Abstract
Introduction
Esophageal cancer consists of two distinct types –esophageal adenocarcinoma (EAC) and squamous cell carcinoma (SCC), both of which differ significantly in their etiology. Freeze dried black raspberry (BRB) has been consistent in its ability to modulate the biomarkers and reduce the incidence of carcinogen-induced SCC in rats. In our previous studies in the esophagoduodenal anastomosis (EDA) model, we have shown that the early modulation of Manganese Superoxide dismutase (MnSOD) significantly correlates with the development of reflux-induced EAC in rats. In this study we looked at the short-term effects of a BRB supplemented diet on the modulation of antioxidant enzymes in reflux-induced esophagitis.
Methods
Male SD rats (8 wo; n=3–5) were randomized into 3 groups- sham-operated, fed control AIN-93M diet (SH-CD), EDA operated and fed either control diet (EDA-CD) or 2.5% (w/w) BRB diet (EDA-BRB). The effect of both reflux and dietary supplementation was analyzed 2 and 4 weeks after EDA surgery.
Results
Animals in EDA groups had significantly lower weight gain and diet intake compared to SH-CD (p<0.05). The sham operated animals, received an average esophagitis score of 0.1 ± 0.1, this increased significantly in EDA –CD animals to 1.8 ± 0.14 (p< 0.001 vs SH-CD) and in EDA-BRB group to 1.7 ± 0.06 (p< 0.001 vs SH-CD), with BE changes also present. However, dietary supplementation of BRB did not alter or ameliorate the grade of esophagitis or the induction of BE. BRB diet caused a 43% increase in MnSOD levels compared to EDA-CD (0.73 ± 0.16; p=0.09), however, this effect was not statistically significant and at 4 weeks, EDA-CD (0.58 ±0.12) showed an increase in MnSOD expression compared to SH-CD (0.34 ± 0.01).
Conclusions
In conclusion, our data suggests that dietary BRB does not increase the levels of cellular antioxidant enzymes or reduce the levels of lipid peroxidation compared to a control diet, in a short-term study of gastroesophageal reflux induction in the EDA animal model. However, it remains to be tested whether this is indicative of its ineffectiveness to inhibit reflux-induced EAC incidence over long-term.
Keywords: Black raspberry, reflux-esophagitis, barrett’s esophagus, esophagoduodenal anastomosis, esophageal adenocarcinoma, superoxide dismutase, cellular antioxidant enzymes
Introduction
Esophageal cancer incidence increased at a rate >300% in the past 3 decades, from 3.8 to 23.3 cases per million[33]. In the year 2000, esophageal cancer contributed to 87,000 and 59,000 person years of life lost (PYLL) due to cancer mortality in men aged under and over 65, respectively. This translated to a total of $22 billion in value of life lost[5, 55]. It is projected that this would increase by 59% in the year 2020, the third highest increase among all cancers, to cost the American economy $35 billion in value[54]. These statistics are important since, esophageal cancer has a higher cost for younger males due to its rapid advancement, late detection and poor prognosis[34,36]. These grim statistics underscore the importance of extensive and urgent research on mechanisms, prevention, detection and therapy of esophageal cancer.
There are 2 distinct types of esophageal cancer. The esophageal squamous cell carcinoma (SCC) has been decreasing in incidence, whereas esophageal adenocarcinoma (EAC) has increased steadily over the last 3 decades[32]. The etiologies of these cancers differ significantly as well. Typically, SCC has been associated with several exogenous factors including smoking, alcohol and other carcinogen exposures, malnourishment along with low body weight, diet lacking in fruits and vegetable and a low socioeconomic status[28]. The strongest risk factor for EAC has been postulated to be gastro esophageal reflux disease (GERD) and its progression to the premalignant lesion- Barrett’s esophagus (BE)[2,4]. Unlike for SCC, quitting tobacco use does not reduce the risk for EAC and alcohol is not strongly associated with EAC risk[6]. Further EAC incidence is also associated with obesity, high meat take and industrialized culture[13,27]. Although, these facts suggests that the two main types of esophageal cancers may differ considerably in their etiology, the low intake of fruits and vegetables is the only risk factor consistently associated with both SCC and EAC[12,29].
Animal models are very helpful in discovering mechanisms of and interventions for a disease. Rat models of SCC typically require carcinogen administration[53], whereas those of EAC require reflux-induction with or without co-carcinogen administration[20]. The research on prevention of EAC using animal models is still nascent. Recently, Hao et al., have shown the efficacy of α-tocopherol and N-acetyl cysteine in the prevention of reflux-induced EAC[11]. In our laboratory we have successfully used the esophagoduodenal anastomosis (EDA) model to induce reflux in male rats and we have consistently shown that EDA surgery produces EAC in 40% of animals within 6 months[25]. A relevant early biomarker for esophageal carcinogenesis is Manganese Superoxide Dismutase (MnSOD), whose levels and activity are significantly reduced 4-weeks after EDA surgery[26], replacement of which results in reduced EAC incidence[30].
Gary Stoner and colleagues have done extensive studies on the effect of freeze dried black raspberries (BRB) in the prevention of carcinogen-induced SCC[47]. It has been consistently and unequivocally shown that dietary BRB at various doses prevents the formation of SCC and reverses the biomarkers of SCC development[37,46]. However, the effects of these berries in a reflux-induced EAC animal model have not been studied. Since, the mechanisms of SCC and EAC development vary we evaluated the effect of dietary BRB on the short-term biomarkers of reflux-induced EAC such as esophagitis, cellular antioxidant enzyme levels and activities, as well as lipid peroxidation markers.
