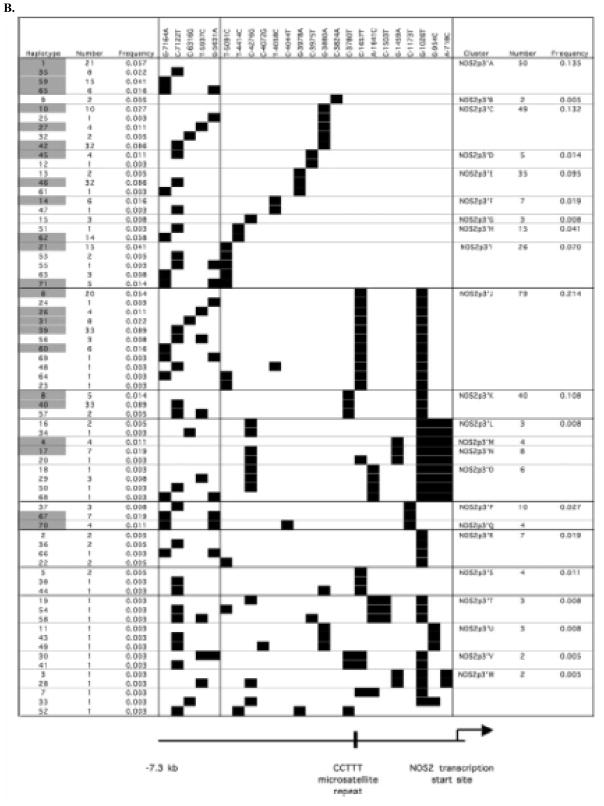
The 18 htSNPs (the 17 htSNPs identified in the original analysis of 13 samples by direct sequencing and the T-4414C SNP) were analyzed in the entire study population of 185 Tanzanian subjects using either restriction enzyme assays, SSCP, TaqMan assays or by direct sequencing. As explained earlier, the direct sequencing of regions between −1310 and −1786 and between −3700 and −4395 identified six further SNPs. Therefore, a total of 24 htSNPs were analyzed in the entire 1994–1995 Tanzanian study population. Figure 2 depicts the inferred haplotypes and the frequency of each haplotype based on an analysis of the data from these 24 htSNPs using the PHASE program (v2.0.2) (Stephens and Donnelly 2003; Stephens et al. 2001). In Fig. 2a, b, the haplotypes are grouped based on SNPs that cluster in the 5′ region (between positions −5631 and −7164) and 3′ region (between positions −1 and −5346), respectively, of the NOS2 promoter. There were a total of 71 different haplotypes (Fig. 2). There were 24 haplotypes with a frequency greater than 1% (highlighted in Fig. 2) and these 24 haplotypes comprised 82% of the 370 sample alleles.
Fig. 2.
Haplotypic structure of NOS2 promoter SNPs. The 24 NOS2 promoter SNPs genotyped in all 185 Tanzanian subjects were analyzed to infer haplotypes using PHASE (v. 2.0.2) (Stephens et al. 2001). The SNPs (marked by black squares) comprising each haplotype are depicted in relation to the CCTTT repeat region and the NOS2 transcription start site. In a and b the haplotypes are clustered in the 5′ and 3′ regions, respectively, of the NOS2 promoter. The 5′ and 3′ regions of the NOS2 promoter are separated by a vertical line. Haplotype numbers highlighted in gray; are greater than 1% in frequency.
The results of our haplotype assignments for the samples in the region of the NOS2 promoter between −1 and −2441 were very similar to those described by Burgner et al. (2003b). We identified all six of the haplotypes (Gam1–6) described by Burgner et al. (2003b). The Gam1– 6 haplotypes are similar to haplotype clusters NOS2p3′A-I, NOS2p3′J, NOS2p3′P-Q, NOS2p3′L-O, NOS2p3′K and NOS2p3′R, respectively, in Fig. 2 and have similar frequencies (0.519, 0.214, 0.038, 0.057, 0.108 and 0.019, respectively) to those described by Burgner et al. (2003b). We found that the Gam1 haplotype could be divided into several different haplotype clusters based on SNPs upstream of the NOS2 promoter region analyzed by Burgner et al. (2003b). We also found that the haplotype groups NOS2p3′J (Gam2) and NOS2p3′K (Gam5) in Fig. 2b extend to and include SNPs C-3051T and C-3780T, respectively. We also identified two other haplotypes in the NOS2 promoter region between bases −1 and −2441 similar to Gam4 that contained SNPs G-1459A and A-1641C (NOS2p3′M–N and NOS2p3′O haplotype groups, respectively, in Fig. 2b).
Identification of a region near the T-5091C NOS2 promoter SNP associated with an increased relative rate of recombination
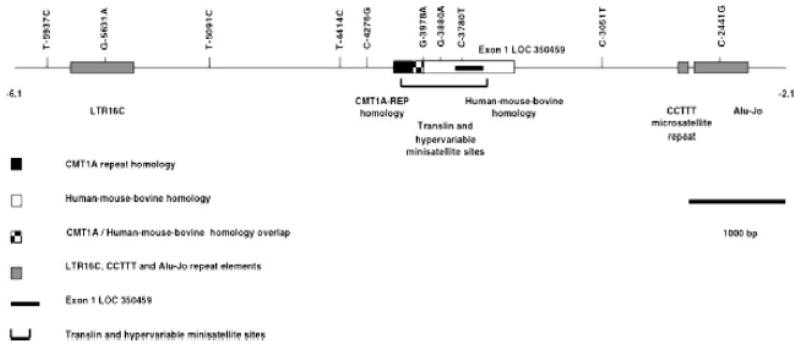
Visual inspection of the haplotype maps in Fig. 2a, b supports the possibility that there is an area of increased relative recombination in the region near SNP T-5091C. The haplotype clusters in Fig. 2 graphically emphasize the clustering of NOS2 promoter SNPs in regions that are 5′ and 3′ of the T-5091C SNP. The haplotype analysis of the NOS2 promoter SNPs using PHASE (v2.0.2) includes an estimate of relative recombination frequency (λ) based on the algorithm by Li and Stephens (2003). An increased relative rate of recombination in the region near the T-5091C SNP is supported by a plot of λ as a function of position within the NOS2 promoter (Fig. 3). Pairwise analyses using the four-gamete test (Figure S1, SOM) and Lewontin’s D′ (Lewontin 1964) (Figure S2, SOM) do not provide clear evidence for an area of increased recombination near SNP T-5091C. The relative recombination frequency (λ) results in Fig. 3 are supported by linkage disequilibrium analyses performed using Haploview (http://www.broad.mit.edu/haploview/haploviewdownloads) and SNPs available in the NCBI database (data not shown) and by the NOS2 promoter haplotype analysis of Burgner et al. (2003a). The region of the NOS2 promoter near the T-5091C SNP contains a cluster of DNA elements associated with increased recombination and translocation (Fig. 4 and Figures S3–S5, SOM).
Fig. 3.
λ-based recombination hot spot analysis of the proximal 7.3 kb region of the NOS2 promoter. Median values of λ generated during analysis of the 24 NOS2 promoter SNPs in Fig. 2 are plotted as a function of the position of each SNP in the NOS2 promoter. λ is the factor by which the recombination rate exceeds the background recombination rate. λ values were generated from SNP analyses using the PHASE program as described in “Methods”
Fig. 4.
