Abstract
Tandemly arrayed genes that belong to gene families characterize genomes of many organisms. Gene duplication and subsequent relaxation of selection can lead to the establishment of paralogous cluster members that may evolve along different trajectories. Here, we report on the structural variation in MADS AFFECTING FLOWERING 2 (MAF2) gene, one member of the tandemly duplicated cluster of MADS-box-containing transcription factors in Arabidopsis thaliana. The altered gene structure at the MAF2 locus is present as a moderate-frequency polymorphism in Arabidopsis and leads to the extensive diversity in transcript patterns due to alternative splicing. Rearrangements at the MAF2 locus are associated with an early flowering phenotype in BC5 lines. The lack of suppression of flowering time in a MAF2-insertion line expressing the MAF2-specific artificial miRNA suggests that these MAF2 variants are behaving as loss-of-function alleles. The variation in gene architecture is also associated with segregation distortion, which may have facilitated the spread and the establishment of the corresponding alleles throughout the Eurasian range of the A. thaliana population.
A single, ancestral gene can give rise to several copies in the genome through the process of gene duplication. Gene duplicates can be found either as dispersed copies or in tandem arrays. While dispersed copies of gene duplicates arise commonly from segmental or whole genome duplication events or retroposition, tandem duplicates can be initiated from single-copy genes by repair of double-stranded DNA breakage and multiplied by unequal crossover events (Sturtevant 1925; Jelesko et al. 1999; Blanc et al. 2003; Soltis and Soltis 2003; Narayanan et al. 2006; Slack et al. 2006; Yandeau-Nelson et al. 2006; Kong et al. 2007; Freeling et al. 2008).
Gene duplication leads to redundancy, which relaxes selection pressure and thereby creates a substrate for functional evolution (Gu and Gu 2003; Shakhnovich and Koonin 2006; Wagner 2008). Due to redundancy, the capacity to sustain nonlethal mutations is enhanced in gene families compared to singleton genes (Clark et al. 2007; Armisen et al. 2008). In plants, successful retention of new gene duplicates is biased toward genes that are involved in plant's ability to respond to environmental cues (Harrison and Gerstein 2002; Sakurai et al. 2007; Korbel et al. 2008). Pathogen response genes that belong to the nucleotide-binding site–leucine-rich repeat (NBS–LRR) gene family showcase functional diversification (Mondragon-Palomino et al. 2002). Although long-lived resistance gene polymorphisms have been noted (Stahl et al. 1999), the large size of gene families associated with pathogen defense in plants may facilitate a response to the rapid evolution of resistance to plant defense mechanisms in pathogens (Bakker et al. 2006; Clark et al. 2007).
Gene families frequently display variation between individuals within a species (Redon et al. 2006; Cutler et al. 2007). This is especially true for tandemly arrayed genes (TAGs). Compared to dispersed paralogs, TAG members are more susceptible to copy number expansion and contraction via unequal crossing over (Jelesko et al. 1999; Leister 2004), sequence exchange via gene conversion (Gao and Innan 2004; Mondragon-Palomino and Gaut 2005; Nei and Rooney 2005; Ganley and Kobayashi 2007; Xu et al. 2008), and the formation of chimeric genes (Jelesko et al. 2004; Kuang et al. 2006), thereby creating lineage-specific diversity in TAG regions (Kuang et al. 2004, 2008). Approximately 1 of 700 seeds produced per plant is estimated to be polymorphic in 1 of the ∼1500 TAGs observed in Arabidopsis thaliana (Zhang and Gaut 2003; Gaut et al. 2007).
In plants, development is highly dependent on the environment. One well-known class of developmental regulators is encoded by the MADS-box gene family. One hundred and seven MADS-box family members in A. thaliana are involved in the morphological diversification of floral and root tissues, development of fruit and seeds, and the transition to flowering (Becker and Theissen 2003; Parenicova et al. 2003). Several of these genes affect flowering time, and among these, FLOWERING LOCUS C (FLC) and a small clade of closely related genes called MADS AFFECTING FLOWERING (MAF) encode repressors of flowering. FLC locus is located at the top of chromosome 5, and MAF1, also known as FLOWERING LOCUS M (FLM) or AGAMOUS-LIKE 27 (AGL27) is on chromosome 1. The remaining members of this group, MAF2 through MAF5, are found in a cluster on the bottom of chromosome 5 (Becker and Theissen 2003; Kofuji et al. 2003; Parenicova et al. 2003). The products of these genes share between 55 and 88% amino acid identity and all clade members are alternatively spliced (Ratcliffe et al. 2003; Scortecci et al. 2003; Lempe et al. 2005; Caicedo et al. 2004). The relevance of alternative splicing for normal function of these transcription factors is currently not known.
