Abstract
Background
Interactions among known risk factors for retinopathy of prematurity (ROP) remain to be clarified.
Objectives
The aim of this study was to identify risk factors associated with ROP and to explore the interrelationships between prominent risk factors for ROP.
Methods
From an institutional cohort of 1,646 very preterm newborns with gestational age <30 weeks or birth weight <1,501 g, we selected infants with a gestational age <30 weeks who met the criteria for ROP screening (n = 622) for a nested case-control analysis.
Results
Of the 622 eligible newborns, 293 (47%) were diagnosed with ROP. From multivariable analyses, gestational age <26 weeks (OR 2.9, CI 1.7–4.9), oxygen exposure at 28 days (OR 1.7, CI 1.0–2.7), and neonatal sepsis (OR 2.1, CI 1.4–3.2) emerged as prominent risk factors for ROP. Oxygen- associated ROP risk was more prominent among infants of 23–25 weeks’ gestational age, while infection-associated ROP risk was higher among infants born at 28–29 weeks. The OR for the joint effect of all 3 risk factors (23.5) was higher than would have been expected under the additive (8.6) and the multiplicative (16.5) patterns of interaction.
Conclusions
Our study suggests that neonatal sepsis, oxygen exposure, and low gestational age are not only independently associated with a significantly increased risk of ROP, but also interact beyond additive and even multiplicative patterns.
Key Words: Infection, Oxygen, Gestational age, Risk factors, Retinopathy of prematurity
Introduction
Retinopathy of prematurity (ROP) is a disorder of the developing retina [1] and an important and potentially preventable cause of blindness in childhood [2]. Although treatment options are available [3], the prevention of ROP is highly desirable. It is widely acknowledged that ROP is a multi-factorial disorder, with low gestational age, low birth weight, oxygen exposure [4,5,6,7,8], neonatal sepsis [9,10,11], and bronchopulmonary dysplasia [12] being important risk factors. Some of the risk factors appear to contribute to ROP by affecting the systemic cytokine and growth factor milieu [13,14,15].
It has long been suggested that risk factors may interact with each other [16]. Interaction is an important concept in biostatistics [17] and epidemiology [18], where it is also known as ‘effect modification’ [19]. The central idea is that exposure to one risk factor alters the impact of second or third risk factors. However, interaction studies of ROP etiology are sparse [20,21].
We recently hypothesized that immaturity and inflammation-associated risk factors of ROP interact with each other [22]. In this paper, we attempt to identify risk factors associated with ROP and elucidate potential interactions.
Methods
Patients
The parent cohort from which our study population was drawn consisted of 1,646 preterm infants who were born at <30 weeks’ gestational age or had a birth weight <1,501 g and were admitted to the neonatal intensive care unit at the Floating Hospital for Children at Tufts Medical Center (Boston, Mass., USA) during the years 1997–2007. Using our local database for research purposes was approved by the Institutional Review Board of Tufts Medical Center.
Clinical data were collected for submission to the Vermont Oxford Network using their data collection forms. The clinical definitions of demographic characteristics, treatment, and outcome measures are published in current [23] and previous manuals of operations. The definition of oxygen at day 28 was the receipt of any supplemental oxygen on the 28th postnatal day. Early and late bacterial sepsis was defined as a bacterial pathogen recovered from a blood and/or cerebrospinal fluid culture obtained on day 1, 2, or 3 of life, or thereafter, respectively. Coagulase-negative Staphylococcus was defined by the presence of all 3 of the following characteristics: (1) a positive blood culture obtained from either a central line or peripheral blood sample and/or recovered from cerebrospinal fluid obtained by lumbar puncture, ventricular tap, or ventricular drain, (2) signs of generalized infection (such as apnea, temperature instability, feeding intolerance, worsening respiratory distress, or hemodynamic instability), and (3) treatment with intravenous antibiotics for ≥5 days after the above cultures were obtained [24]. As per the Vermont Oxford Network protocol, ‘if the infant died, was discharged, or transferred prior to the completion of 5 days of intravenous antibiotics, this condition would still be met if the intention were to treat for 5 or more days’ [24]. Fungal sepsis was defined as a fungus recovered from a blood culture obtained from either a central line or peripheral blood sample after day 3 of life. Any sepsis was defined as sepsis including any of the pathogens mentioned above.
ROP screening was performed by two attending retinal specialists from 1998 to 2007. These same attending specialists performed the laser treatment. Prior to 1998, the infants were screened by retinal fellows with supervision by attending staff. All lasers were staffed by an attending physician. The stages of ROP were classified according to the International Classification of Retinopathy of Prematurity [25, 26]. The ROP stage noted in our database was the highest stage clinically recorded.
