Abstract
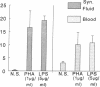
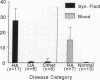
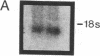
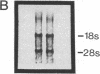
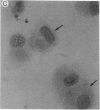
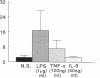
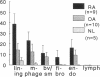
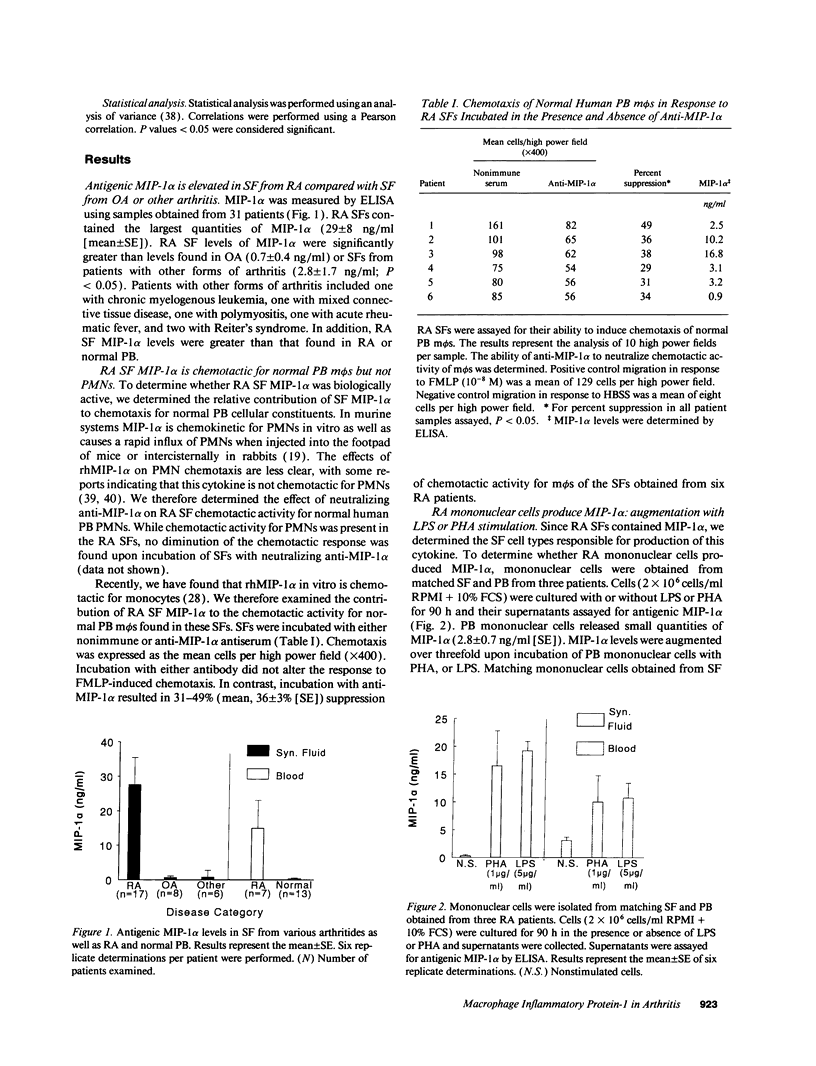
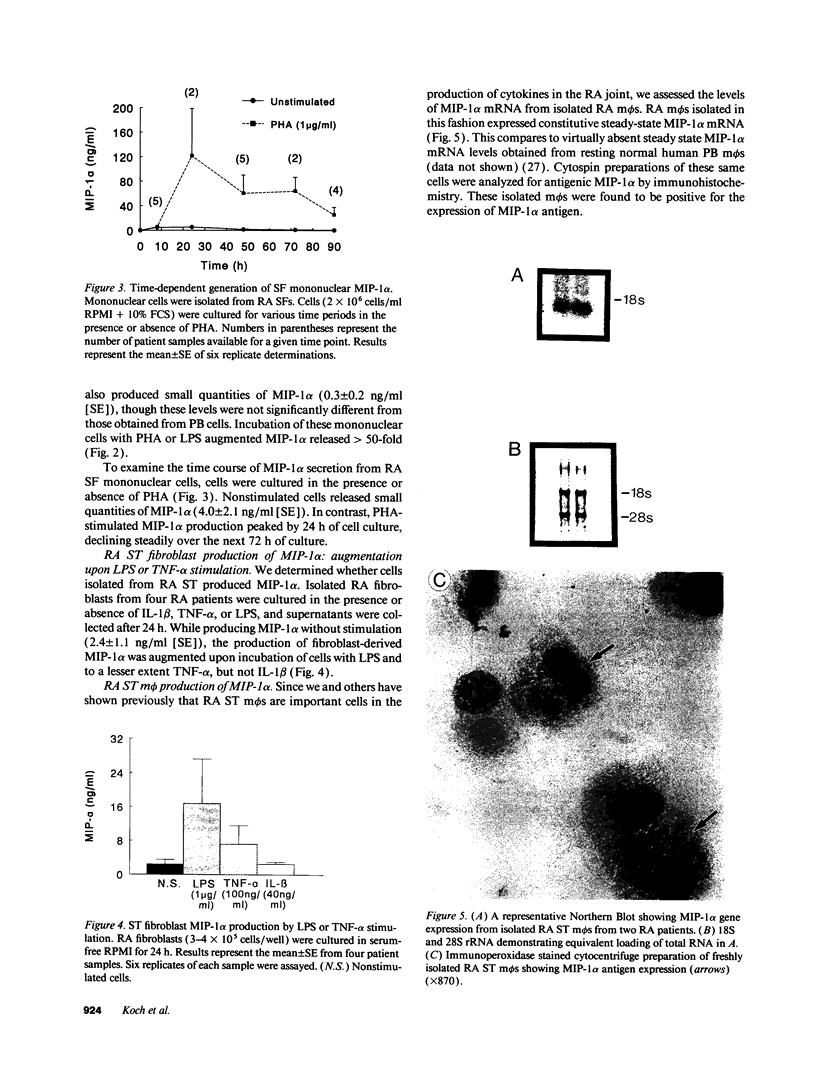
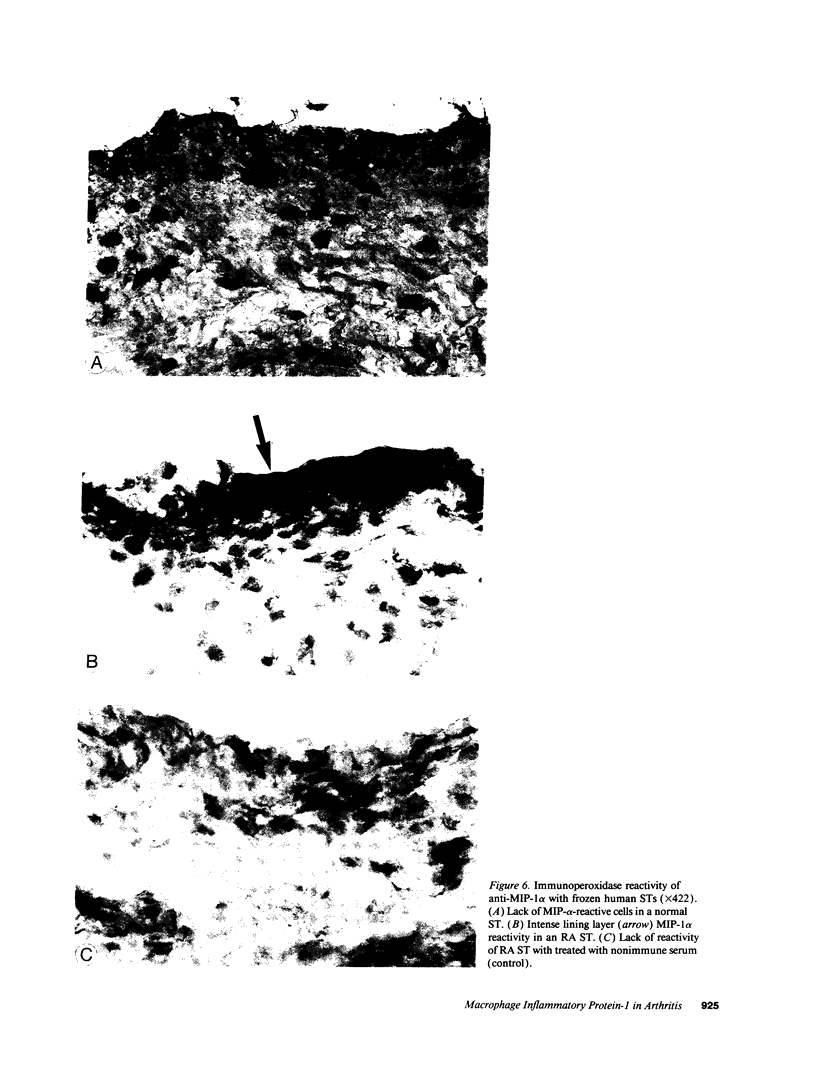
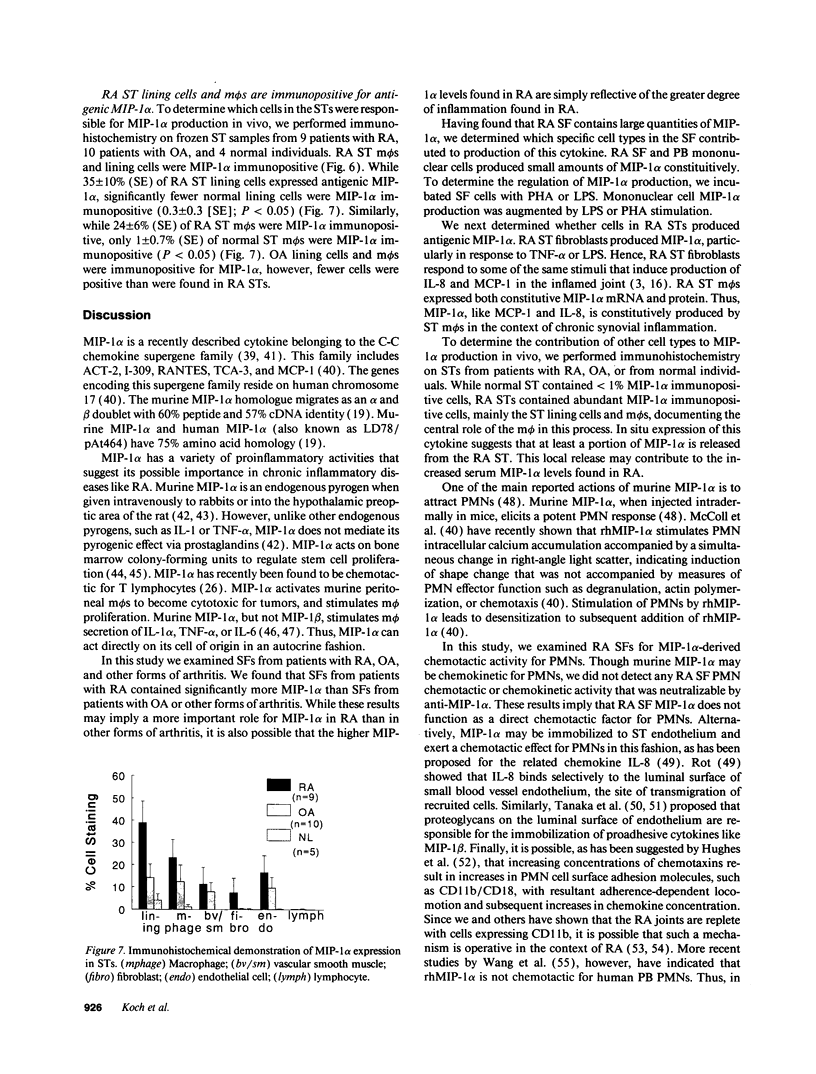
We have shown that human macrophages (m phi s) play an important role in the elaboration of chemotactic cytokines in rheumatoid arthritis (RA) (Koch, A. E., S. L. Kunkel, J. C. Burrows, H. L. Evanoff, G. K. Haines, R. M. Pope, and R. M. Strieter. 1991. J. Immunol. 147:2187; Koch, A. E., S. L. Kunkel, L. A. Harlow, B. Johnson, H. L. Evanoff, G. K. Haines, M. D. Burdick, R. M. Pope, and R. M. Strieter. 1992. J. Clin. Invest. 90:772; Koch, A. E., P. J. Polverini, S. L. Kunkel, L. A. Harlow, L. A. DiPietro, V. M. Elner, S. G. Elner, and R. M. Strieter. 1992. Science (Wash. DC). 258:1798). Recently, m phi inflammatory protein-1 (MIP-1 alpha), a cytokine with chemotactic activity for m phi s and neutrophils (PMNs), has been described. We have examined the production of MIP-1 alpha using sera, synovial fluid (SF), and synovial tissue (ST) from 63 arthritic patients. MIP-1 alpha was higher in RA SF (mean, 29 +/- 8 ng/ml [SE]) compared with other forms of arthritis (2.8 +/- 1.7), or osteoarthritis (0.7 +/- 0.4; P < 0.05). RA SF MIP-1 alpha was greater than that found in either RA or normal peripheral blood (PB) (P < 0.05). Anti-MIP-1 alpha neutralized 36 +/- 3% (mean +/- SE) of the chemotactic activity for m phi s, but not PMNs, found in RA SFs. RA SF and PB mononuclear cells produced antigenic MIP-1 alpha. Mononuclear cell MIP-1 alpha production was augmented with phytohemagglutinin or LPS. Isolated RA ST fibroblast production of antigenic MIP-1 alpha was augmented upon incubation of cells with LPS, and to a lesser extent with tumor necrosis factor-alpha. Isolated RA ST m phi s expressed constitutive MIP-1 alpha mRNA and antigenic MIP-1 