Summary map of the relative positions of DNA elements in the NOS2 promoter associated with a recombination hot spot. The region of the NOS2 promoter associated with the CMT1A repeat and human-mouse-bovine homologies, exon 1 of LOC350459 and the translin and hypervariable minisatellite sites is shown. The relative positions of each element are shown in the map. Locations of several high frequency NOS2 promoter SNPs, the CCTTT microsatellite repeat and the LTR16C and Alu-Jo repeat elements are shown for reference.
Association of NOS2 promoter haplotypes with malaria severity and susceptibility in children from Dar es Salaam, Tanzania
C-1173T haplotypes
We previously published an analysis of the C-1173T SNP in the 1994–1995 study population of children from Dar es Salaam, Tanzania and a cohort of children from Kenya (Hobbs et al. 2002). This analysis identified an association of the C-1173T SNP with protection from CM in the Tanzanian children and severe malarial anemia in the Kenya children and identified an association of the C-1173T SNP with higher levels of systemic NO metabolites. In the present study, we identified three NOS2 promoter haplotypes containing the C-1173T SNP, haplotypes 37, 67 and 70 (Fig. 2). In addition to the C-1173T SNP, haplotype 70 contains the C-4044T SNP and only differs from the more common haplotype 67 on this basis. The distribution of Tanzanian subjects with each C-1173T containing haplotype based on malaria severity is shown in Table 3. Of the three C-1173T containing haplotypes, only haplotype 67 showed a significant association with protection from malaria [odds ratio = 0.12, 95% confidence interval = 0.02–0.66, p = 0.0122 (Pc = 0.0362)]. However, low power precludes drawing firm conclusions about the lack of an association of haplotypes 37 and 70 with protection from malaria due to the low numbers of subjects with these haplotypes.
Table 3.
Distribution of malaria subjects with C-1173T containing haplotypes (haplotypes 37, 67 and 70 described in Fig. 2) based on malaria severity among the 1994–1995 study population of children from Dar es Salaam, Tanzania.
| Haplotype no. | Healthy control | Hospitalized malaria without WHO criteria of severity (MM) | Cerebra malaria |
|---|---|---|---|
| 37 | 2 | 1 | 0 |
| 67a | 5 | 0 | 2 |
| 70 | 5 | 0 | 2 |
| Non-37, 67 or 70 | 38 | 52 | 81 |
Association of haplotype 67 with malaria, odds ratio = 0.12, 95% confidence interval = 0.02–0.66, p = 0.0122 (Pc = 0.0362)
Reports from other regions of Africa have not confirmed an association of the C-1173T SNP with protection from malaria (Burgner et al. 2003b; Cramer et al. 2004; Parikh et al. 2004). In our analysis of the 1994–1995 Tanzanian study population (Hobbs et al. 2002), the association of the C-1173T SNP with protection from CM was related to a high frequency of HC subjects with the C-1173T SNP (10% allelic frequency). Therefore, to reexamine the association of the C-1173T SNP with protection from malaria, we determined the frequency of the C-1173T SNP in a second study population of 250 HC children from Dar es Salaam, Tanzania collected between 2004 and 2005. The two groups of HC subjects were accrued differently; the HC subjects in the 1994–1995 Tanzanian study population were comprised of hospital inpatients aged 6 months to 9 years with orthopedic and elective surgical problems and were ethnically diverse, whereas the HC subjects in the 2004–2005 Tanzanian study population were older school children (aged 3–12 years) and primarily restricted to a single Tanzanian tribe. In contrast to our prior analysis (Hobbs et al. 2002) and the analysis presented in Table 3, we observed a 3.2% allelic frequency of the C-1173T SNP in the 2004–2005 study population of HC Tanzanian children from Dar es Salaam as compared to the 10% allelic frequency observed in the 1994–1995 Tanzanian study population. The 3.2% allelic frequency of the C-1173T SNP in the 2004–2005 Tanzanian study population was comparable to reports by other investigators in other populations (Burgner et al. 2003b; Cramer et al. 2004; Parikh et al. 2004) (4.1, 6.1 and 5.6%, respectively), and also comparable to the overall allelic frequency (3.8%) of the C-1173T SNP in the 1994–1995 MMC study population of children from Dar es Salaam, Tanzania, and the 4.2% allelic frequency of the C-1173T SNP in a study population of pregnant Tanzanian women previously reported by our group (Hobbs et al. 2002). The low allelic frequency of the C-1173T SNP in these healthy cohorts suggested that haplotypes containing the C-1173T SNP might not be associated with protection from CM, and that the previously observed association with protection against CM in Dar es Salaam (Hobbs et al. 2002) may have been due to a high C-1173T SNP frequency occurring by chance in the control group used in that study.
G-954C haplotypes
Our original analysis indicated that there was no association of the G-954C NOS2 promoter SNP with malaria severity in the 1994–1995 MMC study population of children from Dar es Salaam, Tanzania (Levesque et al. 1999). In an effort to reconcile our previous findings with reported associations of the G-954C SNP with protection from clinical and severe malaria (Kun et al. 1998; Parikh et al. 2004), we studied haplotypes containing the G-954C NOS2 promoter SNP, and we clustered haplotypes on the basis of the presence or absence of three other SNPs (G-1026T, G-1459A and A-1641C) in the proximal region of the NOS2 promoter (Table 4). The NOS2 promoter haplotype containing the G-954C, G-1026T and G-1459A SNPs [haplotype NOS2p3′M/N (haplotypes 4, 17 and 20) Fig. 2] was found in 14.9% of HC subjects and 3.6% of subjects with clinical malaria and was significantly associated with protection from clinical malaria (odds ratio = 0.2, 95% confidence interval = 0.1–0.7, p = 0.0127 (Pc = 0.0498)]. Other G-954Ccontaining haplotypes (haplotypes NOS2p3′L/O/U; Table 4) were present in only 1 HC patient and were not associated with protection from clinical malaria (for haplotypes NOS2p3′L/O/U combined, odds ratio = 4.4, confidence interval 0.6–35, p = 0.1894).
Table 4.
The number of Tanzanian subjects with NOS2 promoter haplotypes containing the G-954C, G-1026T, G-1459A and A-1641C SNPs among the 1994–1995 study population of children from Dar es Salaam, Tanzania
| NOS2 promoter haplotypes |
Malaria severity (no. of individuals with haplotype) |
HC versus Malariab (comparison) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Namea | A-1641C | G-1459A | G-1026T | G-954C | HC | MM | CM | OR | 95% CI | p valuec |
| NOS2p3′L | A | G | T | C | 0 | 1 | 3 | 3.2 | 0.2–60 | NS |
| NOS2p3′M/N | A | A | T | C | 7 | 2 | 3 | 0.2 | 0.1–0.7 | 0.0127d |
| NOS2p3′O | C | G | T | C | 1 | 2 | 3 | 1.7 | 0.2–15 | NS |
| NOS2p3′U | A | G | G | C | 0 | 1 | 2 | 2.5 | 0.1–48 | NS |
Haplotype names as described in Fig. 2
Malaria: MM and CM patients combined; MM malaria requiring hospitalization but without WHO criteria for severe malaria, CM cerebral malaria
Fisher’s exact test
Pc = 0.0498
We also determined the frequency of NOS2 promoter haplotypes containing the G-954C and G-1459A SNPs (similar to haplotypes 4, 17 and 20) in the 2004–2005 HC study population of 250 children from Dar es Salaam, Tanzania. We found a lower allelic frequency of haplotypes containing the G-954C and G-1459A SNPs in HC subjects from the 2004–2005 compared to the 1994–1995 study populations from Dar es Salaam, Tanzania (3.5 and 7.4%, respectively). Although not conclusive, these data from the 2004–2005 subjects do not support an association of haplotypes containing the G-954C and G-1459A SNPs with protection from malaria.