We excluded infants who died in the delivery room. We did not exclude 14 infants with non-ocular congenital anomalies. Of these 14 children, 7 were cases (i.e. had ROP) and 7 were controls (i.e. did not have ROP). From the case-control perspective, 2.4% (n = 7) of the cases and 2.1% (n = 7) of the controls had a non-ocular congenital anomaly. Since these anomalies appear to be unrelated to ROP status, we felt comfortable including these 14 children in our analyses.
A total of 736 infants met the American Academy of Pediatrics criteria for ROP screening and remained in the neonatal intensive care unit for longer than 4–6 weeks’ postnatal age, the time point recommended by these criteria for screening [27,28,29]. In order to avoid the potential biases associated with a sample definition based on birth weight [30], we restricted our sample to 622 infants with a gestational age <30 weeks. Two hundred and ninety-three (47%) babies were diagnosed with ROP (cases) and were compared with 329 infants without ROP (controls).
Data Analysis
We used the Statistical Analysis Software (SAS 9.1) to create logistic regression models that yield odds ratios (OR) and 95% CI. After initial exploratory analyses, we focused on 3 variables: low gestational age (<26 weeks), oxygen exposure (operationalized as exposure to oxygen at 28 postnatal days), and any neonatal sepsis (including early bacterial sepsis, late bacterial sepsis, coagulase-negative Staphylococcus infection, and fungal sepsis). OR for combinations of these variables were calculated to identify interactions among them [31].
The most parsimonious model was built by stepwise deletion of independent variables in decreasing order of their p values. All variables from table 1 were included as a starting point, except oxygen at 36 weeks’ postmenstrual age (PMA), early sepsis, and late sepsis. Since information on oxygen exposure at 36 weeks’ PMA was missing for more than half of the cohort due to transfer to home or to another unit before 36 weeks’ PMA, we created a variable for missing information on oxygen exposure at 36 weeks’ PMA. Early and any late sepsis obviously overlap with ‘any sepsis’ and were not included in the multivariable model to avoid collinearity. Variables were retained in the adjusted model if they were significant at p < 0.05. A process of refitting was performed to facilitate the identification of potential collinearity or confounding among the predictors. We checked model fit with Hosmer-Lemeshow's goodness-of-fit statistic. The final model included variables that were significantly associated with ROP (p < 0.05) and their confounders, i.e. variables that changed the β-coefficient of ≥1 covariable in the final model by ≥10%.
Table 1.
Infant characteristics
| Retinopathy of prematurity |
OR and 95% CI |
|||
|---|---|---|---|---|
| yes (n = 293) | no (n = 329) | crude | adjusted for gestational age <26 weeks | |
| Birth weight < 1,000 g (mean) | 79 (835 g) | 48 (1,016 g) | 4.0 (2.8-5.7) | 2.3 (1.6-3.4) |
| Gestational age <26 weeks (mean) | 42 (25.8 weeks) | 9 (27.4 weeks) | 7.1 (4.6-11.0) | − |
| Male | 53 | 56 | 0.9 (0.6-1.2) | 0.9 (0.6-1.2) |
| Multiple birth | 36 | 29 | 1.4 (1.0-1.9) | 1.8 (1.3-2.6) |
| Outborn | 12 | 16 | 0.7 (0.4-1.1) | 0.6 (0.4-1.0) |
| Hispanic | 14 | 19 | 0.7 (0.5-1.1) | 0.6 (0.4-1.0) |
| Race | ||||
| Black | 15 | 15 | 1.0 (0.6-1.5) | 0.8 (0.5-1.4) |
| White | 78 | 75 | 1.2 (0.8-1.8) | 1.3 (0.9-2.0) |
| Other | 7 | 10 | 0.7 (0.4-1.2) | 0.7 (0.4-1.3) |
| Prenatal care | 99 | 98 | 2.4 (0.6-9.2) | 3.0 (0.7-13.2) |
| Early bacterial sepsis | 3 | 0.3 | 9.2 (1.1-74) | 9.9 (1.2-83.4) |
| Late bacterial sepsis | 22 | 11 | 2.3 (1.5-3.7) | 2.0 (1.3-3.3) |
| Coagulase-negative Staphylococcus | 25 | 12 | 2.4 (1.6-3.7) | 1.9 (1.2-3.0) |
| Late sepsis | 42 | 21 | 2.6 (1.9-3.8) | 2.2 (1.5-3.2) |
| Fungal sepsis | 8 | 2 | 4.4 (1.7-10.9) | 3.3 (1.3-8.9) |
| Any sepsis | 44 | 22 | 2.8 (2.0-4.0) | 2.3 (1.5-3.2) |