Materials and Methods
Animals and treatment
Male Sprague-Dawley rats (8 weeks old) were purchased from Harlan, Inc. (Indianapolis, IN) and acclimated for a week before surgery. Food and water were provided ad libitum. Animals were randomized into 3 groups and 2 time points as shown in Table 1. The EDA-operation was performed on those that weighed over 300 g as described[24]. Animals were weighed weekly, before and after surgery. At the end of respective treatment periods (2 or 4 weeks) animals were euthanized and the esophagus was collected for analysis. One half of the EDA junction was fixed in 10% buffered formalin for 24 hours, transferred to 80% ethanol and paraffin embedded. The distal part of the esophagus, proximal to the EDA-junction was collected, the mucosal and smooth muscle layers were separated and flash frozen in liquid nitrogen. Only the mucosal layer was used for immunoblots, enzyme activity analysis and lipid peroxidation assays.
Table 1.
Experimental Design
| Diet | Surgery | Time point | n |
|---|---|---|---|
| CD | Sham | 2 4 |
3 3 |
| CD | EDA | 2 4 |
4 5 |
| BRB | EDA | 2 4 |
4 5 |
Eight week old male SD rats were acclimated for a week and then randomized into 6 groups. EDA surgery was performed as described in Materials and Methods. BRB supplementation was started 2 days before the EDA surgery. Body weight gain and diet intake were measured weekly through out the study.
Esophagitis was scored based on the Hetzel-Dent system (Grade 0- Normal appearing mucosa; grade 1- basal cell hyperplasia, mucosal edema, hyperemia, mucosal friability; grade 2- superficial erosion involving <10% of mucosal surface of the last 5 cm of the esophageal squamous mucosa; grade 3- superficial erosions or ulcerations involving 10–50% of the mucosal surface and grade 4- deep peptic ulceration and confluent erosion of >50% of the esophageal squamous mucosa) on 5 random regions for each sample and the scores averaged.
Diet preparation and Feed intake
Freeze dried black raspberry for this study was provided kindly by Dr. Ramesh Gupta (Department of Pharmacology and Toxicology, University of Louisville, KY) and sourced from Van Drunen Farms (Mommence, IL). The AIN-93M diet was supplemented with 2.5% (w/w) BRB as described[1]. The cornstarch and fiber components of the AIN-93M diet were replaced for the berry diet, based on the nutritional information available for black raspberry (http://www.nal.usda.gov/fnic/foodcomp). Previous proximate analysis of the 2.5% BRB diet has shown it to be isocaloric to regular AIN-93M diet[1]. The feed intake was measured for each cage over a period of 5 days and the feed intake/rat/day was calculated as described[1]. This procedure was repeated every week to evaluate diet intake. Berry diet was started 2 days before the EDA surgery, which mimics simultaneous feeding rather than pre- or post-feeding of chemopreventive agent.
Immunoblot analysis
The antibodies for MnSOD and gluatathione peroxidase (GPX) were purchased from Millipore (Temecula, CA) and the antibodies for catalase (CAT) and β-actin was purchased from Sigma Corp. (St Louis, MO). Fresh tissue homogenate of esophageal mucosa was prepared in ice-cold lysis buffer (20 mM HEPES, 1.5 mM MgCl2, 400 mM Nacl and 20% glycerol; pH 7.5) containing protease inhibitors as described[23]. Insoluble cellular material was removed and the total protein levels were determined using a spectrophotometer. Thirty μg of protein was subjected to SDS/PAGE using a 10% gel, followed by transfer to a PVDF membrane. The membrane was blocked with 5% milk, incubated with the primary antibody at a dilution of 1: 2000, 1 h at room temperature and detected with chemiluminescent detection using appropriate secondary antibodies (1:5,000–10,000 dilution depending on previous standardization). The specific protein bands were detected based on their molecular weight with reference to pre-labeled markers and quantitated using UnScanIt software (Silk Scientific Inc., Orem, UT). The average pixel of each protein band was divided by that of the β-actin band so that the expression levels for all proteins were normalized using respective β-actin levels.
Lipid peroxidation assay (Thiobarbituric acid reactive substances-TBARS assay)
TBARS was quantified using an OXItek TBARS assay kit (Zeptometrix Corporation, Buffalo, NY) according to manufacturer’s instruction. The insoluble cellular material that primarily consists of cell membrane components obtained during the protein isolation was used for these analyses and equivalent amount of protein was loaded for each sample. Malondialdehyde (MDA) levels were measured using a spectrophotometer and the MDA concentration of each sample was derived using values from a standard curve.
SOD activity assays
SOD activity was determined using a SOD assay kit-WST (Dojindo Molecular Technologies, Inc., Gaithesburg, MD) as described[22]. This kit uses xanthine-oxidase to generate superoxide radicals which can then react with either the total SOD present in the samples or a water-soluble tetrazolium salt, which then forms a formazan dye that can be detected spectrophotometrically. The total SOD was quantitated based on a standard curve generated concurrently. To selectively measure the MnSOD activity, the Cu/ZnSOd activity was inhibited using KCN (10 μM) and measured simultaneously.
Statistics
All data are represented as mean ± SEM. The weight gain data was compared using a Two-way ANOVA. For the other analyses, the mean value was compared using a one way ANOVA followed by a Tukey’s post hoc test and a p-value < 0.05 was considered significant. All analyses were done using the Graphpad Prizm software (San Diego, CA).