alpha. Using ST immunohistochemistry, MIP-1 alpha+ cells from RA compared with normal were predominantly m phi s and lining cells (P < 0.05). These results suggest that MIP-1 alpha plays a role in the selective recruitment of m phi s in synovial inflammation associated with RA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Blum S., Forsdyke R. E., Forsdyke D. R. Three human homologs of a murine gene encoding an inhibitor of stem cell proliferation. DNA Cell Biol. 1990 Oct;9(8):589–602. doi: 10.1089/dna.1990.9.589. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Sherry B., Cooper S., Ruscetti F. W., Williams D. E., Arosio P., Kwon B. S., Cerami A. Macrophage inflammatory protein (MIP)-1 beta abrogates the capacity of MIP-1 alpha to suppress myeloid progenitor cell growth. J Immunol. 1991 Oct 15;147(8):2586–2594. [PubMed] [Google Scholar]
- Davatelis G., Tekamp-Olson P., Wolpe S. D., Hermsen K., Luedke C., Gallegos C., Coit D., Merryweather J., Cerami A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med. 1988 Jun 1;167(6):1939–1944. doi: 10.1084/jem.167.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatelis G., Wolpe S. D., Sherry B., Dayer J. M., Chicheportiche R., Cerami A. Macrophage inflammatory protein-1: a prostaglandin-independent endogenous pyrogen. Science. 1989 Feb 24;243(4894 Pt 1):1066–1068. doi: 10.1126/science.2646711. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey T. J., 3rd, Tracey K. J., Tekamp-Olson P., Cousens L. S., Jones W. G., Shires G. T., Cerami A., Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992 May 1;148(9):2764–2769. [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Firestein G. S., Berger A. E., Tracey D. E., Chosay J. G., Chapman D. L., Paine M. M., Yu C., Zvaifler N. J. IL-1 receptor antagonist protein production and gene expression in rheumatoid arthritis and osteoarthritis synovium. J Immunol. 1992 Aug 1;149(3):1054–1062. [PubMed] [Google Scholar]
- Firestein G. S. The immunopathogenesis of rheumatoid arthritis. Curr Opin Rheumatol. 1991 Jun;3(3):398–406. doi: 10.1097/00002281-199106000-00012. [DOI] [PubMed] [Google Scholar]