| Necrotizing enterocolitis | 13 | 11 | 1.2 (0.7-2.0) | 1.3 (0.7-2.1) |
| Ventilation | 98 | 91 | 4.8 (2.0-11.7) | 3.0 (1.2-7.5) |
| High-frequency ventilation | 48 | 20 | 3.6 (2.5-5.2) | 2.3 (1.6-3.4) |
| Oxygen at day 28 | 87 | 65 | 3.6 (2.4-5.3) | 2.4 (1.6-3.7) |
| Oxygen at 36 weeks' PMA | 54 | 31 | 2.6 (1.7-4.0) | 2.1 (1.3-3.3) |
| Oxygen at 36 weeks' PMA missing data | 20 | 54 | 0.3 (0.2-0.5) | 0.3 (0.2-0.4) |
| Respiratory distress syndrome | 98 | 91 | 5.8 (2.2-15.1) | 4.0 (1.5-10.7) |
| Pneumothorax | 10 | 5 | 2.3 (1.2-4.4) | 1.7 (0.9-3.5) |
| Antenatal steroid | 91 | 88 | 1.4 (0.8-2.3) | 1.3 (0.7-2.2) |
| Postnatal steroid | 27 | 13 | 2.6 (1.7-3.9) | 1.7 (1.1-2.7) |
| Patent ductus arteriosus | 54 | 29 | 2.8 (2.0-3.8) | 2.0 (1.4-2.9) |
| Duct ligation | 13 | 5 | 2.8 (1.5-5.2) | 1.5 (0.8-2.9) |
| Indomethacin | 43 | 22 | 2.6 (1.9-3.7) | 2.0 (1.3-2.9) |
| Surfactant at any time | 97 | 89 | 3.6 (1.8-7.3) | 2.7 (1.3-5.6) |
| Intraventricular hemorrhage (any) | 153 | 139 | 1.5 (1.1-2.0) | 1.4 (0.9-2.0) |
Three-way interactions between the association of low gestational age, oxygen exposure, and any sepsis were evaluated using the Bartlett test for 2 × 2 × 2 tables [32]. Some cells in this scenario might have small numbers with n < 5. The Bartlett test was designed to accommodate exactly this situation. Any cell with n < 5 works well in the Bartlett test (as it does in Fisher's exact test, for example).
Stratified analysis based on gestational age categories was used to explore the relationship between ROP and oxygen or infection exposure. Homogeneity of the OR was assessed using the Breslow-Day test for stratified analysis.
Results
Of the 293 infants with ROP, 125 (43%) were diagnosed with stage 1, 76 (26%) with stage 2, 83 (28%) with stage 3, and 9 (3%) with stage 4 ROP. No child in this cohort had stage 5 ROP.
Univariable Analysis (table 1)
Newborns diagnosed with ROP were more likely than controls to have a birth weight <1,000 g and a gestational age <26 weeks. The impact of birth weight <1,000 g persisted when controlling for gestational age <26 weeks (OR 2.3, CI 1.6–3.4). Multiple birth was associated with a two-fold increased risk for ROP (OR 1.8, CI 1.3–2.6). Gender, Hispanic ethnicity, outborn status, prenatal care and vaginal delivery were not associated with ROP.
Among infants with ROP, early sepsis, late bacterial sepsis, coagulase-negative staphylococcal infection, fungal sepsis and any sepsis were observed more frequently than in the control group. We did not find an association between necrotizing enterocolitis and ROP.
Infants with ROP were much more likely than controls to have received respiratory support and to be exposed to oxygen at 28 days and at 36 weeks’ PMA. The absence of information about oxygen at 36 weeks’ PMA due to the child's discharge before this age was associated with a prominently decreased risk. Moreover, ROP cases had more patent ductus arteriosus and indomethacin treatment than controls.
After adjusting for gestational age <26 weeks, respiratory distress syndrome (OR 4.0, CI 1.5–10.7), mechanical ventilation (OR 3.0, CI 1.2–7.5), and the need for high frequency ventilation (OR 2.3, CI 1.6–3.4) were strongly associated with ROP. Both oxygen at 28 postnatal days and oxygen at 36 weeks’ PMA were significantly associated with ROP (OR 2.4, CI 1.6–3.7 and OR 2.0, CI 1.3–3.3, respectively).
Postnatal steroid use was associated with a two-fold increased risk of ROP (OR 1.7, CI 1.1–2.7), while patent ductus arteriosus and indomethacin were associated with a two-fold increase in the risk of ROP (OR 2.0, CI 1.4–2.9 and OR 2.0, CI 1.3–2.9, respectively). Surfactant use at any time was associated with a three-fold increased risk of ROP (OR 2.7, CI 1.3–5.6). We found no significant association between pneumothorax, duct ligation and antenatal steroid use and ROP. Intraventricular hemorrhage was associated with ROP at the univariable level, but not when adjusted for gestational age.