Results
Effect of EDA surgery on short-term weight loss and feed intake
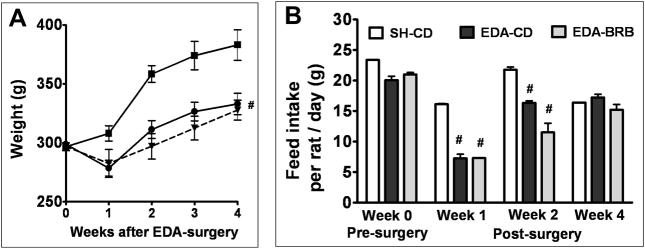
There were no significant differences in initial weight between any groups of animals. Animals that underwent EDA surgery, regardless of the diet, had significant weight loss 1 week following surgery (Figure 1A) and also had significantly lower feed intake (Figure 1B). However, feed intake recovered 2 weeks after surgery (Figure 1B). Although both groups of EDA animals began to gain weight starting at 2 weeks, this weight gain did not match that of the sham surgery group and continued to remain significantly different until 4 weeks (Figure 1A).
Figure 1. Comparison of weight gain (A) and diet intake (B) in rats that underwent EDA surgery.
EDA surgery to induce mixed-reflux was performed in 8 weeks-old male SD rats as described in Materials and Methods. (A) Weight gain and (B) diet intake were measured weekly after surgery and then compared using either two-way ANOVA (weight gain) or one-way ANOVA (diet intake).
#- significantly different from SH-CD (p<0.05).
Effect of BRB supplementation on histopathological changes in the esophagus after EDA surgery
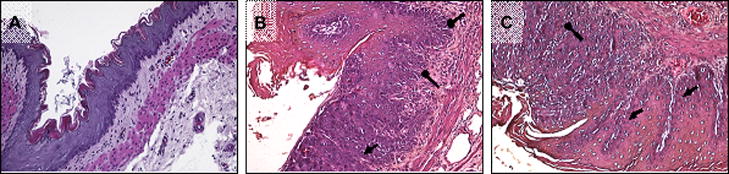
Esophagitis was observed in all EDA animals, regardless of diet, both 2 and 4 weeks after surgery (Figure 2). BE was assessed based on the presence of columnar lined esophagus consisting of absorptive and goblet cells. The sham operated animals, received an average esophagitis score of 0.1 ± 0.1. This increased significantly in EDA –CD animals to 1.8 ± 0.14 (p< 0.001 vs SH-CD) and in EDA-BRB group to 1.7 ± 0.06 (p< 0.001 vs SH-CD). Early BE changes were also present in certain samples (Figure 2B and C; blunt arrows). Dietary supplementation of BRB did not alter or ameliorate the grade of esophagitis or the induction of BE.
Figure 2. Histopathological changes observed in the esophageal epithelium 4 weeks after reflux-induction.
Representative H&E stained sections of esophageal mucosa (200X magnification) from (A) SH-CD –normal mucosa (B) EDA-PD and (C) EDA-BRB. Esophagitis (denoted by triangular arrowhead) and Barrett’s esophagus (blunt arrow head) can be visualized in both EDA-operated groups (B and C).
Effect of BRB supplementation on antioxidant enzyme levels in the esophagus
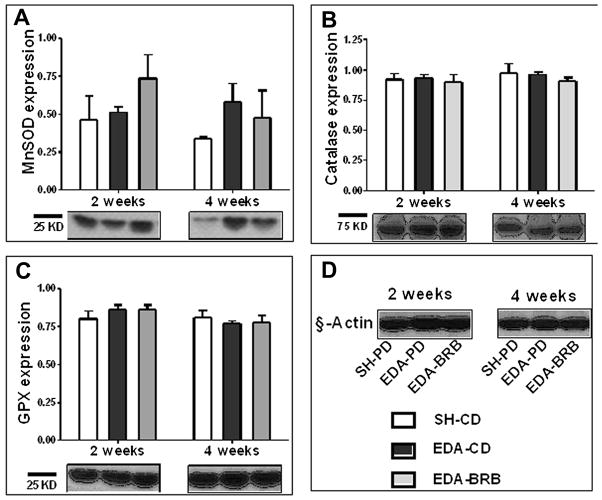
The protein levels of 3 cellular antioxidant enzymes- MnSOD, CAT and GPX were measured using immunoblot analysis. At 2 weeks after surgery, compared to SH-CD (0.46 ± 0.16) group, MnSOD levels were unaltered in EDA-CD (0.51 ± 0.04; Figure 3A). BRB diet caused a 43% increase in MnSOD levels compared to EDA-CD (0.73 ± 0.16; Figure 3A) (p=0.09), however, this effect was not statistically significant. At 4 weeks, EDA-CD (0.58 ±0.12) showed an increase in MnSOD expression compared to SH-CD (0.34 ± 0.01; Figure 3A). This increase was higher than in EDA-BRB (0.48 ± 0.2), but not statistically significant (p = 0.09). EDA surgery did not affect the levels of the other antioxidant enzymes (CAT and GPX) measured at either time points (Figure 3B and C).
Figure 3. Effect of dietary BRB on antioxidant enzyme expression levels in the esophageal mucosa in the presence of reflux.