- Graham G. J., Wright E. G., Hewick R., Wolpe S. D., Wilkie N. M., Donaldson D., Lorimore S., Pragnell I. B. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990 Mar 29;344(6265):442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- Hughes B. J., Hollers J. C., Crockett-Torabi E., Smith C. W. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J Clin Invest. 1992 Nov;90(5):1687–1696. doi: 10.1172/JCI116041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Synovial localization of tumor necrosis factor in patients with rheumatoid arthritis. J Autoimmun. 1988 Aug;1(4):363–371. doi: 10.1016/0896-8411(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Johnson B. A., Haines G. K., Harlow L. A., Koch A. E. Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 1993 Feb;36(2):137–146. doi: 10.1002/art.1780360203. [DOI] [PubMed] [Google Scholar]
- Kasama T., Strieter R. M., Standiford T. J., Burdick M. D., Kunkel S. L. Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1 alpha. J Exp Med. 1993 Jul 1;178(1):63–72. doi: 10.1084/jem.178.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Burrows J. C., Haines G. K., Carlos T. M., Harlan J. M., Leibovich S. J. Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991 Mar;64(3):313–320. [PubMed] [Google Scholar]
- Koch A. E., Burrows J. C., Marder R., Domer P. H., Leibovich S. J. Reactivity of human tissues with monoclonal antibodies to myeloid activation and differentiation antigens. An immunohistochemical study. Pathobiology. 1990;58(5):241–248. doi: 10.1159/000163594. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Haines G. K., Rizzo R. J., Radosevich J. A., Pope R. M., Robinson P. G., Pearce W. H. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990 Nov;137(5):1199–1213. [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Burrows J. C., Evanoff H. L., Haines G. K., Pope R. M., Strieter R. M. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991 Oct 1;147(7):2187–2195. [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Chensue S. W., Haines G. K., Strieter R. M. Expression of interleukin-1 and interleukin-1 receptor antagonist by human rheumatoid synovial tissue macrophages. Clin Immunol Immunopathol. 