FLC and the MAFs mediate responsiveness to the environment (Michaels and Amasino 1999; Ratcliffe et al. 2003; Balasubramanian et al. 2006b; Sung et al. 2006). FLC expression is repressed by a long, cold treatment comparable to winter (vernalization), thereby promoting flowering after winter has passed (Michaels and Amasino 1999). Variation in FLC expression is responsible for ∼40% of variation in flowering time in a survey of global A. thaliana accessions (Lempe et al. 2005; Shindo et al. 2005). MAF1 acts predominately to repress flowering under short-day conditions (Scortecci et al. 2003; Werner et al. 2005; Balasubramanian et al. 2006b). MAF2 prevents early flowering in response to short periods of cold, which may be an adaptation to brief cold episodes followed by transient warmer temperatures in the autumn, thereby avoiding initiation of flowering prior to the onset of winter (Ratcliffe et al. 2003). A T-DNA knockout of MAF2 is early flowering, indicating that MAF2 is not completely redundant to other TAG members, MAF3-5. Sequence analysis at the population level suggested purifying selection at MAF4 and MAF5 in contrast to MAF2 and MAF3, which are characterized by a high degree of polymorphism (Caicedo et al. 2009). One common polymorphism at MAF2 locus has been described as structural variation that associates with early flowering in a set of A. thaliana accessions (Caicedo et al. 2009). The extent and functional significance of this structural variation at MAF2 is currently not well understood.
Here, we address the natural diversity, geographic distribution, and functional consequences of the widespread, natural MAF2 allelic series. By screening a large accession population for both gene expression and structural variation, we demonstrate extensive and previously unknown, natural variation at MAF2. Using a backcrossing strategy, we demonstrate that a series of rearrangements at the MAF2 locus are associated with an early flowering phenotype under long-day conditions. Using MAF2-specific, artificial miRNA (amiRNA) knock-down lines, we show that early flowering associated with a rearranged MAF2 allele is conferred by a loss of MAF2 function. In addition, consistent with the widespread geographical distribution, we show that segregation distortion, linked to the MAF locus, favors chimeric MAF2 alleles. Association of MAF2 to a locus capable of distorting the segregation ratio provides an alternative explanation for the widespread distribution of MAF2 structural variants.
MATERIALS AND METHODS
Plant material:
Accessions were obtained from the Arabidopsis Biological Resource Center and are listed in the supporting information, Tables S1 and S2, respectively. maf2, a T-DNA knock-out allele in the Col background derived from the Salk collection (SALK-045625, s206432) was kindly provided by Oliver Ratcliffe, Mendel Biotechnology.
Growth conditions:
Seeds were sterilized with 20% bleach, followed by 70% ethanol, washed in sterile water, plated on half-strength MS medium (Sigma) with 0.7% agarose, and stratified for 3 days at 4°. Seeds were then resuspended in half-strength MS medium and were planted in the corners of square 85-mm pots containing PRO-MIX soil (Plant Products) using a Pasteur pipette. Four seedlings were grown in each pot. Plants were grown under 100- to 150-mmol m−2 s−1 cool-white fluorescent light at 22° in 16 hr light/8 hr dark long-day (LD) or 8 hr light/16 hr dark short-day (SD) cycles in controlled growth chambers. Plants for RNA extraction were stratified at 4° for 3 days and harvested after growth for 8 days at 21° on half-strength MS medium with 0.7% agarose and 1% sucrose under LD.