The enzymes (A) Manganese superodxide dismutase (MnSOD), (B) Catalase and (C) Glutathione peroxidase (GPX) in the esophageal mucosa 2 and 4 weeks after EDA surgery were measured using immunoblot and normalized to tissue β-actin levels (D). SH-CD - sham operated on control diet; EDA-CD – EDA-operated on control diet and EDA-BRB – EDA-operated on BRB diet.
Effect of BRB supplementation on SOD activity
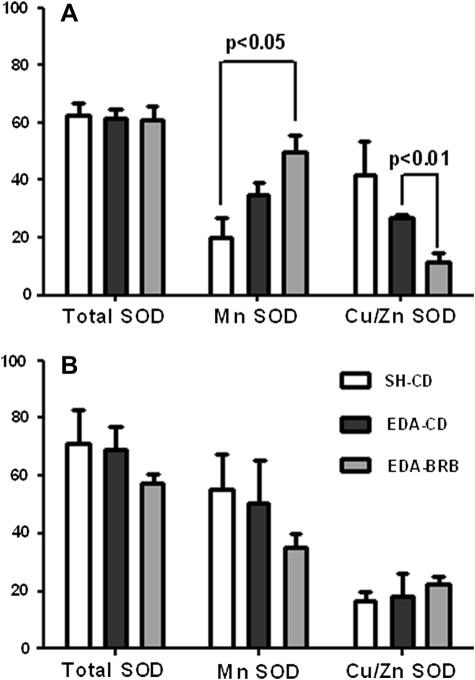
At 2 weeks after EDA-surgery, the total SOD activity was similar among the 3 groups. However, the MnSOD activity mirrored the expression levels and increased with the presence of EDA–surgery. It was the highest in EDA-BRB group and significantly different from SH-CD group (p = 0.02). On the other hand, the reverse trend was seen for Cu/ZnSOD, with EDA-BRB having the lowest activity (Figure 4A). At 4 weeks, there was an insignificant reduction in total SOD activity for the EDA-BRB group (Figure 4B). Once again, the MnSOD activity mimicked the expression level and there was a slight reduction in MnSOD activity for EDA-BRB groups compared to the other 2 (Figure 4B). CuZnSOD activity was the same for all groups at 4 weeks.
Figure 4. Effect of dietary BRB on SOD enzyme activities in the esophageal mucosa in the presence of reflux.
SOD enzyme activities in the esophageal mucosa 2 (A) and 4 weeks (B) after EDA surgery were measured using an SOD assay kit according to attached instructions. Cu/ZnSOD activity was inhibited using KCN (10 μM) and the MnSOD was measured alongside total SOD activity. Cu/ZnSOD activity was then derived by subtracting MnSOD from total SOD activity.
Effect of BRB supplementation on lipid peroxidation
In these studies, reflux induction did not increase lipid peroxidation either at 2 or 4 weeks after surgery. In fact, 4 weeks after EDA-surgery, EDA-PD group had a lower lipid peroxidation level than SH-PD. BRB supplementation significantly increased the measured MDA concentration compared to EDA-PD at 2 weeks (p <0.05), but had no effect at 4 weeks (Figure 5).
Figure 5. Effect of dietary BRB on lipid peroxidation of the esophageal mucosa in the presence reflux.
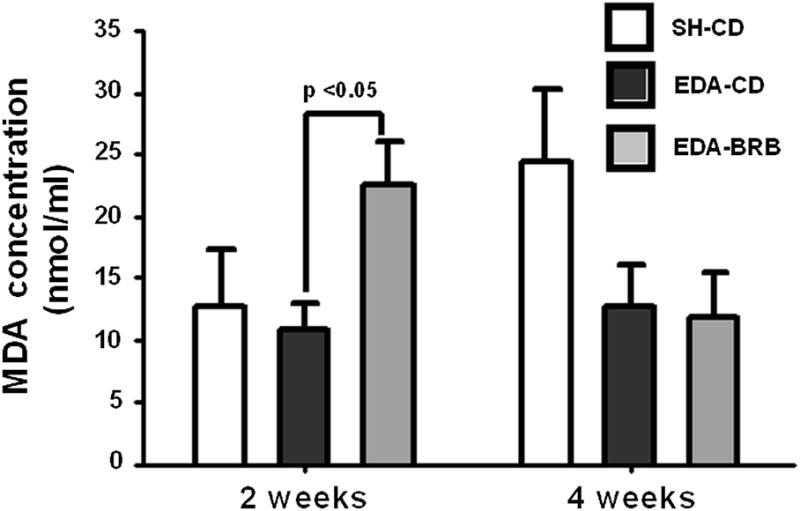
MDA concentration (nmol/ml) was measured in the esophageal mucosa 2 and 4 weeks after EDA surgery using a commercially available TBARS assay kit as described in Materials and Methods.
Discussion
Extensive studies by Stoner and colleagues have shown repeatedly that freeze-dried dietary black raspberry is highly effective in the prevention of N′-nitrosomethyl benzylamine (NMBA)-induced esophageal SCC[1,38,45]. However, the effect of these berries in reflux-induced EAC has not been studied. This is the first study to report the effects of dietary BRB in an animal model of reflux-induced esophagitis. Compared to the control diet, BRB diet did not show any effect on the development of esophagitis. BRB did demonstrate an increased in the level and activity of MnSOD as well as TBARS at 2 weeks, but had no effect on the other antioxidant enzymes (CAT and GPX), with this effect lost at 4 weeks.