1992 Oct;65(1):23–29. doi: 10.1016/0090-1229(92)90243-h. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Harlow L. A., Johnson B., Evanoff H. L., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992 Sep;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Functional heterogeneity of human rheumatoid synovial tissue macrophages. J Rheumatol. 1988 Jul;15(7):1058–1063. [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986 Apr;29(4):471–479. doi: 10.1002/art.1780290403. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Robinson P. G., Radosevich J. A., Pope R. M. Distribution of CD45RA and CD45RO T-lymphocyte subsets in rheumatoid arthritis synovial tissue. J Clin Immunol. 1990 Jul;10(4):192–199. doi: 10.1007/BF00918651. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Davis L. S., Cush J. J., Oppenheimer-Marks N. The role of cytokines in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1989;11(2):123–162. doi: 10.1007/BF00197186. [DOI] [PubMed] [Google Scholar]
- Lotz M., Moats T., Villiger P. M. Leukemia inhibitory factor is expressed in cartilage and synovium and can contribute to the pathogenesis of arthritis. J Clin Invest. 1992 Sep;90(3):888–896. doi: 10.1172/JCI115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. A., Dorf M. E. Differential regulation of interleukin-6, macrophage inflammatory protein-1, and JE/MCP-1 cytokine expression in macrophage cell lines. Cell Immunol. 1991 Jun;135(1):245–258. doi: 10.1016/0008-8749(91)90269-h. [DOI] [PubMed] [Google Scholar]
- McColl S. R., Hachicha M., Levasseur S., Neote K., Schall T. J. Uncoupling of early signal transduction events from effector function in human peripheral blood neutrophils in response to recombinant macrophage inflammatory proteins-1 alpha and -1 beta. J Immunol. 1993 May 15;150(10):4550–4560. [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Goto M., Sasano M., Natsuyama M., Inoue K., Nishioka K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988 Apr;31(4):480–486. doi: 10.1002/art.1780310404. [DOI] [PubMed] [Google Scholar]