Multivariable Analysis (table 2)
Table 2.
Multivariable logistic regression model predicting any ROP with confounders
| Variables | OR and 95% CI | p value |
|---|---|---|
| Birth weight <1,000 g | 2.2 (1.4-3.4) | 0.0003 |
| Gestational age <26 weeks | 2.9 (1.7-4.9) | <0.0001 |
| Multiple birth | 2.1 (1.4-3.2) | 0.0004 |
| Any sepsis | 2.1 (1.4-3.2) | 0.0003 |
| High-frequency ventilation | 1.7 (1.1-2.6) | 0.0106 |
| Oxygen at 28 days | 1.7 (1.0-2.7) | 0.0347 |
| Patent ductus arteriosus | 1.7 (1.1-2.5) | 0.0104 |
| Black | 1.2 (0.7-2.1) | 0.4676 |
| Other race | 0.8 (0.4-1.5) | 0.4399 |
| White | ref. | − |
| Years 2003-2007 | 1.0 (0.7-1.5) | 0.9079 |
| Years 1997-2002 | ref. | − |
Twenty-seven variables served as a starting point for our most parsimonious model for ROP that included only significant factors for ROP (any grade). The final model retained birth weight <1,000 g, gestational age <26 weeks, multiple birth, any sepsis, high-frequency ventilation, oxygen at day 28 and patent ductus arteriosus as significant predictors, with race as a confounder.
Interaction (table 3)
Table 3.
Three-way interaction of low gestational age, oxygen at 28 neonatal days, and any sepsis for ROP
| ROP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Gestational age <26 weeks | Oxygen at 28 days | Any sepsis | yes, n | no, n | yes, % | Bartlett interaction test p value | Expected OR under multiplicative assumption | OR1 |
| 1 | + | + | + | 60 | 11 | 85 | 0.46 | 16.5 | 23.5 |
| 2 | + | + | − | 61 | 16 | 79 | <0.0001 | 3.9 | 16.4 |
| 3 | + | − | + | 2 | 1 | 67 | 0.0149 | 6.6 | 8.6 |
| 4 | + | − | − | 1 | 3 | 25 | 1.4 | ||
| 5 | − | + | + | 53 | 46 | 54 | 0.22 | 11.8 | 5.0 |
| 6 | − | + | − | 79 | 137 | 37 | 2.5 | ||
| 7 | − | − | + | 13 | 12 | 52 | 4.7 | ||
| 8 | − | − | − | 23 | 99 | 19 | ref. | ||
From univariate comparisons of children with one of the first 7 combinations of characteristics to infants without any of these.
We looked at all possible combinations of the 3 risk factors gestational age <26 weeks, oxygen exposure at 28 days, and sepsis in comparison to infants who were not exposed to any of the three. The observed OR for the joint effect of all 3 risk factors (23.5) was considerably higher than expected under the additive (8.6) and multiplicative pattern (16.5) of interaction.
An examination of each of the two-factor interactions showed that the joint effect of gestational age <26 weeks and oxygen exposure was significantly higher than would have been expected under the multiplicative model (16.4 compared to 3.9). The joint effect of gestational age <26 weeks and any sepsis was higher than the expected multiplicative model (8.6 compared to 6.6). The joint effect of oxygen exposure and any sepsis was much lower than expected under the multiplicative model (5.0 compared to 11.8). Hence, the main sub-multiplicative interaction appeared to be the two-way interaction between gestational age <26 weeks and oxygen exposure (p < 0.0001), with the sub-multiplicative interaction between gestational age <26 weeks and any sepsis (p = 0.0149) also contributing significantly.
Stratified Analysis (table 4)
Table 4.
Oxygen exposure at 28 days and any sepsis predicting ROP in strata defined by gestational age at birth/post-menstrual age at 28 days
| Age, weeks | OR and 95% CI | ||
|---|---|---|---|
| gestational | post-menstrual (at 28 days) | oxygen at 28 days | any sepsis |
| 23–25 | 27–29 | 5.9 (1.3-28.3) | 1.6 (0.7-3.5) |
| 26–27 | 30–31 | 1.5 (0.8-2.8) | 1.7 (1.01-3.0) |
| 28–29 | 32–33 | 1.9 (0.9-3.9) | 4.5 (2.2-9.3) |
In stratified analyses, oxygen exposure was associated with a six-fold increased risk of ROP (5.9, 1.3–28.3) in infants with a gestational age <26 weeks, but with a less prominent risk increase in older gestational age strata (26–27 weeks: OR 1.5, CI 0.8–2.8; 28–29 weeks: OR 1.9, CI 0.9–3.9). Conversely, any sepsis was associated with a 4.5-fold risk increase for ROP among infants >27 weeks’ gestation, but not in those 23–27 weeks’ gestation.