Genotyping backcrossed and segregating lines:
Backcrossed (BC) lines were generated by crossing UWO, Tu-1, Sha, or Kas-1 accessions to Ler five times. Progeny of successive crosses were grown and their genotypes at the MAF2 locus were determined in each generation by PCR (Table S6 lists primers used in this study). DNA was extracted using the method of Edwards et al. (1991). Cosegregation of the flowering-time phenotype with MAF2 alleles was determined in a BC5 F2 segregating population. After counting leaves, DNA was extracted from individual plants and 2 μl of this suspension was subjected to PCR. A set of three unique primers was designed to diagnostically amplify each MAF2 insertion allele in conjunction with MAF2–Ler in backcross and segregating lines. Control amplifications were conducted with plants of known genotype.
cDNA cloning, sequencing, and predicted protein analysis:
RT–PCR products were cloned into pGEM-T-easy TA vector (Promega) using manufacturer's instructions. Plasmids were introduced into Escherichia coli DH5α by the heat-shock method. Plasmid DNA was isolated from individual clones and sequenced. Sequences were aligned to the genomic region using SIM4 (Florea et al. 1998) and visualized in LalnView (Duret et al. 1996). Protein sequences were predicted with Transeq (Rice et al. 2000) at EBI (Labarga et al. 2007).
Generation of amiRNA lines:
The Web MicroRNA Designer platform (WMD2, http://wmd.weigelworld.org/bin/mirnatools.pl) (Schwab et al. 2006) was used to design an amiRNA specific to MAF2 (At5g65050) transcripts from MAF2–Ler and the MAF2 insertion alleles. The plasmid pRS300 containing mir319a precursor was used as a template to replace the endogenous miRNA and miRNA* sequences by the 21mers of MAF2–amiRNA and MAF2–miRNA* (Schwab et al. 2006). The three overlapping PCR products were generated by using primers 57, 58, 59, and 60. The three PCR products were joined in a single PCR reaction by using the miR319a backbone specific primers (61 and 62). The resulting fragment containing amiRNA and the backbone of miRNA 319a were digested with EcoRI and BamHI and cloned into pJLBLue (−) entry vector. An LR reaction was performed to move this fragment into the binary vector pEarlyGate101. Transgenic plants were selected with phosphoinothricine (BASTA).
Linkage analysis of MAF2–UWO transcript pattern:
The MAF2–UWO transcript pattern was mapped in the F2 generation of a UWO × Col cross. RNA and DNA were collected from 35 individual plants and subjected to either RT–PCR or PCR, as described above. Plants were genotyped at MAF2 locus using primers 44 and 45, at ATTED2 marker using primers 47 and 48, and at EG7F2 using primers 49 and 50. Map distances were determined by MAPMAKER version 3.0 (Lander et al. 1987; Lincoln et al. 1992).
Sequencing of MAF2 insertion alleles:
In the genomic survey, accessions were first analyzed for the 3′ of the UWO insertion allele using primers 7, 8, and 9. The 5′ border of positive accessions was sequenced by amplifying the region of interest with Pfu polymerase (Promega) using primers 15 and 16. The PCR fragments were purified and directly sequenced.
The entire MAF2 region was sequenced on both strands from six accessions representing different insertion classes after PCR amplification using Pfu polymerase (Promega) with overlapping primer sets. Because of sequence similarity with the downstream MAF3 gene, the entire MAF3 insertion region within MAF2 was amplified. This fragment was sequenced directly using primers and PCR conditions reported in Table S6.
Gene expression experiments:
Eight-day-old seedlings were harvested and pulverized in liquid nitrogen. RNA was extracted using the RNeasy kit (Qiagen). RNA (2 μg) was subjected to RT–PCR using the Superscript II reverse transcriptase (Invitrogen) in a 20-μl reaction using manufacturer's instructions. A 2-μl sample of this reaction was subjected to RT–PCR. Amplification of genomic DNA was undetectable using all primer combinations.
Phylogenetic analysis:
A 1128-bp DNA sequence of the MAF3 gene was derived from 20 accessions that tested negatively for the MAF2 insertion allele using PCR primers 55 and 56 or from sequence information previously deposited in GENBANK (PopSet: EU980614–EU980630). The same region of the MAF3 gene was sequenced or acquired from preexisting MAF GENBANK PopSet data from seven accessions bearing the MAF2 insertion allele. Additionally, the paralogous MAF3-derived sequence was acquired from the MAF3 insertion region from eight accessions using sequencing primers reported above or from the MAF GENBANK PopSet. These sequence data were used to generate a UPGMA dendrogram in PHYLIP version 3.66 using 10,000 replicates (Felsenstein 2005).