BRB diet increased the levels of both MnSOD and TBARS at 2 weeks. There is evidence to suggest that the SOD/CAT ratio plays a role in the ultimate levels of cellular oxidative stress[8] and this oxidative stress may be a precursor to other cellular effects such as apoptosis[15]. Since MnSOD generates the substrate for CAT- H2O2, an oxidant, an increase in MnSOD without a concurrent increase in CAT levels can lead to increased oxidative stress and lipid peroxidation as seen in our results. Nevertheless, this effect is not sustained until 4 weeks, suggesting that this is an early modulatory response to BRB diet. Several reports have shown the effects of various black raspberry phytochemicals such as ellagic acid and anthocyanins on antioxidant enzymes[7,10,19,50]. However, it is difficult to compare our results to these data since studies were done in cell types other than esophageal epithelium, used phytochemicals in black raspberry but extracted from a different food source and exposed to different treatment conditions or methodologies. There are at least 2 human short-term studies that show lack of antioxidant effect in peripheral blood cells when berry juices were provided orally[9,31].
Studies done by Stoner and colleagues are the most relevant to our work. However, these investigators have primarily looked at a dietary BRB dose of 5 and 10% in NMBA-induced squamous cell carcinoma[17,40,44]. Given the decided different animal model and completely different histology, comparisons to Stoners work is limited at best. Also, they have focused primarily on xenobiotic metabolism, signal transduction and cell regulation[18,41,51]. In their recent report analyzing the early molecular changes induced by BRB diet in the esophageal epithelium, in response to NMBA treatment, they do not report any changes in the expression of the antioxidant enzymes SOD, CAT and GPX[42].
Kresty et al., have shown that lyophilized black raspberry reduces the level of urinary 8-epi-prostaglandin-F2α in the BE patients, they also report that BRB had no effect on the length of BE. Further, they show that there is a high variability in the reduction of urinary 8-hydroxyguanosine and do not report changes in the biomarkers of the actual BE mucosa[16]. Dr. Gary Stoner in his most recent review has suggested that this lack of effect may be due to the rapid transit of the berry slurry through the esophagus[39]. The effect of BRB phytochemicals in a preclinical model of mixed gastroesophageal reflux has not yet been shown. Reflux is the operative word, since reports on the effect of ellagic acid and BRB diet on phase I and II enzymes and other antioxidant biomarkers in the esophageal mucosa in vivo are published[41,43,52].
Additional molecular markers, specifically P53 have begun to be evaluated as a potential molecular predictive marker in BE carcinogenesis. P53 expression has been demonstrated as early as 2004, when Segal et al demonstrated pos P53 staining in 39% of cases with IM and 60% with BE, with the degree of dysplasia not presented[35]. This was one of the single highest reported expression of P53 in BE without dysplasia, since more recent reports have demonstrated positive staining in 0% of BE[14], 10% of BE[49], and 62.5% of BE[3]. Thus the true predictive valuate of P53 in BE without dysplasia remains to be proven and was one of the reasons this molecular marker was not studied in this experiment. Similarly the effects of BRB on P53 expression remain to be evaluated.
It is possible that dietary BRB is ineffective in this study due to the following reasons. First, there is ineffective digestion of BRB due to lack of stomach and the rapid transit of food through the EDA junction. Due to the lack of a functional stomach, the EDA junction in this animal model expands to accommodate food storage. It is known that anthocyanins are partially absorbed in the stomach of rats[48]. Thus this lack of stomach may lead to both reduced digestion and absorption of protective phytonutrients, assuming that systemically absorbed phytonutrients provide protective effect on esophageal mucosa. Second, BRB constituents are known to affect enzymes of carcinogen metabolism[50]. Hence the high effectiveness of BRB in the SCC animal model may arise out of its effect on carcinogen metabolism as opposed to its effect on bile/acid reflux. Third, the dose of BRB supplemented may be too low to be effective. Finally, even though BRB has no effect on the antioxidant enzymes, it may be effective through other mechanisms such as inhibition of cell proliferation and induction of apoptosis etc. These biomarkers are yet to be analyzed in our study. Currently, a pilot clinical trial to study the effects of oral BRB on esophageal dysplasia is ongoing (34). The results from this study may contextualize our data regarding the effects of BRB on the esophageal dysplasia or vice versa.
The lack of effectiveness of BRB further underscores the differences in the mechanisms of SCC and EAC development[21]. However, these results are from a short-term study and it remains to be seen whether BRB when fed over longer term is effective in reducing reflux-induced EAC incidence. Also, data on other molecular markers such as apoptosis and cell proliferation, which are altered by BRB, must also be gathered. Currently we are conducting long-term esophageal tumorigenesis studies and analysis of other potential biomarkers to evaluate this.
In conclusion, our data suggests that dietary BRB does not increase the levels of cellular antioxidant enzymes or reduce the levels of lipid peroxidation compared to a control diet, in a short-term study of gastroesophageal reflux induction in the EDA animal model. However, it remains to be tested whether this is indicative of its ineffectiveness to inhibit reflux-induced EAC incidence over long-term.