CCTTT microsatellite repeat haplotypes
We determined whether NOS2 promoter haplotypes containing the G-954C SNP were associated with specific NOS2 promoter pentanucleotide microsatellite repeat lengths. We hypothesized that the NOS2p3′M/N haplotype would be associated with 8 CCTTT repeats in the NOS2 promoter pentanucleotide microsatellite given the strong association of NOS2 promoter alleles with the G-954C SNP and 8 CCTTT repeats with disease severity in a cohort of African American patients with systemic lupus erythematosus (SLE) (Oates et al. 2003). Indeed, the data in Table 5 indicated a strong significant association of 8 CCTTT repeats with the NOS2p3′M/N haplotype (row 2) compared to other NOS2 haplotype clusters containing the G-954C SNP (NOS2p3′L/O/U haplotypes; Table 5) (p = 0.0001). Calculation of D0 supported linkage disequilibrium between 8 CCTTT repeats and the NOS2p3′M/N haplotype (D′ = 0.81, p<0.0001). Haplotype inferences using the PHASE program (Stephens and Donnelly 2003) supported an association of 8 CCTTT repeats with the NOS2p3′M/N haplotype (data not shown).
Table 5.
The number of subjects and corresponding CCTTT repeat values for Tanzanian subjects with NOS2 promoter haplotypes containing the G-954C, G-1026T, G-1459A and A-1641C SNPs among the 1994–1995 study population of children from Dar es Salaam, Tanzania
| NOS2 promoter haplotypes |
Numbers of CCTTT repeats for individual subjectsb | Numbers of subjects wilh |
|||||
|---|---|---|---|---|---|---|---|
| Namea | A-1641C | G-1459A | G-1026T | G-954C | 8 CCTTT repeats | >8 CCTTT repeats | |
| NOS2p3′L | A | G | T | C | (10,10) (11,12) (13,15) (12,13) | 0 | 4 |
| NOS2p3′M/N | A | A | T | C | (8,12) (8,8) (8,9) (8,15) (8,13) (9,14) (9,11) (8,13) (8,10) (8,14) (8,10) (8,9) | 10c,d | 2 |
| NOS2p3′O | C | G | T | C | (11,13) (12,12) (11,14) (10,14) (9,14) (11,12) | 0 | 6 |
| NOS2p3′U | A | G | G | C | (10,11) (9,11) (9,11) | 0 | 3 |
Haplotype names as described in Fig. 2
Numbers in parentheses refer to number of CCTTT repeats for each allele of individual subjects
p = 0.0001 for association of 8 CCTTT repeats with haplotypes containing the G-954C, G-1026T and G-1459A compared to other NOS2 haplotypes containing the G-954C SNP
D′ = 0.81 and p<0.0001 for linkage disequilibrium of 8 CCTTT repeats to haplotypes containing the G-954C, G-1026T and G-1459A SNPs
C-1657T haplotypes
In contrast to the results of Burgner et al. (2003b), we did not observe an association of haplotype cluster NOS2p3′J (Gam2) containing the C-1657T and G-1026T NOS2 promoter SNPs with CM or the development of malaria (data not shown). Our power to detect an association of Gam2 with CM in our study of Tanzanian children ranged from 32 to 87% with 79 Gam2 alleles in the 185 subjects and using odds ratios as reported by Burgner et al. (2003b) between 1.3 and 1.8, respectively.
Association of NOS2 promoter haplotypes with frequency of severe malaria in an inception cohort of Tanzanian children from Muheza-Tanga, Tanzania
To better understand the association of NOS2 promoter haplotypes and malaria severity, we determined whether haplotypes containing the G-1459A SNP (similar to haplotypes 4, 17 and 20) and the C-1173T SNP (similar to haplotype 67) were associated with protection or susceptibility to malaria in a third study population, a cohort of children followed longitudinally from birth in Muheza- Tanga, Tanzania (Mutabingwa et al. 2005) (Tables 6, 7). There were no statistically significant associations with any of the pre-defined clinical outcomes and the data did not support an association of haplotypes containing the C-1173T SNP with protection from malaria hospitalization (Table 6) or severe anemia (Table 7). In addition, the data in Table 6 did not support an association of haplotypes containing the G-1459A and G-954C SNPs with protection from malaria hospitalization or severe malaria in the cohort of Tanzanian children from Muheza-Tanga.
Table 6.
NOS2 haplotype associations with hospitalization outcomes in Muheza, Tanzania
| Na (% total) | Hospitalization type |
|||||
|---|---|---|---|---|---|---|
| WHO severe hospitalizationb | Clinically severe hospitalizationd | WHO/clinically severe hospitalizatione | Non-malaria hospitalizationf | Any Malaria Hospitalizationg | ||
| OR (95% CI)c | OR (95% CI)c | OR (95% CI)c | OR (95% CI)c | OR (95% CI)c | ||
| C-1173T haplotype (at least 1 vs. none | ||||||
| Univariate model | 49 (6.7) | 0.62 (0.24, 1.59) | 1.36 (0.74, 2.50) | 1.07 (0.63, 1.81) | 1.21 (0.74, 2.00) | 0.70 (0.46, 1.05) |
| p = 0.32 | p = 0.32 | p = 0.81 | p = 0.45 | p = 0.08 | ||
| Multivariate model | 49 (6.7) | 0.62 (0.24, 1.62) | 1.48 (0.82, 2.67) | 1.13 (0.66, 1.94) | 1.21 (0.74, 2.00) | 0.70 (0.47, 1.03) |
| p = 0.33 | p = 0.20 | p = 0.66 | p = 0.45 | p = 0.07 | ||
| G-954C/G-1459A haplotype (at leasl 1 vs. none) | ||||||
| Univariate model | 36 (5.0) | 2.04 (0.95, 4.39) | 0.86 (0.36, 2.03) | 1.31 (0.79, 2.17) | 0.71 (0.41, 1.23) | 1.38 (0.87, 2.18) |
| p = 0.07 | p = 0.72 | p = 0.29 | p = 0.23 | p = 0.17 | ||
| Multivariate model | 36 (5.0) | 1.67 (0.80, 3.51) | 0.88 (0.37, 2.10) | 1.25 (0.82, 1.90) | 0.72 (0.42, 1.24) | 1.32 (0.95, 1.85) |
| p = 0.17 | p = 0.78 | p = 0.30 | p = 0.24 | p = 0.10 | ||
| Number of cases | 99 | 154 | 252 | 255 | 642 | |
Number of infants with at least one of the specified haplotype(s)
Defined as positive bloodsmear and one or more of the following: respiratory distress (clinician indication of respiratory distress and, for infants < 2 months of age a RR > 50, for infants ≥ 2 months of age a RR > 40), two or more convulsions occurring in past 24 h, prostration, hypoglycemia, or severe anemia (hemoglobin < 5)
Odds ratio and 95% confidence interval acquired from GEE univariate models and acquired from GEE multivariate models adjusted for: gravidity (primigravid, secundigravid, or multigravid), placental malaria (positive or negative), residence location (four geographical areas), bed net usage (treated, untreated, none, or unknown), birth weight (low or normal) season at birth and at clinic visit (high or low), age at clinic visit. Models were also adjusted for HbS (AA or AS) and thalassemia as well as their interaction
Defined as positive bloodsmear and one or more of the following: respiratory distress (no clinician indication of respiratory distress and, for infants < 2 months of age a RR > 50, for infants ≥ 2 months of age a RR > 40), one convulsion occurring in the past 24 h, hyperpyrexia, or moderate anemia (hemoglobin ≥ 5,< 8)
Defined as positive bloodsmear and one or more of the following: respiratory distress (for infants < 2 months of age a > RR 50, for infants > 2 months of age a RR > 40, with or without clinician indication of respiratory distress), one or more convulsions in the past 24 h, prostration, hypoglycemia, hyperpyrexia, or moderate-to-severe anemia (hemoglobin < 8)
Defined as negative bloodsmear at time of hospitalization
Defined as positive bloodsmear at time of hospitalization
Table 7.