- Miñano F. J., Sancibrian M., Vizcaino M., Paez X., Davatelis G., Fahey T., Sherry B., Cerami A., Myers R. D. Macrophage inflammatory protein-1: unique action on the hypothalamus to evoke fever. Brain Res Bull. 1990 Jun;24(6):849–852. doi: 10.1016/0361-9230(90)90150-x. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Peichl P., Ceska M., Effenberger F., Haberhauer G., Broell H., Lindley I. J. Presence of NAP-1/IL-8 in synovial fluids indicates a possible pathogenic role in rheumatoid arthritis. Scand J Immunol. 1991 Sep;34(3):333–339. doi: 10.1111/j.1365-3083.1991.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Pope R. M., Landay A., Modlin R. L., Lessard J., Koch A. E. Gamma/delta T cell receptor positive T cells in the inflammatory joint: lack of association with response to soluble antigens. Cell Immunol. 1991 Oct 1;137(1):127–138. doi: 10.1016/0008-8749(91)90063-h. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Schall T. J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991 May;3(3):165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Seitz M., Dewald B., Gerber N., Baggiolini M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J Clin Invest. 1991 Feb;87(2):463–469. doi: 10.1172/JCI115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Liebler J. M., Burdick M. D., Gilbert A. R., Strieter R. M. Gene expression of macrophage inflammatory protein-1 alpha from human blood monocytes and alveolar macrophages is inhibited by interleukin-4. Am J Respir Cell Mol Biol. 1993 Aug;9(2):192–198. doi: 10.1165/ajrcmb/9.2.192. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Rolfe M. W., Kunkel S. L., Lynch J. P., 3rd, Burdick M. D., Gilbert A. R., Orringer M. B., Whyte R. I., Strieter R. M. Macrophage inflammatory protein-1 alpha expression in interstitial lung disease. J Immunol. 1993 Sep 1;151(5):2852–2863. [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993 Mar;14(3):111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Conlon K., Lloyd A. R., Oppenheim J. J., Kelvin D. J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993 Apr 16;260(5106):355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Villiger P. M., Terkeltaub R., Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992 Jul 15;149(2):722–727. [PubMed] [Google Scholar]