Acknowledgments
The project described was supported by Award Number R03CA137801 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phillips C. The Curious Rise of Esophageal Adenocarcinoma. NCI Cancer Bulletin. 2006 May 16;3(20):4. [Google Scholar]
- 2.Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. The Annals of thoracic surgery. 2003 Oct;76(4):S1367–9. doi: 10.1016/s0003-4975(03)01202-5. [DOI] [PubMed] [Google Scholar]
- 3.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008 Dec 17;100(24):1763–70. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008 Dec 17;100(24):1755–62. doi: 10.1093/jnci/djn383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds JC, Waronker M, Pacquing MS, Yassin RR. Barrett’s esophagus. Reducing the risk of progression to adenocarcinoma. Gastroenterology clinics of North America. 1999 Dec;28(4):917–45. doi: 10.1016/s0889-8553(05)70098-4. [DOI] [PubMed] [Google Scholar]
- 6.Stahl M, Wilke H. Curative management of adenocarcinoma of the oesophagus--pro multimodal approach. Acta gastro-enterologica Belgica. 2000 Jul–Sep;63(3):309–11. [PubMed] [Google Scholar]
- 7.Altorki NK, Oliveria S, Schrump DS. Epidemiology and molecular biology of Barrett’s adenocarcinoma. Seminars in surgical oncology. 1997 Jul–Aug;13(4):270–80. doi: 10.1002/(sici)1098-2388(199707/08)13:4<270::aid-ssu9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007 Jan;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Lukanich JM. Section I: epidemiological review. Seminars in thoracic and cardiovascular surgery. 2003 Apr;15(2):158–66. [PubMed] [Google Scholar]
- 10.Wargovich MJ, Imada O. Esophageal carcinogenesis in the rat: a model for aerodigestive tract cancer. J Cell Biochem Suppl. 1993;17F:91–4. doi: 10.1002/jcb.240531013. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Martin RC., 2nd Reflux injury of esophageal mucosa: experimental studies in animal models of esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2007;20(5):372–8. doi: 10.1111/j.1442-2050.2007.00713.x. [DOI] [PubMed] [Google Scholar]
- 12.Hao J, Zhang B, Liu B, Lee M, Hao X, Reuhl KR, et al. Effect of alpha-tocopherol, N-acetylcysteine and omeprazole on esophageal adenocarcinoma formation in a rat surgical model. Int J Cancer. 2009 Mar 15;124(6):1270–5. doi: 10.1002/ijc.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Alterations in manganese superoxide dismutase expression in the progression from reflux esophagitis to esophageal adenocarcinoma. Annals of surgical oncology. 2007 Jul;14(7):2045–55. doi: 10.1245/s10434-007-9387-7. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Loss of manganese superoxide dismutase expression and activity in rat esophagus with external esophageal perfusion. Surgery. 2007 Mar;141(3):359–67. doi: 10.1016/j.surg.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Martin RC, Liu Q, Wo JM, Ray MB, Li Y. Chemoprevention of carcinogenic progression to esophageal adenocarcinoma by the manganese superoxide dismutase supplementation. Clin Cancer Res. 2007 Sep 1;13(17):5176–82. doi: 10.1158/1078-0432.CCR-07-1152. [DOI] [PubMed] [Google Scholar]
- 16.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008 Sep;29(9):1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008 May 1;104(1):339–56. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 18.Aiyer H, Srinivasan C, Gupta R. Dietary berries and ellagic acid diminish estrogen–induced mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60(2) doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 19.Stoner GD, Dombkowski AA, Reen RK, Cukovic D, Salagrama S, Wang LS, et al. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both black raspberries and phenylethyl isothiocyanate. Cancer Res. 2008 Aug 1;68(15):6460–7. doi: 10.1158/0008-5472.CAN-08-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dani C, Pasquali MA, Oliveira MR, Umezu FM, Salvador M, Henriques JA, et al. Protective effects of purple grape juice on carbon tetrachloride-induced oxidative stress in brains of adult Wistar rats. J Med Food. 2008 Mar;11(1):55–61. doi: 10.1089/jmf.2007.505. [DOI] [PubMed] [Google Scholar]
- 21.Klamt F, Dal-Pizzol F, Gelain DP, Dalmolin RS, Birnfeld de Oliveira R, Bastiani M, et al. Vitamin A treatment induces apoptosis through an oxidant-dependent activation of the mitochondrial pathway. Cell Biol Int. 2008 Jan;32(1):100–6. doi: 10.1016/j.cellbi.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC, Lee WC. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006 Aug;41(8):797–803. doi: 10.1007/s11745-006-5033-6. [DOI] [PubMed] [Google Scholar]
- 23.Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006 Sep–Oct;26(5A):3601–6. [PubMed] [Google Scholar]
- 24.Li CY, Xu HD, Zhao BT, Chang HI, Rhee HI. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol. 2008 Dec;42(8):683–7. doi: 10.1016/j.alcohol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Vattem DA, Shetty K. Biological functionality of ellagic acid: A review. Journal of Food Biochemistry. 2005 Jun;29(3):234–66. [Google Scholar]
- 26.Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. 2006 Mar;45(2):113–22. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 27.Moller P, Loft S, Alfthan G, Freese R. Oxidative DNA damage in circulating mononuclear blood cells after ingestion of blackcurrant juice or anthocyanin-rich drink. Mutat Res. 2004 Jul 13;551(1–2):119–26. doi: 10.1016/j.mrfmmm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Stoner GD, Aziz RM. Prevention and therapy of squamous cell carcinoma of the rodent esophagus using freeze-dried black raspberries. Acta Pharmacol Sin. 2007 Sep;28(9):1422–8. doi: 10.1111/j.1745-7254.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 29.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001 Aug 15;61(16):6112–9. [PubMed] [Google Scholar]