NOS2 haplotype associations with anemia outcomes in Muheza, Tanzania
| Na (% total) | Anemia |
|||
|---|---|---|---|---|
| HGB <5 | HGB < 6 | HGB < 8 | ||
| OR (95% CI)b | OR (95% CI)b | OR (95% CI)b | ||
| C-1173T haplotype (at least 1 vs. none) | ||||
| Univariate model | 49 (6.7) | 1.97(0.47, 8.29) | 1.14 (0.37, 3.49) | 1.49(0.85, 2.61) |
| p =0.36 | p = 0.82 | p = 0.16 | ||
| Multivariate model | 49 (6.7) | 2.68 (0.67, 10.70) | 1.32 (0.44, 4.01) | 1.74 (1.00, 3.02) |
| p =0.16 | p = 0.62 | p = 0.05 | ||
| Number of cases | 17 | 42 | 174 | |
Number of infants with at least one of the specified haplotype(s)
Odds ratio and 95% confidence interval acquired from GEE univariate models and acquired from GEE multivariate models adjusted for: gravidity (primigravid, secundigravid, or multigravid), placental malaria (positive or negative), residence location (four geographical areas), bed net usage (treated, untreated, none, or unknown), birth weight (low or normal) season at birth and at clinic visit (high or low), age at clinic visit. Models were also adjusted for HbS (AA or AS) and thalassemia as well as their interaction
Luciferase reporter construct assays of polymorphic NOS2 promoter sequences
Prior studies using luciferase reporter construct assays that included the proximal 7.2 kb region of the NOS2 promoter indicated that the G-954C SNP does not alter NOS2 promoter function (Coia et al. 2005). To test the functional relevance of NOS2 promoter SNPs, we cloned the proximal 7.3 kb region of the NOS2 promoter into a pGL4 luciferase construct and introduced the G-954C, C-1173T, G-1459A and T-5091C SNPs using site-directed mutagenesis. The G-954C and G-1459A SNPs were also introduced into the NOS2 promoter construct in combination to reproduce the NOS2p3′M/N haplotype. The C-7076T and C-7122T SNPs (SNPs not expected to alter promoter activity) were introduced into NOS2 promoter constructs as controls.
We found that NOS2 promoter constructs produced a low level of basal luciferase activity following transfection into A549 cells (human bronchial epithelial cell line cells) that did not differ between constructs (data not shown). Increased expression of IFNγ and TNFα are associated with malaria severity and P. falciparum glycosylphosphatidylinositol (GPI) induces expression of IL-1β and TNFα (Robinson et al. 2009; Schofield and Grau 2005). With this in mind, we tested whether 7.3 kb NOS2 promoter luciferase reporter constructs containing the G-954C, C-1173T, G-1459A and T-5091C SNPs were associated with differences in activity following transfection and co-culture with IL-1β, TNFα and IFNγ. As shown in Table 8, luciferase activity was markedly increased following transfection of NOS2 promoter constructs into A549 cells and co-culture with IL-1β, TNFα and IFNγ.We did not observe significant differences in cytokine-stimulated luciferase activity between NOS2 promoter constructs with and without the G-954C, C-1173T, G-1459A, T-5091C, C-7076T and C-7122T NOS2 promoter SNPs. In addition, we did not observe differences in luciferase activity between transfected NOS2 promoter constructs using individual cytokines or mixtures of cytokines (Table 8).
Table 8.
Luciferase reporter construct assay results for polymorphic NOS2 promoters containing the G-954C, C-1173T, G-1459A and T-5091C SNPs
| Cytokine(s) | NOS2 SNP(s) |
|||||||
|---|---|---|---|---|---|---|---|---|
| None | G-954C | G-1459A | G-954C G-1459A | C-1173T | T-5091C | C-7076T | C-7122T | |
| IL-1β | 2.5 ± 0.7b | 2.1 ± 0.4 | 3.2 ± 0.6 | – | 3.6 ± 2.4 | 2.9 ± 1.1 | 2.7 ± 0.8 | 3.0 ± 1.1 |
| 4c | 4 | 4 | 4 | 4 | 4 | 4 | ||
| TNFα | 1.6 ± 0.5 | 2.1 ± 1.7 | 1.7 ± 0.3 | – | 2.0 ± 1.2 | 1.8 ± 0.9 | 2.2 ± 1.0 | 1.7 ± 0.3 |
| 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||
| IFNγ | 1.8 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.3 | – | 1.5 ± 0.5 | 1:5 ± 0.6 | 1.6 ± 0.1 | 1.6 ± 0.4 |
| 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||
| IL-1/TNFα | 2.5 ± 0.2 | 3.5 ± 1.1 | 4.3 ± 1.7 | – | 4.8 ± 3.6 | 4.7 ± 2.6 | 3.5 ± 1.2 | 4.3 ± 3.0 |
| 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||
| TNFα/IFNγ | 8.5 ± 3.6 | 5.7 ± 0.7 | 5.6 ± 0.6 | – | 12 ± 7.3 | 9.4 ± 7.6 | 11 ± 8.2 | 7.7 ± 2.9 |
| 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||
| IL-1/IFNγ | 38 ± 15 | 36 ± 11 | 29 ± 3.4 | – | 46 ±21 | 29 ± 10 | 34 ±20 | 33 ± 16 |
| 4 | 4 | 4 | - | 4 | 4 | 4 | ||
| IL-1β/TNFα/IFNγ | 36 ± 14 | 40 ± 15 | 46 ± 17 | 42 ± 20 | 48 ±20 | 53 ± 11 | 59 | 66 |
| 9 | 8 | 4 | 4 | 7 | 7 | 2 | 2 | |
There were no statistically significant differences between groups (ANOVA and Tukey–Kramer)
Mean ± SD of fold luciferase activity versus transfected cells not stimulated with cytokines
N (number of transfections performed on different days)
These studies suggested that the G-954C, C-1173T, G-1459A and T-5091C SNPs may not functionally alter NOS2 promoter activity and that any associations of these SNPs with malaria severity or NO production may be due to other functional polymorphisms in linkage disequilibrium with these NOS2 promoter SNPs. With this in mind, we sequenced all 26 exons and flanking intronic regions of the NOS2 gene from three subjects with the C-1173T haplotype and from three subjects with the G-954C and G-1459A haplotype. In this analysis, we did not identify any known or new NOS2 gene SNPs that were associated with these haplotypes and that resulted in non-synonymous or splice region base changes (data not shown).