Statistical analysis:
Allelic Aggregation Index Analysis was performed using the Alleles in Space (AIS) software (Miller 2005). Only coordinates of Eurasian accessions from the genomic survey were used. Differences in rosette leaf number in the BC5 segregating populations were analyzed by ANOVA implemented in PAST version 1.81 (http://folk.uio.no/ohammer/past/index.html). Segregation distortion in BC5 populations was assessed by chi-square analysis.
RESULTS
Natural variation in transcript patterns of MAF2:
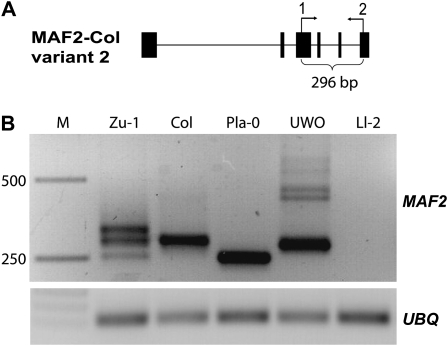
To assess the spectrum and the frequency of natural variation in transcript patterns at MAF2 locus we surveyed the transcript variation across 147 accessions using RT–PCR with primers 1 and 2 (Figure 1A). Approximately half (72/147) of accessions had a banding pattern characteristic of the Col reference strain (Ratcliffe et al. 2003). We grouped the remaining accessions into four additional classes that are named after a representative accession: 16% (23/147) had an additional larger RT–PCR product (exemplified by Zu-1), 9% (13/147) had a single, smaller product (exemplified by Pla-0), 24% (36/147) of accessions had a slightly smaller product accompanied by a series of larger products (exemplified by UWO), and in three accessions (exemplified by Ll-2) we failed to obtain RT–PCR products (Table S1). Upon sequencing the corresponding PCR products in accessions representative for each class, we were able to confirm in all cases, except Zu-1, that these novel bands contain MAF2 sequence (data not shown). Therefore, in 52/147 accessions tested, the major transcript encoded by the MAF2 locus deviates in size and sequence from the MAF2–Col reference allele, indicating that MAF2 harbors extensive natural transcript variation.
Figure 1.—
Expression survey for transcript variation at MAF2. (A) Diagram of the MAF2–Col variant 2 transcript with position of primers used. Primer 1 is specific for the alternatively spliced exon present only in this single MAF2 transcript. Solid boxes, exons; lines, introns. (B) Expression classes observed in the cDNA screen of Arabidopsis accessions. RT–PCR products for MAF2 (top) and ubiquitin, UBQ, (bottom). M, molecular marker; Zu-1; Col (reference); Pla-0, UWO, and Ll-2 are shown as class representatives.
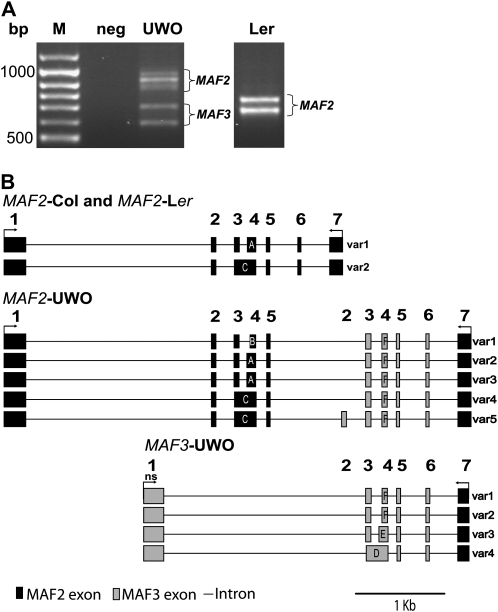
The divergent RT–PCR banding pattern in the UWO class is associated with the production of chimeric transcripts containing both MAF2 and MAF3 sequences. As the UWO class had the greatest number of accessions, we further investigated the nature of MAF2 gene expression characteristic for this set. To ensure that extra bands generated in this class are derived from MAF2 cDNAs, we used another set of primers that specifically amplify two cDNAs in the Col and Ler backgrounds (Figure 2A). As previously seen, numerous extra fragments can be amplified from accessions of this class, suggesting