‘Multi-Hit’ Analysis
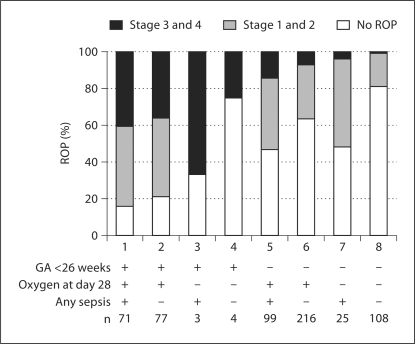
When displayed by the presence or absence of low gestational age (<26 weeks), any sepsis, and oxygen exposure, fully 85% (95% CI for proportion 74–92%) of the newborns in our database who had all 3 ‘hits’ had a ROP diagnosis (fig. 1). Only 19% (13–27%) of the newborns without any of these factors had ROP. Among the 3 factors, gestational age had the most prominent effect for ROP occurrence and progression. The incidence of high-grade ROP tended to decrease with exposure to a decreasing number of these 3 risk factors.
Fig. 1.
Low gestational age, oxygen at 28 day, and any sepsis as risk factors in different combinations for ROP in the whole sample.
Discussion
We observed prominent interaction patterns among the 3 known risk factors of ROP, low gestational age, oxygen exposure, and neonatal sepsis. This is the first report that quantifies these patterns.
Our first major finding is that we confirmed the known strong association between low gestational age, low birth weight, and ROP [33,34,35]. Our finding that neonatal sepsis is a significant risk factor for ROP is also in keeping with previous studies [9, 10, 36, 37]. In our univariable analyses, both bacterial sepsis and fungal sepsis were associated with increased risk of ROP, confirming one meta-analysis [38] and multiple observational studies [10, 11, 36]. As expected based on received knowledge, oxygen exposure was a strong predictor of ROP.
Oxygen Exposure
Our second major finding is that oxygen exposure is more strongly associated with ROP risk among infants with the lowest gestational age than in older infants, probably because low gestational age stands in for increased vulnerability of the developing retina at lower gestational ages.
The first phase of ROP occurs from birth to PMA approximately 30–32 weeks trigged by hyperoxia [39]. Oxygen exposure in this phase suppresses vascular endothelial growth factor (VEGF) expression, resulting in the cessation of normal vessel growth and regression of existing vessels, explaining the longstanding recognition that oxygen exposure in the first few weeks of life increases the likelihood of ROP occurrence [4,5,6, 40,41,42,43,44]. Our finding that the risk of ROP in association with oxygen exposure at 28 days is modified by gestational age at birth (table 4) is likely to be related to the different PMA of infants at 28 days, since the gestational age plus 28 days equals the PMA at which the infant received oxygen. In our data, oxygen exposure loses its strong risk information at around 30–33 weeks’ PMA (table 4).
The second phase of ROP is characterized by hypoxia-induced retinal neovascularization and begins around 32–34 weeks’ PMA [39]. This raises the possibility that supplemental oxygen at this PMA range might improve retinal oxygenation and down-regulate retinal neovascularization in phase II of ROP. Randomized clinical trials and cohort studies indicated that high oxygen supplementation during the second phase of ROP has protective effects [45,46,47,48,49]. Indeed, the STOP-ROP trial showed that higher supplemental oxygen saturation in infants with a mean PMA of 35 weeks reduced disease progression to threshold by 28%, although the 95% confidence limits of this estimate include the null (OR 0.72, CI 0.52–1.01) [46]. We recently performed a meta-analysis on this issue [50] which further supports our results reported here that a risk reduction by supplemental oxygen should probably not be expected until approximately 33 weeks’ PMA. A recent study reported that low oxygen supplementation before 34 weeks’ PMA and higher oxygen supplementation after 34 weeks’ PMA significantly decreased the severity and incidence of ROP [51]. Unfortunately, actual oxygen concentrations for individual babies are not available in our database.
Much of our discussion revolves around the concept of a two-stage etiology of ROP. Even if this concept was unequivocally accepted, it would not need to invoke a clear-cut threshold between the two phases of ROP at a certain fixed PMA that is similar for all infants. At least part of both phases, and also the transition from the first to the second, are now attributed to the effects of VEGF [52] and insulin-like growth factor I [13, 53]. Thus, developmentally regulated differences among newborns with respect to such growth factors might help explain inter-individual differences regarding the timing of the two phases of ROP, or even simpler, regarding the two biologically different mechanisms that are part of the ROP etiology at different stages of development.
Infection Exposure
Our third major finding is that sepsis yields stronger associations with ROP at higher gestational ages (table 4), probably by standing in for a reduced capability of younger infants to mount a potentially harmful inflammatory response.