Discussion
The purpose of this study was to identify SNPs in the NOS2 promoter, to construct haplotypes from these SNPs, and to test for associations of these haplotypes with malaria severity and susceptibility in Tanzanian children (Anstey et al. 1996; Mutabingwa et al. 2005). We identified 34 SNPs in the 7.3 kb region of the NOS2 promoter proximal to the NOS2 transcription start site, and analyzed 24 of these SNPs in the entire 1994–1995 study population of 185 children from Dar es Salaam, Tanzania. 71 haplotypes were inferred from the genotype data and used to calculate the relative rates of recombination (λ) in this region of the NOS2 promoter. The analysis method of Li and Stephens (2003) suggested the presence of a region of increased relative recombination approximately 4 kb upstream of the NOS2 transcription start site and near the CCTTT microsatellite repeat. The region approximately 4 kb upstream of the NOS2 transcription start site is characterized by the presence of DNA elements associated with recombination and translocation in other studies (Aoki et al. 1995; Jeffreys et al. 1985; Jurka et al. 1996; Reiter et al. 1996; Smith et al. 1981; Wahls et al. 1990) and is conserved based on a comparison of human, bovine and murine NOS2 promoter sequences. We found that a NOS2 promoter haplotype containing the G-954C, G-1026T and G-1459A SNPs, and pentanucleotide microsatellites with 8 CCTTT repeats was associated with protection from malaria in the 1994–1995 study population of Tanzanian children from Dar es Salaam, but these findings were not supported by an analysis of samples from a longitudinal cohort of Tanzanian children from Muheza-Tanga. In addition, our analyses did not support an association of haplotypes containing the C-1173T with lower rates of malaria hospitalization or severe malaria in the Muheza-Tanga cohort. Functional assays using NOS2 promoter reporter construct assays did not identify increased promoter activity associated with the C-1173T SNP or with the G-954C and G-1459A SNPs.
Our calculations of π (nucleotide diversity) and θn (Watterson’s estimate of the mutation parameter) and Tajima’s D for SNPs in the NOS2 promoter yielded different results depending on the extent of the SNP discovery methods utilized (Table 2). The extent of these disparities is not surprising since the calculations of π, n and Tajima’s D are dependent on the number of low frequency SNPs identified using a given search strategy for SNP discovery. Therefore, it is clear that comparisons of p, hn and Tajima’s D between different regions of the genome are highly dependent on factors that may have little to do with the intrinsic mutation rate or the influences of selective pressures on a given region of DNA.
We used the PHASE program for haplotype construction because Version 2 of this algorithm takes into account possible recombination events and the relative locations of SNPs (Stephens and Donnelly 2003); this algorithm has also been shown to be superior to other available methods. When used in combination with an algorithm described by the same group, the PHASE program also permits an estimation of relative rates of recombination (Li and Stephens 2003). The resulting haplotypes derived from the PHASE program are consistent with haplotypes in the proximal 2.5 kb region of the NOS2 promoter described by Burgner et al. (2003b) in their cohort of individuals in Gambia. Their Gambian samples included parent and child DNA samples that allowed relatively unambiguous construction of NOS2 promoter haplotypes. In the proximal portion of the NOS2 promoter analyzed in Burgner’s study and our study, there was excellent agreement on haplotypes and the frequency of haplotypes. A major difference in our study compared to Burgner’s was our discovery of haplotypes containing the G-1459A and A-1641C SNPs; these SNPs define different haplotypes from the parent haplotype containing the G-954C NOS2 promoter SNP.
We used a variety of different analyses to examine recombination in the NOS2 promoter. The analysis of Li and Stephens (2003) depicted in Fig. 3 provides the clearest quantitative picture of relative recombination frequency. A visual inspection of NOS2 promoter haplotypes as presented in Fig. 2 also provides a clear view of recombination events and their relative frequencies. However, the patterns of positive four-gamete tests in Figure S1 (SOM) and significant pairwise linkage disequilibrium in Figure S2 (SOM) do not show strong evidence of a recombination hot spot. This is in contrast to the sequence data for the LPL gene in which both pairwise linkage disequilibrium and the model-based approach of Li and Stephens (2003) show evidence of a recombination hot spot (Clark et al. 1998; Templeton et al. 2000).
Results in Figs. 2 and 3, S1 and S2 (SOM), depend on the haplotype assignments made by the PHASE program. For ethnically diverse samples in which the assumptions of random mating may not be appropriate, alternative methods have been used to resolve phase ambiguity. For example, Clark et al. (1998) used allele-specific PCR in their study of the LPL gene. The method of Li and Stephens (2003) can be more powerful than pairwise linkage disequilibrium analysis because their approach exploits the pattern of linkage disequilibrium for all the markers simultaneously rather than just for a pair of markers. This is particularly important for hotspots of low to moderate intensity since the linkage disequilibrium between markers on each side of the hot spot may not be adequately degraded to reveal an interpretable pattern such as that seen with NOS2. In contrast, in the LDL gene the pairwise pattern is clear.
Certain studies suggest that the human genome may be divided into regions of high linkage disequilibrium separated by regions of low linkage disequilibrium (Jeffreys et al. 2001). Although our analysis suggests an area of increased recombination near SNP T-5091C, other possible mechanisms for the 5′ and 3′ SNP clusters include gene conversion, selection involving the 5′ and 3′ SNP clusters, or epistatic selection. Therefore, it remains unclear how the rates of recombination in regions such as those described in this manuscript for the NOS2 promoter compare to other regions of the genome designated as “hot spots.”
The region of the human NOS2 promoter approximately 4 kb upstream of the NOS2 transcription start site that is near the putative NOS2 promoter recombination hot spot has a high degree of homology with murine and bovine NOS2 sequences. This suggests that this region has been conserved and as such likely serves an important function. This region of the human NOS2 promoter can be deleted in luciferase promoter construct assays without decreasing promoter activity (Taylor et al. 1998). This is in contrast to the other region of the NOS2 promoter that is homologous in humans and mice and which is immediately proximal to the NOS2 transcription start site (Chartrain et al. 1994); this region is important for promoter function in in vitro assays (Sakitani et al. 1998). Interestingly, the region of the NOS2 promoter approximately 4 kb upstream of the NOS2 transcription start site has not been previously identified as important for mouse NOS2 promoter activity. It is possible that this DNA region may function to regulate NOS2 gene transcription by interacting with elements such as nucleosomes or the 30UTR that are not assessed in typical luciferase reporter construct assays. Alternatively, this region may have been conserved because it is a recombination hot spot, and it confers an important selective advantage. Finally, it is also possible that this region of the NOS2 promoter forms an exon for a protein that serves an important function in mice, cows and humans even though it is not coordinately regulated with NOS2.