that there is variation at MAF2 transcripts in these accessions (Figure 2A). To determine the identity of amplified products, we cloned and sequenced bands amplified from a representative strain, UWO (Figure 2B). Sequencing revealed that bands in the higher-molecular-weight range, 800–1100 bp, are derived from a chimeric cDNAs containing exonic regions of MAF3 inserted between the penultimate and last MAF2 exons, relative to Col reference sequence (Figure 2B). Alternative splicing, characteristic of both MAF2 and MAF3, resulted in the recovery of five MAF2–UWO splice variants. Surprisingly, the lower-molecular-weight bands, 600 and 750 bp, recovered from the UWO strain were derived from the MAF3 locus, even though the MAF3 cDNAs are not amplified with these primers in Col or Ler. Sequence analysis, however, revealed that the final exon containing the 3′ UTR of the MAF3–UWO is derived from MAF2 (Figure 2B) allowing for the nonspecificity of the PCR reaction in this genotype. Therefore, the divergent RT–PCR banding pattern in this class of accessions is associated with the production of chimeric transcripts containing both MAF2 and MAF3 sequences. In addition to size differences of cDNAs recovered from the MAF2–UWO allele, it is worth noting that the band intensity of RT–PCR products is considerably lighter than that observed in MAF2–Ler. Therefore, a reduced expression level of individual transcripts also accompanies the polymorphism in MAF2–UWO transcripts.
Figure 2.—
Chimeric nature of MAF2 and MAF3 cDNAs from the UWO accession. (A) RT–PCR product profiles of UWO and Ler lines using primers 44 and 45. (B) Exon and intron structure of cDNAs sequenced from MAF2–Col, MAF2–Ler, MAF2–UWO, and MAF3–UWO. Primers 44 and 45 are shown as arrows; ns, nonspecific primer binding site; solid boxes, MAF2 exons; shaded boxes, MAF3 exons. In alternatively spliced regions, exons labeled with the same letter have the same 5′ and 3′ boundaries.
MAF2–MAF3 chimeric transcripts are generated from a rearranged locus in the UWO class:
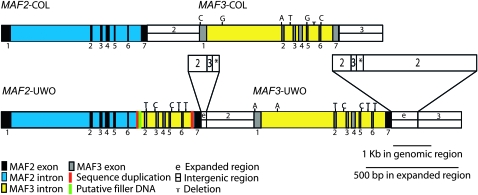
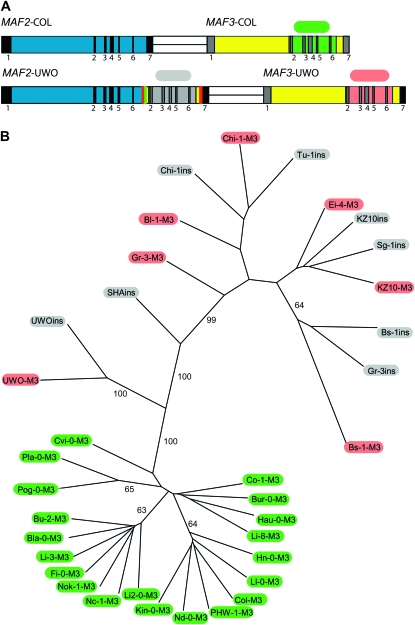
Rearrangements at the MAF2 and MAF3 loci have been recently described in accessions of A. thaliana (Caicedo et al. 2009). Therefore, we wondered whether the transcript pattern we observed could be due to a rearrangement of MAF2 and MAF3 in the UWO strain. Sequencing revealed a 1371-bp insertion of MAF3 genomic sequences into the final intron of MAF2 (Figure 3). In addition, consistent with the chimeric sequence of MAF3–UWO cDNAs, the final exon and the 3′ UTR of MAF3–UWO were replaced by the corresponding MAF2 sequences (Figure 3). While MAF2–UWO is similar in its structure to the MAF2 rearranged alleles previously reported by Caicedo et al. (2009), MAF2–UWO does not correspond to any of the six MAF2 allelic groups described. Thus, MAF2–UWO allele is a newly identified form of the MAF2 insertion allele.