Infections and sepsis are frequent complications among preterm infants [54, 55], and both are associated with neurodevelopmental and vision impairment [56]. Fungal sepsis and any (non-specified) sepsis are significant risk factors in preterm newborns, both for threshold and all degrees of ROP [9,10,11, 20, 38, 57, 58]. In our study, the excess relative risk among individuals with both sepsis and low gestational age is greater than the product of the two factors. However, stratified analyses do not support the interpretation of increased vulnerability at lower gestational ages. To the contrary, the sepsis-associated risk of ROP is higher at older gestational ages. It is possible that the capability of mounting an inflammatory response to neonatal sepsis is developmentally regulated and that it is not the infection but the inflammation that contributes to ROP occurrence. For example, at least part of the protective effect of omega-3-polyunsaturated fatty acids in an experimental model of ROP is mediated through a down-regulation of the inflammatory cytokine tumor necrosis factor-α [59].
Interaction
Our fourth major finding is that the 3 risk factors (gestational age <26 weeks, oxygen exposure and any sepsis) have a joint effect that exceeds what would be expected if the individual effects were multiplicative (defined as the mathematical product of the potential interacting risk factors’ OR). Further exploration revealed that the main-sub-multiplicative interaction appeared to be the two-way interaction between low gestational age and oxygen exposure, with the sub-multiplicative interaction between low gestational age and any sepsis also contributing significantly.
Interestingly, the co-occurrence of oxygen exposure and any sepsis was associated with a risk for ROP smaller than expected under the multiplicative assumption, indicating that either exposure might reduce the effect of the other. One possibility is that timing of oxygen administration fell into the second phase ROP for some infants exhibiting its potentially protective effects. Another possibility is that such an effect might be similar to the protection by hyperbaric oxygen from sepsis mortality via an interleukin-10-dependent mechanism in mice [60].
One major disadvantage of our study is that it is a secondary analysis of an existing single center database, designed for outcome research and quality control. Thus, the variables available to us lack the detail desirable for more thorough analyses. Also, the sample size of n = 622 is too small to model interactions in a more elaborate fashion. The database incorporates infants who stayed in our neonatal intensive care unit for more than 4–6 weeks and who were assessed for ROP. Our results might be biased because we did not include infants who were transferred to other hospitals before 4–6 weeks of age, the time of ROP screening. There were no practice changes regarding oxygen administration during the duration of the study other than a more pro-active use towards the end of the study period of high-flow nasal cannula. During the study years, the oxygen saturation target range was 92–96% with alarm limits set at 90 and 97%. This policy was in effect until 2008 and this change should, therefore, not affect our sample. Otherwise, there were no new types of ventilators or ventilation techniques brought into the unit and patients were routinely extubated to nasal CPAP. In keeping with these issues, the two halves of the study period were not significantly different with regard to ROP risk (table 2).
In conclusion, our study suggests that neonatal sepsis, oxygen exposure and low gestational age are not only independently associated with a significantly increased risk for ROP, but also interact in a fashion that suggests synergistic effects that go beyond additive and even multiplicative patterns between low gestational age and any sepsis. We also might have detected antagonistic effects between oxygen exposure and sepsis. Finally, we offer data in support of the notion that among very preterm infants, oxygen exposure might be a more prominent risk factor at extremely low gestational ages, while infection appears to gain importance later. Future observational and intervention studies of ROP should consider such interaction patterns.
Acknowledgements
We are grateful to Dr. Lois Smith for helpful comments on this paper. The authors are supported by grants from the National Eye Institute (1R21EY019253-01; O.D.), Deutsche Forschungsgemeinschaft (Da 378/3-1; C.E.L.D.), and the Richard Saltonstall Charitable Foundation (M.C.). The sponsors have no involvement in study design, data collection, analysis and interpretation of the data, the writing of the report or the decision to submit the paper for publication.