- 30.Lechner JF, Reen RK, Dombkowski AA, Cukovic D, Salagrama S, Wang LS, et al. Effects of a black raspberry diet on gene expression in the rat esophagus. Nutr Cancer. 2008;60 (Suppl 1):61–9. doi: 10.1080/01635580802393118. [DOI] [PubMed] [Google Scholar]
- 31.Stoner GD, Chen T, Kresty LA, Aziz RM, Reinemann T, Nines R. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr Cancer. 2006;54(1):33–46. doi: 10.1207/s15327914nc5401_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008 Oct 8;269(2):281–90. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett’s esophagus patients. Nutr Cancer. 2006;54(1):148–56. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 34.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila Pa) 2009 Mar;2(3):187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talavera S, Felgines C, Texier O, Besson C, Lamaison JL, Remesy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J Nutr. 2003 Dec;133(12):4178–82. doi: 10.1093/jn/133.12.4178. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Martin RC., 2nd . Apoptosis in Carcinogenesis and Chemotherapy: Esophageal cancer. In: George G, Chen M, editors. Apoptosis in Carcinogenesis and Chemotherapy Netherlands. Springer Publishing; 2009. [Google Scholar]
Reference List
- 1.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60(2):227–34. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 2.Altorki NK, Oliveria S, Schrump DS. Epidemiology and molecular biology of Barrett’s adenocarcinoma. Semin Surg Oncol. 1997;13(4):270–80. doi: 10.1002/(sici)1098-2388(199707/08)13:4<270::aid-ssu9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Binato M, Gurski RR, Fagundes RB, Meurer L, Edelweiss MI. P53 and Ki-67 overexpression in gastroesophageal reflux disease--Barrett’s esophagus and adenocarcinoma sequence. Dis Esophagus. 2009;22(7):588–95. doi: 10.1111/j.1442-2050.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- 4.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008;100(24):1763–70. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008;100(24):1763–70. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown LM. The role of race/ethnicity in the epidemiology of esophageal cancer. J Assoc Acad Minor Phys. 2000;11(2–3):32–7. [PubMed] [Google Scholar]
- 7.Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC, Lee WC. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006;41(8):797–803. doi: 10.1007/s11745-006-5033-6. [DOI] [PubMed] [Google Scholar]
- 8.Dani C, Pasquali MA, Oliveira MR, Umezu FM, Salvador M, Henriques JA, Moreira JC. Protective effects of purple grape juice on carbon tetrachloride-induced oxidative stress in brains of adult Wistar rats. J Med Food. 2008;11(1):55–61. doi: 10.1089/jmf.2007.505. [DOI] [PubMed] [Google Scholar]
- 9.Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, Yap LS, Christen P, Duthie GG. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. 2006;45(2):113–22. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 10.Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006;26(5A):3601–6. [PubMed] [Google Scholar]
- 11.Hao J, Zhang B, Liu B, Lee M, Hao X, Reuhl KR, Chen X, Yang CS. Effect of alpha-tocopherol, N-acetylcysteine and omeprazole on esophageal adenocarcinoma formation in a rat surgical model. Int J Cancer. 2009;124(6):1270–5. doi: 10.1002/ijc.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Hritz I, Gyorffy H, Molnar B, Lakatos G, Sipos F, Pregun I, Juhasz M, Pronai L, Schaff Z, Tulassay Z, Herszenyi L. Increased p53 expression in the malignant transformation of Barrett’s esophagus is accompanied by an upward shift of the proliferative compartment. Pathol Oncol Res. 2009;15(2):183–92. doi: 10.1007/s12253-008-9095-z. [DOI] [PubMed] [Google Scholar]
- 15.Klamt F, Dal-Pizzol F, Gelain DP, Dalmolin RS, Birnfeld de OR, Bastiani M, Horn F, Fonseca Moreira JC. Vitamin A treatment induces apoptosis through an oxidant-dependent activation of the mitochondrial pathway. Cell Biol Int. 2008;32(1):100–6. doi: 10.1016/j.cellbi.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, Fromkes JJ. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett’s esophagus patients. Nutr Cancer. 2006;54(1):148–56. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 17.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, Blackwood M, Stoner GD. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61(16):6112–9. [PubMed] [Google Scholar]
- 18.Lechner JF, Reen RK, Dombkowski AA, Cukovic D, Salagrama S, Wang LS, Stoner GD. Effects of a black raspberry diet on gene expression in the rat esophagus. Nutr Cancer. 2008;60 (Suppl 1):61–9. doi: 10.1080/01635580802393118. [DOI] [PubMed] [Google Scholar]
- 19.Li CY, Xu HD, Zhao BT, Chang HI, Rhee HI. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol. 2008;42(8):683–7. doi: 10.1016/j.alcohol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Martin RC. Reflux injury of esophageal mucosa: experimental studies in animal models of esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2007;20(5):372–8. doi: 10.1111/j.1442-2050.2007.00713.x. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Martin RC. Apoptosis in Carcinogen and Chemotherapy: Esophageal Cancer. In: Chen GC, editor. Apoptosis in Carcinogenesis and Chemotherapy. Netherlands: Springer Publishing; 2009. [Google Scholar]