Figure 3.—
Configuration of the MAF2–UWO and MAF3–UWO loci. MAF2–UWO has an insertion of 1371 bp of MAF3 genomic DNA into the last intron. Polymorphisms differentiating MAF3–Col from MAF2–UWO, and MAF3–UWO are shown above. The expanded regions reveal rearrangements downstream of MAF2–UWO and MAF3–UWO. Numbers 2 and 3 refer to DNA sequence homologous to the downstream region of MAF2–Col or MAF3–Col, respectively; * refers to a region that cannot be definitively assigned to MAF2 or MAF3 because of sequence identity.
Chimeric transcripts map at the MAF locus:
The configuration of MAF2–UWO and MAF3–UWO genomic sequences can account for all cDNAs detected in this genotype through a genomic rearrangement combined with alternative splicing. However, both this study and previous study of genetic variations associated with the MAF locus are based on the sequence of contiguous PCR products, which are untethered to a chromosome region (Caicedo et al. 2009). Furthermore, a chimeric transcript containing MAF2 and MAF3 sequences has been reported in the Col-0 background (Ratcliffe et al. 2003). Therefore, in principle, a newly formed MAF2 paralog situated elsewhere in the A. thaliana genome, or a trans-splicing event, could lead to the observed transcription profile. To ensure that the observed pattern is due to the rearrangement at MAF2 locus, we mapped the novel transcript pattern. Analysis of UWO × Col-0 F1 plants revealed that this transcript pattern is codominant (Figure S1). We next tested linkage between the chimeric banding pattern and MAF2 using ATTED2 and EG7F2 markers that are linked to the MAF locus (Figure S2). The RT–PCR profile was linked to these markers, indicating that transcript variation is controlled in cis, arising as a result of the rearrangement at the MAF2 locus.
A series of rearranged MAF2 alleles in A. thaliana populations:
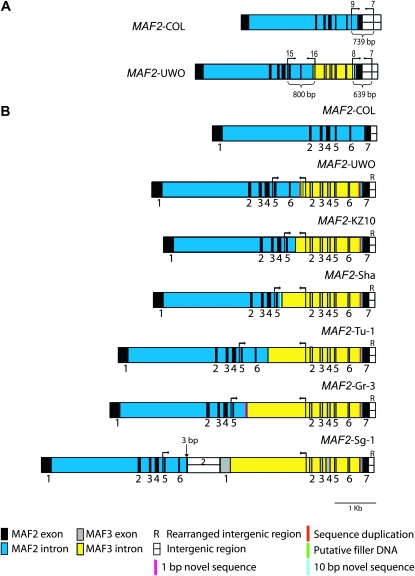
Discovery of the novel MAF2 insertion allele in a screen of 147 accessions suggests that there may be even greater genetic diversity than previously reported (Caicedo et al. 2009). To determine the frequency of the novel MAF2–UWO insertion allele and to discover additional insertion alleles, we surveyed 323 accessions for diagnostic PCR fragments at the 5′ and 3′ boundary of the insertion. We used primers 7, 8, and 9 to amplify DNA at the 3′ boundary of the insertion (Figure 4A). These primers are expected to generate a 639-bp fragment at MAF2–UWO and a 739-bp fragment in MAF2–Col. All 31 accessions initially identified in the expression screen that had a similar RT–PCR pattern as UWO tested positively for the 639-bp, 3′ boundary fragment. Sixty-one accessions belonging to other RT–PCR classes were also retested; they all amplified the 739-bp, Col-like fragment. The congruence between the genomic and RT–PCR results indicates that our assay is diagnostic for the MAF2 insertion allele. Of 225 accessions not previously tested, 127 accessions amplified the 739-bp Col-like fragment and 98 amplified the 639-bp fragment at the 3′ insertion boundary. Therefore, a total of 129 out of 317 accessions, or 41%, had the insertion allele.
Figure 4.—
A natural series of MAF2 insertion alleles. (A) Schematic of MAF2–Col and MAF2–UWO genomic regions showing primers used in the genomic survey. (B) Natural MAF2 allelic series based on the genomic DNA sequence of insertion allele subclasses. Genomic regions of the subclasses are aligned at the 3′ end to demonstrate length variability in MAF3 insertion region. Binding sites of primers 15 and 16 are depicted as arrows. Exons of MAF2 variant 1 (black boxes) and MAF3 variant 1 (gray boxes) are depicted for reference purposes.