Our haplotype analysis of NOS2 promoter polymorphisms may help to explain the apparent discrepancy between the analysis of the G-954C SNP in Gabonese children presented by Kun et al. (1998) and our published analysis of the 1994–1995 study population of children from Dar es Salaam, Tanzania (Levesque et al. 1999). We believe that the relatively large proportion of Tanzanian individuals with the G-954C SNP, but with haplotypes that do not also include the G-1026T and G-1459A SNPs and pentanucleotide microsatellites with 8 CCTTT repeats (NOS2p3′L/O/U haplotypes), make it difficult to identify an association of the G-954C SNP with malaria severity in the 1994–1995 study population of children from Dar es Salaam, Tanzania. Our data may also explain why an association of haplotypes containing the G-954C and G-1459A SNPs and pentanucleotide microsatellites with 8 CCTTT repeats was not seen in an analysis of Gambians from western Africa with malaria (Burgner et al. 2003b). In these Gambian studies, the G-1459A SNP was apparently not observed in this population. Therefore, it will be important to test whether the G-1459A SNP is present in central Africans from Gabon and whether it is associated with a haplotype including the G-954C and G-1026T SNPs and pentanucleotide microsatellites with 8 CCTTT repeats, and whether this haplotype is associated with protection from clinical or severe malaria.
Previously, we observed an association of the G-954C NOS2 promoter SNP with disease severity in African Americans from North and South Carolina with SLE (Oates et al. 2003). In this African American population with SLE, there was an association of the G-954C SNP with NOS2 pentanucleotide microsatellites containing 8 CCTTT repeats. The association of the G-954C NOS2 promoter SNP with 8 CCTTT repeats in African Americans from the southern United States may be particularly relevant to prior findings in Gabon (Kun et al. 1998) because many African Americans originated from central and west Africa. Importantly, the results of our analysis of the G-954C SNP and 8 CCTTT repeats with SLE help to validate the importance of the G-954C containing NOS2p3′M/N haplotype to human disease. We believe that selective pressures that may have favored the NOS2p3′M/N haplotype for malaria resistance via increased production of NO, may have also fostered susceptibility to SLE and perhaps other inflammatory diseases associated with increased NO production.
It also remains unclear whether the G-1459A SNP is functional since we only identified two individuals with haplotypes that included the G-1459A SNP but not the G-954C SNP; one of these individuals was an HC subject, and the other a CM subject. Both of these individuals had an allele that contained 8 CCTTT repeats. In addition, results of the NOS2 promoter luciferase reporter construct assays (Table 8) do not support a functional role for the G-954C and G-1459A NOS2 promoter SNPs. However, the promoter constructs used for these assays did not contain other polymorphisms that were normally in linkage disequilibrium with the G-954C and G-1459A NOS2 promoter SNPs. Therefore, any associations of the G-954C and G-1459A SNPs with protection from malaria may be due to other functional polymorphisms in linkage disequilibrium with the G-954C and G-1459A NOS2 promoter SNPs.
The results of the NOS2 promoter reporter construct assays did not identify differences in NOS2 promoter activity for constructs containing the C-1173T SNP or other NOS2 promoter SNPs. While this suggests that the C-1173T SNP may be in linkage disequilibrium with other functional polymorphisms and may not directly alter NOS2 promoter activity, there are several limitations of these types of reporter construct assays that do not preclude a functional role for the C-1173T SNP in NOS2 promoter activity. For example, the cell line and cytokines used for the transfections and stimulation may not be applicable to the C-1173T SNP. The histone and transcription factor concentrations in the A549 cells used for these transfections may limit the ability of these cells to regulate expression of extra-physiologic levels of transfected DNA or regulation may be limited due to the lack of epigenetic modifications such as acetylation or methylation of the transfected DNA. Another important limitation of the reporter constructs may be the absence of upstream or downstream elements that interact with the C-1173T region of the promoter; for example, similar reporter construct studies of the TNFα promoter have indicated that the TNFα 3U′TR is necessary to identify increased promoter activity in reporter constructs containing the G-308A TNFα promoter polymorphism, which is associated with several inflammatory disorders (Abraham and Kroeger 1999).
The number of CCTTT pentanucleotide microsatellite repeats also seems unlikely to result in functional differences in NOS2 promoter activity. For example, in addition to NOS2 promoter haplotypes containing the G-954C and G-1459A SNPs, we identified other haplotypes associated with 8 CCTTT repeats that were present in all malaria groups including CM patients. Moreover, our recent studies in malaria-exposed patients in Papua New Guinea found no evidence to support functionality of 8 CCTTT repeats; there was no relationship between the number of CCTTT repeats with either NO production or mononuclear cell NOS activity in malaria-exposed Papuans (Boutlis et al. 2003a). The association of alleles containing 8 CCTTT repeats with the G-954C, G-1026T and G-1459A SNPs and protection from malaria may also explain our failure to identify an association of short (<11 repeats) pentanucleotide microsatellites with severe malaria (Levesque et al. 1999) as originally described by Burgner et al. (1998). Despite the absence of a clear association of any of the individual SNPs described in our current study with malaria severity, it is possible that these NOS2 promoter polymorphisms act together to promote resistance to malaria.
In summary, we identified 34 SNPs and inferred 71 haplotypes in the region of the NOS2 promoter proximal to the NOS2 transcription start site. Analysis of haplotypes in this region is complicated by the presence of a region of increased relative recombination. An understanding of recombination events in the NOS2 promoter should aid the search for functional SNPs in linkage disequilibrium with haplotypes associated with human disease and NO production. The construction of haplotypes permitted identification of a novel G-954C-containing haplotype that was associated with protection from malaria in the study population from Dar es Salaam but not in a cohort from Muheza- Tanga, Tanzania. The haplotype analysis of the G-954C SNP may explain discrepancies in prior publications regarding the association of the G-954C SNP with malaria severity (Jallow et al. 2009; Kun et al. 1998; Levesque et al. 1999). In addition, our results in children from three different study populations in Tanzania did not support a consistent association between haplotypes containing the C-1173T SNP with protection from malaria hospitalization or severe anemia. However, our prior analysis in a Kenyan cohort (Hobbs et al. 2002) still suggests that the C-1173T SNP may protect against the development of severe malarial anemia in some circumstances. The absence of associations between NOS2 promoter SNPs and malaria severity are limited to the Tanzanian populations we analyzed and may not be similar in other populations, especially those from other regions of Africa. Furthermore, larger sample sizes may have revealed weaker associations of these SNPs with malaria severity. Taken together, our results suggest that the relationship between NOS2 promoter polymorphisms and malaria severity is more complex than previously described.
Supplementary Material
Acknowledgments
This work funded in part by grants from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative and the U.S. National Institutes of Health R01 AI52059 (PED), R01 AI041764 (JBW), and by the Veterans Affairs Research Service. The authors gratefully acknowledge the contributions of Bernard John, R.N., Study Manager, Ann Shambuso, R.N. and Sophia Mbangukira, R.N. at Hubert Kairuki Memorial University, Mikocheni Mission Hospital in Dar es Salaam, Tanzania.