- 22.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Alterations in manganese superoxide dismutase expression in the progression from reflux esophagitis to esophageal adenocarcinoma. Ann Surg Oncol. 2007;14(7):2045–55. doi: 10.1245/s10434-007-9387-7. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Alterations in manganese superoxide dismutase expression in the progression from reflux esophagitis to esophageal adenocarcinoma. Ann Surg Oncol. 2007;14(7):2045–55. doi: 10.1245/s10434-007-9387-7. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Alterations in manganese superoxide dismutase expression in the progression from reflux esophagitis to esophageal adenocarcinoma. Ann Surg Oncol. 2007;14(7):2045–55. doi: 10.1245/s10434-007-9387-7. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Alterations in manganese superoxide dismutase expression in the progression from reflux esophagitis to esophageal adenocarcinoma. Ann Surg Oncol. 2007;14(7):2045–55. doi: 10.1245/s10434-007-9387-7. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Loss of manganese superoxide dismutase expression and activity in rat esophagus with external esophageal perfusion. Surgery. 2007;141(3):359–67. doi: 10.1016/j.surg.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Lukanich JM. Section I: epidemiological review. Semin Thorac Cardiovasc Surg. 2003;15(2):158–66. [PubMed] [Google Scholar]
- 28.Lukanich JM. Section I: epidemiological review. Semin Thorac Cardiovasc Surg. 2003;15(2):158–66. [PubMed] [Google Scholar]
- 29.Lukanich JM. Section I: epidemiological review. Semin Thorac Cardiovasc Surg. 2003;15(2):158–66. [PubMed] [Google Scholar]
- 30.Martin RC, Liu Q, Wo JM, Ray MB, Li Y. Chemoprevention of carcinogenic progression to esophageal adenocarcinoma by the manganese superoxide dismutase supplementation. Clin Cancer Res. 2007;13(17):5176–82. doi: 10.1158/1078-0432.CCR-07-1152. [DOI] [PubMed] [Google Scholar]
- 31.Moller P, Loft S, Alfthan G, Freese R. Oxidative DNA damage in circulating mononuclear blood cells after ingestion of blackcurrant juice or anthocyanin-rich drink. Mutat Res. 2004;551(1–2):119–26. doi: 10.1016/j.mrfmmm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg. 2003;76(4):S1367–S1369. doi: 10.1016/s0003-4975(03)01202-5. [DOI] [PubMed] [Google Scholar]
- 33.Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg. 2003;76(4):S1367–S1369. doi: 10.1016/s0003-4975(03)01202-5. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds JC, Waronker M, Pacquing MS, Yassin RR. Barrett’s esophagus. Reducing the risk of progression to adenocarcinoma. Gastroenterol Clin North Am. 1999;28(4):917–45. doi: 10.1016/s0889-8553(05)70098-4. [DOI] [PubMed] [Google Scholar]
- 35.Segal F, Kaspary AP, Prolla JC, Leistner S. p53 protein overexpression and p53 mutation analysis in patients with intestinal metaplasia of the cardia and Barrett’s esophagus. Cancer Lett. 2004;210(2):213–8. doi: 10.1016/j.canlet.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Stahl M, Wilke H. Curative management of adenocarcinoma of the oesophagus--pro multimodal approach. Acta Gastroenterol Belg. 2000;63(3):309–11. [PubMed] [Google Scholar]
- 37.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104(1):339–56. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 38.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104(1):339–56. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 39.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila Pa) 2009;2(3):187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoner GD, Aziz RM. Prevention and therapy of squamous cell carcinoma of the rodent esophagus using freeze-dried black raspberries. Acta Pharmacol Sin. 2007;28(9):1422–8. doi: 10.1111/j.1745-7254.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 41.Stoner GD, Chen T, Kresty LA, Aziz RM, Reinemann T, Nines R. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr Cancer. 2006;54(1):33–46. doi: 10.1207/s15327914nc5401_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoner GD, Dombkowski AA, Reen RK, Cukovic D, Salagrama S, Wang LS, Lechner JF. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both black raspberries and phenylethyl isothiocyanate. Cancer Res. 2008;68(15):6460–7. doi: 10.1158/0008-5472.CAN-08-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29(9):1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29(9):1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29(9):1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29(9):1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29(9):1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talavera S, Felgines C, Texier O, Besson C, Lamaison JL, Remesy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J Nutr. 2003;133(12):4178–82. doi: 10.1093/jn/133.12.4178. [DOI] [PubMed] [Google Scholar]
- 49.Vaninetti NM, Geldenhuys L, Porter GA, Risch H, Hainaut P, Guernsey DL, Casson AG. Inducible nitric oxide synthase, nitrotyrosine and p53 mutations in the molecular pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Mol Carcinog. 2008;47(4):275–85. doi: 10.1002/mc.20382. [DOI] [PubMed] [Google Scholar]
- 50.Vattem DA, Ghaedian R, Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pac J Clin Nutr. 2005;14(2):120–30. [PubMed] [Google Scholar]
- 51.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, McIntyre C, Rocha C, Lechner JF, Stoner GD. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila Pa) 2009;2(1):84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, McIntyre C, Rocha C, Lechner JF, Stoner GD. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila Pa) 2009;2(1):84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wargovich MJ, Imada O. Esophageal carcinogenesis in the rat: a model for aerodigestive tract cancer. J Cell Biochem Suppl. 1993;17F:91–4. doi: 10.1002/jcb.240531013. [DOI] [PubMed] [Google Scholar]
- 54.Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008;100(24):1755–62. doi: 10.1093/jnci/djn383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008;100(24):1755–62. doi: 10.1093/jnci/djn383. [DOI] [PMC free article] [PubMed] [Google Scholar]