The identified 129 accessions were further tested for the presence of the MAF3 insertion by amplification and sequencing of the fragment generated with primers 15 and 16 at the 5′ boundary of the insertion (Figure 4A). All 129 accessions also contained this fragment, suggesting that these accessions contain insertions of MAF3 into the MAF2 genomic region. However, the size of amplified genomic fragments was not uniform. Eighteen accessions yielded the fragment at the 5′ boundary of the insertion that was identical in size and sequence to the MAF2–UWO. Sequencing PCR fragments from the remaining accessions revealed the presence of different MAF3 inserts into MAF2, sequestering the 129 accessions into six subclasses (Tables S2 and S3). All accessions within a single subclass have an identical sequence of the PCR fragment amplified at the 5′ boundary of the insertion.
Subsequent sequencing of the entire MAF2 genomic sequence of a representative accession from each of the six groups provided insight into the topology of these MAF2 alleles (Figure 4B). The five additional alleles have longer insertions of MAF3 into the last intron of MAF2 compared to MAF2–UWO. The MAF3 insertion is accompanied by deletions of varying lengths of the MAF2 sequences adjacent to the 5′ insertion boundary (Table S3). The coincident insertions of MAF3 sequences and deletions of MAF2 sequences create divergent product sizes with primers 15 and 16 (Figure 4B). Two of six identified alleles, from the Sha and Gr-3 subgroups, were previously identified as the S2 and S4 insertion types, respectively, by Caicedo et al. (2009). However, the UWO, Sg-1, Tu-1, and KZ10 alleles identified four new MAF2 allelic subclasses.
Geographic distribution of the MAF2 insertion allele class and subclasses:
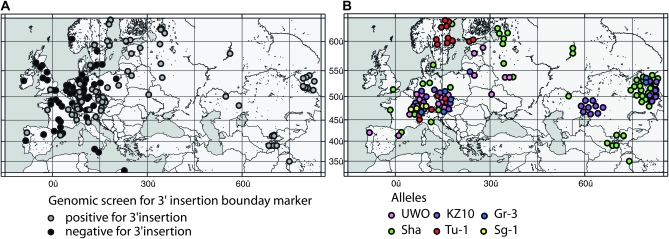
Inclusion of accessions collected throughout the Eurasian range of Arabidopsis allowed us to study the allelic distribution of both allelic classes and subclasses. MAF2 insertion alleles were found predominately in accessions from the central and eastern Eurasian range (Figure 5A, shaded circles). Interestingly, each subclass also had a distinct geographical distribution (Figure 5B). The Sha subclass is ubiquitous across Eurasia with greater representation among accessions in the eastern Eurasian range (Figure 5B). In contrast to the wide distribution of the Sha subclass, the Sg-1 subclass is confined to the Alps region, while the KZ-10 and Gr-3 subclasses appear to be confined between 45° and 55° north latitude. The UWO subclass is mostly present in central Europe, and the Tu-1 subclass has a disjunct distribution with population centers in the Alps and Northern Scandinavia. Allelic Aggregation Index Analysis (Miller 2005) showed significant clustering of all allele subclasses, except the Sha class (Rave = 17.3122, P ≤ 0.048*; RSha = 26.307, P = 0.207). Thus, MAF2 structural variation has a strong tendency to exhibit population structure, suggesting that these alleles may be phylogenetically related.
Figure 5.—
Geographic distribution of accessions surveyed. (A) Location of accessions testing negatively (black circles) and positively (gray circles) for the MAF3 insertion in MAF2. One dot often signifies more than one accession due to proximity of collection locations. (B) Subclasses of the MAF2 insertion allele class. Accessions were grouped on the basis of sequence data from the 5′ insertion boundary and mapped. Circles are slightly shifted where accession locations overlap.
To test the phylogenetic relatedness among MAF2 insertion alleles, we compared MAF3 sequences originating from the MAF3 locus itself and/or from the paralogous MAF3 sequences inserted into the MAF2 (Figure 6). Accessions were chosen randomly and, combined with preexisting accession sequence data, represent a set with a broad distribution across the A. thaliana range.