References
- Abraham LJ, Kroeger KM. Impact of the −308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: relevance to disease. J Leukoc Biol. 1999;66:562–566. doi: 10.1002/jlb.66.4.562. [DOI] [PubMed] [Google Scholar]
- Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D, McDonald MI, Granger DL. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Suzuki K, Sugano T, Tasaka T, Nakahara K, Kuge O, Omori A, Kasai M. A novel gene, Translin, encodes a recombination hotspot binding protein associated with chromosomal translocations. Nat Genet. 1995;10:167–174. doi: 10.1038/ng0695-167. [DOI] [PubMed] [Google Scholar]
- Boutlis CS, Hobbs MR, Marsh RL, Misukonis MA, Tkachuk AN, Lagog M, Booth J, Granger DL, Bockarie MJ, Mgone CS, Levesque MC, Weinberg JB, Anstey NM. Inducible nitric oxide synthase (NOS2) promoter CCTTT repeat polymorphism: relationship to in vivo nitric oxide production/NOS activity in an asymptomatic malaria-endemic population. Am J Trop Med Hyg. 2003a;69:569–573. [PubMed] [Google Scholar]
- Boutlis CS, Tjitra E, Maniboey H, Misukonis MA, Saunders JR, Suprianto S, Weinberg JB, Anstey NM. Nitric oxide production and mononuclear cell nitric oxide synthase activity in malaria-tolerant Papuan adults. Infect Immun. 2003b;71:3682–3689. doi: 10.1128/IAI.71.7.3682-3689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Burgner D, Xu WM, Rockett K, Gravenor M, Charles IG, Hill AV, Kwiatkowski D. Inducible nitric oxide synthase polymorphism and fatal cerebral malaria. Lancet. 1998;352:1193–1194. doi: 10.1016/S0140-6736(05)60531-4. [DOI] [PubMed] [Google Scholar]
- Burgner D, Rockett K, Ackerman H, Hull J, Usen S, Pinder M, Kwiatkowski DP. Haplotypic relationship between SNP and microsatellite markers at the NOS2A locus in two populations. Genes Immun. 2003a;4:506–514. doi: 10.1038/sj.gene.6364022. [DOI] [PubMed] [Google Scholar]
- Burgner D, Usen S, Rockett K, Jallow M, Ackerman H, Cervino A, Pinder M, Kwiatkowski DP. Nucleotide and haplotypic diversity of the NOS2A promoter region and its relationship to cerebral malaria. Hum Genet. 2003b;112:379–386. doi: 10.1007/s00439-002-0882-4. [DOI] [PubMed] [Google Scholar]
- Chartrain NA, Geller DA, Koty PP, Sitrin NF, Nussler AK, Hoffman EP, Billiar TR, Hutchinson NI, Mudgett JS. Molecular cloning, structure, and chromosomal localization of the human inducible nitric oxide synthase gene. J Biol Chem. 1994;269:6765–6772. [PubMed] [Google Scholar]
- Chu SC, Marks-Konczalik J, Wu HP, Banks TC, Moss J. Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters. Biochem Biophys Res Commun. 1998;248:871–878. doi: 10.1006/bbrc.1998.9062. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Abramson SB. Nitric oxide: a novel mediator of inflammation. Proc Soc Exp Biol Med. 1995;210:93–101. doi: 10.3181/00379727-210-43927aa. [DOI] [PubMed] [Google Scholar]
- Clark IA, Rockett KA. Nitric oxide and parasitic disease. Adv Parasitol. 1996;37:1–56. doi: 10.1016/s0065-308x(08)60218-3. [DOI] [PubMed] [Google Scholar]
- Clark AG, Weiss KM, Nickerson DA, Taylor SL, Buchanan A, Stengard J, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF. Haplotype structure and population genetic inferences from nucleotide-sequence variation in human lipoprotein lipase. Am J Hum Genet. 1998;63:595–612. doi: 10.1086/301977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coia V, Juliger S, Mordmuller B, Kreidenweis A, Stroh A-L, Ortega C, Vindigni A, Dengjel J, Lukyanov DV, Destro-Bisol G, Fedorov A, Podgornaya OI, Kun JFJ. Analysis of polymorphic sites in the promoter of the nitric oxide synthase 2 gene. Biochem Biophys Res Commun. 2005;335:1123–1131. doi: 10.1016/j.bbrc.2005.07.178. [DOI] [PubMed] [Google Scholar]
- Cramer JP, Mockenhaupt FP, Ehrhardt S, Burkhardt J, Otchwemah RN, Dietz E, Gellert S, Bienzle U. iNOS promoter variants and severe malaria in Ghanaian children. Trop Med Int Health. 2004;9:1074–1080. doi: 10.1111/j.1365-3156.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- de Vera ME, Shapiro RA, Nussler AK, Mudgett JS, Simmons RL, Morris SM, Jr, Billiar TR, Geller DA. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc Natl Acad Sci USA. 1996;93:1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens WJ. Mathematical Population Genetics. Springer; Berlin: 1979. [Google Scholar]
- Fang FC. Perspectives series: host/pathogen interactions Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganster RW, Taylor BS, Shao LF, Geller DA. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12:1417–1422. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- Halushka MK, Fan JB, Bentley K, Hsie L, Shen N, Weder A, Cooper R, Lipshutz R, Chakravarti A. Patterns of singlenucleotide polymorphisms in candidate genes for blood-pressure homeostasis. Nat Genet. 1999;22:239–247. doi: 10.1038/10297. [DOI] [PubMed] [Google Scholar]
- Hobbs MR, Udhayakumar V, Levesque MC, Booth J, Tkachuk AN, Pole A, Roberts JM, Kariuki S, Nahlen BL, Mwaikambo ED, Lal AL, Granger DL, Anstey NM, Weinberg JB. A novel NOS2 promoter polymorphism associated with protection from severe malaria in children from Tanzania and Kenya. Lancet. 2002;360:1468–1475. doi: 10.1016/S0140-6736(02)11474-7. [DOI] [PubMed] [Google Scholar]
- Huang X, Miller W. A time-efficient, linear-space local similarity algorithm. Adv Appl Math. 1991;12:337–357. [Google Scholar]
- Hudson RR, Kaplan NL. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics. 1985;111:147–164. doi: 10.1093/genetics/111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, Kivinen K. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nature. 2009 doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys AJ, Wilson V, Thein SL. Hypervariable ‘minisatellite’ regions in human DNA. Nature. 1985;314:67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys AJ, Kauppi L, Neumann R. Intensely punctate meiotic recombination in the class II region of the major histocompatibility complex. Nat Genet. 2001;29:217–222. doi: 10.1038/ng1001-217. [DOI] [PubMed] [Google Scholar]
- Jurka J. Repeats in genomic DNA: mining and meaning. Curr Opin Struct Biol. 1998;8:333–337. doi: 10.1016/s0959-440x(98)80067-5. [DOI] [PubMed] [Google Scholar]
- Jurka J, Klonowski P, Dagman V, Pelton P. CENSOR a program for identification and elimination of repetitive elements from DNA sequences. Comput Chem. 1996;20:119–121. doi: 10.1016/s0097-8485(96)80013-1. [DOI] [PubMed] [Google Scholar]
- Kun JF, Mordmuller B, Lell B, Lehman LG, Luckner D, Kremsner PG. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria [letter] Lancet. 1998;351:265–266. doi: 10.1016/S0140-6736(05)78273-8. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Zhang CX, Russell SW, Murphy WJ, Sherman PA. Analysis of expression and promoter function of the human inducible nitric oxide synthase gene in DLD-1 cells and monkey hepatocytes. Biochim Biophys Acta. 1997;1351:287–295. doi: 10.1016/s0167-4781(96)06909-6. [DOI] [PubMed] [Google Scholar]