References
- 1.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116:15–23. doi: 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 2.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J Aapos. 1999;3:26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 3.Clark D, Mandal K. Treatment of retinopathy of prematurity. Early Hum Dev. 2008;84:95–99. doi: 10.1016/j.earlhumdev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Kinsey VE, Arnold HJ, Kalina RE, Stern L, Stahlman M, Odell G, et al. PaO2 levels and retrolental fibroplasia: a report of the cooperative study. Pediatrics. 1977;60:655–668. [PubMed] [Google Scholar]
- 5.Patz A, Hoeck LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol. 1952;35:1248–1253. doi: 10.1016/0002-9394(52)91140-9. [DOI] [PubMed] [Google Scholar]
- 6.Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F106–F110. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol. 2004;24:164–168. doi: 10.1038/sj.jp.7211067. [DOI] [PubMed] [Google Scholar]
- 8.Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br J Ophthalmol. 1953;37:513–520. doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maheshwari R, Kumar H, Paul VK, Singh M, Deorari AK, Tiwari HK. Incidence and risk factors of retinopathy of prematurity in a tertiary care newborn unit in New Delhi. Natl Med J India. 1996;9:211–214. [PubMed] [Google Scholar]
- 10.Liu PM, Fang PC, Huang CB, Kou HK, Chung MY, Yang YH, et al. Risk factors of retinopathy of prematurity in premature infants weighing less than 1600 g. Am J Perinatol. 2005;22:115–120. doi: 10.1055/s-2005-837276. [DOI] [PubMed] [Google Scholar]
- 11.Manzoni P, Maestri A, Leonessa M, Mostert M, Farina D, Gomirato G. Fungal and bacterial sepsis and threshold ROP in preterm very low birth weight neonates. J Perinatol. 2006;26:23–30. doi: 10.1038/sj.jp.7211420. [DOI] [PubMed] [Google Scholar]
- 12.Holmstrom G, Broberger U, Thomassen P. Neonatal risk factors for retinopathy of prematurity – a population-based study. Acta Ophthalmol Scand. 1998;76:204–207. doi: 10.1034/j.1600-0420.1998.760216.x. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98:5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dordelmann M, Kerk J, Dressler F, Brinkhaus MJ, Bartels DB, Dammann CE, et al. Interleukin-10 high producer allele and ultrasound-defined periventricular white matter abnormalities in preterm infants: a preliminary study. Neuropediatrics. 2006;37:130–136. doi: 10.1055/s-2006-924554. [DOI] [PubMed] [Google Scholar]
- 15.Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163:879–887. doi: 10.1016/S0002-9440(10)63448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucey JF, Dangman B. A reexamination of the role of oxygen in retrolental fibroplasia. Pediatrics. 1984;73:82–96. [PubMed] [Google Scholar]
- 17.Berrington De Gonzalez A, Cox DR. Interpretation of interaction: a review. Ann Appl Stat. 2007;1:371–385. [Google Scholar]
- 18.Kenneth Rothman SG. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 19.Aschengrau A, Seage GR. Essentials of Epidemiology in Public Health. Sudbury: Jones and Bartlett; 2003. [Google Scholar]
- 20.Noyola DE, Bohra L, Paysse EA, Fernandez M, Coats DK. Association of candidemia and retinopathy of prematurity in very low birthweight infants. Ophthalmology. 2002;109:80–84. doi: 10.1016/s0161-6420(01)00841-7. [DOI] [PubMed] [Google Scholar]
- 21.Karlowicz MG, Giannone PJ, Pestian J, Morrow AL, Shults J. Does candidemia predict threshold retinopathy of prematurity in extremely low birth weight (</= 1000 g) neonates? Pediatrics. 2000;105:1036–1040. doi: 10.1542/peds.105.5.1036. [DOI] [PubMed] [Google Scholar]
- 22.Dammann O, Brinkhaus MJ, Bartels DB, Dordelmann M, Dressler F, Kerk J, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009;85:325–329. doi: 10.1016/j.earlhumdev.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Vermont Oxford Network . Manual of operations (for infants born in 2008: release 12.1 2000) Burlington: Vermont; 2008. www.vtoxford.org/home.aspx?p=/tools/downloads.htm. [Google Scholar]
- 24.Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114:348–355. doi: 10.1542/peds.114.2.348. [DOI] [PubMed] [Google Scholar]
- 25.International Committee for the Classification of Retinopathy of Prematurity The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 26.An international classification of retinopathy of prematurity. Pediatrics. 1984;74:127–133. [PubMed] [Google Scholar]
- 27.Screening examination of premature infants for retinopathy of prematurity. A joint statement of the American Academy of Pediatrics, the American Association for Pediatric Ophthalmology and Strabismus, and the American Academy of Ophthalmology. Pediatrics. 1997;100:273. [PubMed] [Google Scholar]
- 28.American Academy of Pediatrics Section on Ophthalmology: Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001;108:809–811. doi: 10.1542/peds.108.3.809. [DOI] [PubMed] [Google Scholar]
- 29.Section on Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–576. doi: 10.1542/peds.2005-2749. erratum in: Pediatrics 2006;118:324. [DOI] [PubMed] [Google Scholar]