- Wang J. M., Sherry B., Fivash M. J., Kelvin D. J., Oppenheim J. J. Human recombinant macrophage inflammatory protein-1 alpha and -beta and monocyte chemotactic and activating factor utilize common and unique receptors on human monocytes. J Immunol. 1993 Apr 1;150(7):3022–3029. [PubMed] [Google Scholar]
- Widmer U., Yang Z., van Deventer S., Manogue K. R., Sherry B., Cerami A. Genomic structure of murine macrophage inflammatory protein-1 alpha and conservation of potential regulatory sequences with a human homolog, LD78. J Immunol. 1991 Jun 1;146(11):4031–4040. [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Davatelis G., Sherry B., Beutler B., Hesse D. G., Nguyen H. T., Moldawer L. L., Nathan C. F., Lowry S. F., Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988 Feb 1;167(2):570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Hamerman D. Release of interleukin-1 from human synovial tissue in vitro. Arthritis Rheum. 1985 Aug;28(8):853–862. doi: 10.1002/art.1780280804. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Hattori T., Obaru K., Sakai K., Asou N., Takatsuki K., Ohmoto Y., Nomiyama H., Shimada K. Synthesis of a novel cytokine and its gene (LD78) expressions in hematopoietic fresh tumor cells and cell lines. J Clin Invest. 1989 Dec;84(6):1707–1712. doi: 10.1172/JCI114353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum D. E., Esparza L., Dubry S., Benjamin J. B., Volz R., Scuderi P. Characteristics of tumor necrosis factor production in rheumatoid arthritis. Cell Immunol. 1989 Aug;122(1):131–145. doi: 10.1016/0008-8749(89)90154-8. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Balke J., Irving S. G., Kelly K., Siebenlist U. Mitogenic activation of human T cells induces two closely related genes which share structural similarities with a new family of secreted factors. J Immunol. 1989 Mar 1;142(5):1582–1590. [PubMed] [Google Scholar]