- Levesque MC, Hobbs MR, Anstey NM, Vaughn TN, Chancellor JA, Pole A, Perkins DJ, Misukonis MA, Chanock SJ, Granger DL, Weinberg JB. Nitric oxide synthase type 2 promoter polymorphisms, nitric oxide production and disease severity in Tanzanian children with malaria. J Infect Dis. 1999;180:1994–2002. doi: 10.1086/315140. [DOI] [PubMed] [Google Scholar]
- Lewontin RC. The interaction of selection and linkage. I. General considerations; heterotic models. Genetics. 1964;49:49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin RC. The detection of linkage disequilibrium in molecular sequence data. Genetics. 1995;140:377–388. doi: 10.1093/genetics/140.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WH. Molecular evolution. Sinauer Associates; Sunderland: 1997. [Google Scholar]
- Li N, Stephens M. Modelling linkage disequilibrium, and identifying recombination hot spots using SNP data. Genetics. 2003;165:2213–2233. doi: 10.1093/genetics/165.4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Stephens M. Modelling linkage disequilibrium, and identifying recombination hot spots using SNP data. Genetics. 2003;165:2213–2233. doi: 10.1093/genetics/165.4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Linn SC, Morelli PJ, Edry I, Cottongim SE, Szabo C, Salzman AL. Transcriptional regulation of human inducible nitric oxide synthase gene in an intestinal epithelial cell line. Am J Physiol. 1997;272:G1499–G1508. doi: 10.1152/ajpgi.1997.272.6.G1499. [DOI] [PubMed] [Google Scholar]
- Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Marks-Konczalik J, Chu SC, Moss J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaB-binding sites. J Biol Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- Mellott JK, Nick HS, Waters MF, Billiar TR, Geller DA, Chesrown SE. Cytokine-induced changes in chromatin structure and in vivo footprints in the inducible NOS promoter. Am J Physiol Lung Cell Mol Physiol. 2001;280:L390–L399. doi: 10.1152/ajplung.2001.280.3.L390. [DOI] [PubMed] [Google Scholar]
- Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mutabingwa TK, Bolla MC, Li J-L, Domingo GJ, Li X, Fried M, Duffy PE. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med Publ Lib Sci. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunokawa Y, Ishida N, Tanaka S. Promoter analysis of human inducible nitric oxide synthase gene associated with cardiovascular homeostasis. Biochem Biophys Res Commun. 1994;200:802–807. doi: 10.1006/bbrc.1994.1522. [DOI] [PubMed] [Google Scholar]
- Oates JC, Levesque MC, Hobbs MR, Smith EG, Molano ID, Page GP, Hill BS, Weinberg JB, Cooper GS, Gilkeson GS. Nitric oxide synthase 2 promoter polymorphisms and systemic lupus erythematosus in African-Americans. J Rheumatol. 2003;30:60–67. [PubMed] [Google Scholar]
- Parikh S, Dorsey G, Rosenthal PJ. Host polymorphisms and the incidence of malaria in Ugandan children. Am J Trop Med Hyg. 2004;71:750–753. [PubMed] [Google Scholar]
- Perkins DJ, Kremsner PG, Schmid D, Misukonis MA, Kelly MA, Weinberg JB. Blood mononuclear cell nitric oxide production and plasma cytokine levels in healthy gabonese children with prior mild or severe malaria. Infect Immun. 1999;67:4977–4981. doi: 10.1128/iai.67.9.4977-4981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter LT, Murakami T, Koeuth T, Pentao L, Muzny DM, Gibbs RA, Lupski JR. A recombination hotspot responsible for two inherited peripheral neuropathies is located near a mariner transposon-like element. Nat Genet. 1996;12:288–297. doi: 10.1038/ng0396-288. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, D’Ombrain MC, Stanisic DI, Taraika J, Bernard N, Richards JS, Beeson JG, Tavul L, Michon P, Mueller I, Schofield L. Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect Immun. 2009;77:3033–3043. doi: 10.1128/IAI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakitani K, Nishizawa M, Inoue K, Masu Y, Okumura T, Ito S. Synergistic regulation of inducible nitric oxide synthase gene by CCAAT/enhancer-binding protein beta and nuclear factor-kappaB in hepatocytes. Genes Cells. 1998;3:321–330. doi: 10.1046/j.1365-2443.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- Sebastiani P, Lazarus R, Weiss ST, Kunkel LM, Kohane IS, Ramoni MF. Minimal haplotype tagging. Proc Natl Acad Sci USA. 2003;100:9900–9905. doi: 10.1073/pnas.1633613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GR, Kunes SM, Schultz DW, Taylor A, Triman KL. Structure of chi hotspots of generalized recombination. Cell. 1981;24:429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Spitsin SV, Farber JL, Bertovich M, Moehren G, Koprowski H, Michaels FH. Human- and mouse-inducible nitric oxide synthase promoters require activation of phosphatidylcholinespecific phospholipase C and NF-kappa B. Mol Med. 1997;3:315–326. [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyaev SR, Lathe WC, 3rd, Ramensky VE, Bork P. SNP frequencies in human genes an excess of rare alleles and differing modes of selection. Trends Genet. 2000;16:335–337. doi: 10.1016/s0168-9525(00)02058-8. [DOI] [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusova TA, Madden TL. BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, Billiar TR, Geller DA. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- Templeton AR, Clark AG, Weiss KM, Nickerson DA, Boerwinkle E, Sing CF. Recombinational and mutational hotspots within the human lipoprotein lipase gene. Am J Hum Genet. 2000;66:69–83. doi: 10.1086/302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls WP, Wallace LJ, Moore PD. Hypervariable minisatellite DNA is a hotspot for homologous recombination in human cells. Cell. 1990;60:95–103. doi: 10.1016/0092-8674(90)90719-u. [DOI] [PubMed] [Google Scholar]
- Weinberg JB. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Lopansri BK, Mwaikambo E, Granger DL. Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria. Curr Opin Infect Dis. 2008;21:468–475. doi: 10.1097/QCO.0b013e32830ef5cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Liu L, Emson PC, Harrington CR, Charles IG. Evolution of a homopurine-homopyrimidine pentanucleotide repeat sequence upstream of the human inducible nitric oxide synthase gene. Gene. 1997;204:165–170. doi: 10.1016/s0378-1119(97)00538-6. [DOI] [PubMed] [Google Scholar]
- Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, Price RN, Duffull SB, Celermajer DS, Anstey NM. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci USA. 2008;105:17097–17102. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.