- 30.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134:604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 31.Khoury M. Modern Epidemiology. ed 2. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 32.Bartlett MS. Contingency table interaction. J R Stat Soc. 1935;(suppl 2):248–252. [Google Scholar]
- 33.Kim TI, Sohn J, Pi SY, Yoon YH. Postnatal risk factors of retinopathy of prematurity. Paediatr Perinat Epidemiol. 2004;18:130–134. doi: 10.1111/j.1365-3016.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 34.Seiberth V, Linderkamp O. Risk factors in retinopathy of prematurity: a multivariate statistical analysis. Ophthalmologica. 2000;214:131–135. doi: 10.1159/000027482. [DOI] [PubMed] [Google Scholar]
- 35.Brown BA, Thach AB, Song JC, Marx JL, Kwun RC, Frambach DA. Retinopathy of prematurity: evaluation of risk factors. Int Ophthalmol. 1998;22:279–283. doi: 10.1023/a:1006326008909. [DOI] [PubMed] [Google Scholar]
- 36.Chye JK, Lim CT, Leong HL, Wong PK. Retinopathy of prematurity in very low birth weight infants. Ann Acad Med Singapore. 1999;28:193–198. [PubMed] [Google Scholar]
- 37.Gunn TR, Easdown J, Outerbridge EW, Aranda JV. Risk factors in retrolental fibroplasia. Pediatrics. 1980;65:1096–1100. [PubMed] [Google Scholar]
- 38.Bharwani SK, Dhanireddy R. Systemic fungal infection is associated with the development of retinopathy of prematurity in very low birth weight infants: a meta-review. J Perinatol. 2008;28:61–66. doi: 10.1038/sj.jp.7211878. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 40.Flynn JT, Bancalari E, Snyder ES, Goldberg RN, Feuer W, Cassady J, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326:1050–1054. doi: 10.1056/NEJM199204163261603. [DOI] [PubMed] [Google Scholar]
- 41.Wright KW, Sami D, Thompson L, Ramanathan R, Joseph R, Farzavandi S. A physiologic reduced oxygen protocol decreases the incidence of threshold retinopathy of prematurity. Trans Am Ophthalmol Soc. 2006;104:78–84. [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace DK, Veness-Meehan KA, Miller WC. Incidence of severe retinopathy of prematurity before and after a modest reduction in target oxygen saturation levels. J Aapos. 2007;11:170–174. doi: 10.1016/j.jaapos.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderveen DK, Mansfield TA, Eichenwald EC. Lower oxygen saturation alarm limits decrease the severity of retinopathy of prematurity. J Aapos. 2006;10:445–448. doi: 10.1016/j.jaapos.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Deulofeut R, Critz A, Adams-Chapman I, Sola A. Avoiding hyperoxia in infants < or = 1250 g is associated with improved short- and long-term outcomes. J Perinatol. 2006;26:700–705. doi: 10.1038/sj.jp.7211608. [DOI] [PubMed] [Google Scholar]
- 45.McGregor ML, Bremer DL, Cole C, McClead RE, Phelps DL, Fellows RR, et al. Retinopathy of prematurity outcome in infants with prethreshold retinopathy of prematurity and oxygen saturation >94% in room air: the high oxygen percentage in retinopathy of prematurity study. Pediatrics. 2002;110:540–544. doi: 10.1542/peds.110.3.540. [DOI] [PubMed] [Google Scholar]
- 46.Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 47.Gaynon MW, Stevenson DK, Sunshine P, Fleisher BE, Landers MB. Supplemental oxygen may decrease progression of prethreshold disease to threshold retinopathy of prematurity. J Perinatol. 1997;17:434–438. [PubMed] [Google Scholar]
- 48.Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349:959–967. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- 49.Seiberth VLO, Akkoyun-Vardarli I, Jendritza W, Voegele C. Oxygen therapy in acute retinopathy of prematurity stage 3. Invest Ophthalmol Vis Sci. 1998;39:S820. [Google Scholar]
- 50.Chen M, Guo L, Smith L, Dammann C, Dammann O. High or low oxygen saturation and severe retinopathy of prematurity. Pediatrics. 2010;125:e1483–e1492. doi: 10.1542/peds.2009-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009;116:513–518. doi: 10.1016/j.ophtha.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 52.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 53.Hellstrom A, Carlsson B, Niklasson A, Segnestam K, Boguszewski M, de Lacerda L, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87:3413–3416. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 54.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 55.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 56.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 57.Cats BP, Tan KE. Retinopathy of prematurity: review of a four-year period. Br J Ophthalmol. 1985;69:500–503. doi: 10.1136/bjo.69.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussain N, Clive J, Bhandari V. Current incidence of retinopathy of prematurity, 1989–1997. Pediatrics. 1999;104:e26. doi: 10.1542/peds.104.3.e26. [DOI] [PubMed] [Google Scholar]
- 59.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buras JA, Holt D, Orlow D, Belikoff B, Pavlides S, Reenstra WR. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit Care Med. 2006;34:2624–2629. doi: 10.1097/01.CCM.0000239438.22758.E0. [DOI] [PubMed] [Google Scholar]