Figure 6.—
The MAF2 insertion alleles are phylogenetically related. (A) Location of MAF3 or MAF3-homologus regions sequenced to generate an UPGMA dendrogram. Sequence sites are designated by a colored oval: green, MAF3 gene sequence proper, from accessions that do not have an insertion of MAF3 into MAF2; pink, MAF3 gene sequence proper, from accessions containing MAF2 insertion allele; gray, MAF3-homologous region derived from the insertion of MAF3 into the MAF2 gene. (B) UPGMA dendrogram of MAF3 and MAF3-homologous sequences. Bootstrap values are reported when they exceed 50%. Colored ovals on branch termini reflect the origin of the MAF3 sequence, as depicted in A.
All MAF3 sequences from noninsertion allele accessions were found in a single, well-supported clade. The MAF3 sequences from the MAF3 region of the insertion allele or the linked MAF3 gene, clustered in another well-supported clade (Figure 6B), suggesting that these accessions share a common ancestor and overlapping geographic distribution of the allele subclasses. The relatedness of these alleles suggest that formation of the diverse MAF2 alleles was likely a reiterative process resulting in the expansion, via gene conversion, or contraction, via repetitive deletion, of the MAF3 insertion from a single precursor allele.
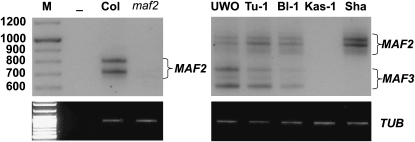
Polymorphism in MAF3 gene is linked to some MAF2 insertion alleles:
The Sha subclass of the MAF2 insertion alleles is the most numerous, and thus we characterized it further. Using primers 44 and 45, a canonical RT–PCR banding pattern can be obtained for an accession in this subclass, as exemplified by the Bl-1 (Figure 7). However, Sha and Kas-1 accessions displayed variation in their RT–PCR bands. Sha is lacking bands corresponding to rearranged MAF3 RT–PCR products, and Kas-1 expresses neither MAF2 nor MAF3 RT–PCR bands. Further analysis established that the MAF3 locus is deleted in both Sha and Kas-1 (Figure S3A). The deletion of this region, which is supported by RT–PCR data, was not detected in the published sequence of MAF2–Sha (Caicedo et al. 2009). In addition, MAF2–Kas-1 has a 466-bp internal deletion (Figure S3B). It is unclear if this deletion prevents the expression of the MAF2–Kas-1 RNA; however, the RT–PCR primer sequences are intact. MAF2–Sha and MAF2–Kas-1 demonstrate that there may be further sequence diversity within the allele subclasses. Thus, putative loss-of-function MAF3 alleles are linked to some MAF2 insertion alleles.
Figure 7.—
Expression of MAF2 and MAF3 across insertion allele subclasses. MAF2 RT–PCR showing expression divergence among Col, UWO, Tu-1, Bl-1, Kas-1, and Sha accessions. RT–PCR was performed with primers 44 and 45.
Proteins encoded by the MAF2 insertion alleles:
To determine the predicted proteins encoded by these novel alleles, we cloned and sequenced cDNAs encoded by MAF2–UWO, MAF2–Tu-1 and MAF2–Sha alleles. Alternative splicing is characteristic for the MAF2 locus, and four splice variants have been reported for the MAF2–Col allele. Of these four transcripts, only two are amplified with the primers 44 and 45 used for the RT–PCR reaction. Corresponding transcripts of MAF2 insertion alleles are all chimeras between MAF2 and MAF3 (Figure S4). Insertion of MAF3 sequences, that are also alternatively spliced, contributed to the proliferation of transcripts generated by MAF2 insertion alleles. Some of the canonical splice sites appear inefficient in the context of the insertion alleles, leading to the synthesis of new splice variants that include changes in transcript composition such as reduction in size of MAF2 exon 4, skipping of MAF2 exon 6, and frequently MAF3 exon 2. However, despite a large transcript sequence dissimilarity of the MAF2 insertion alleles to MAF2–Col, the protein sequences appear more similar due to the incorporation of the stop codon upstream of the MAF3 insertion in most transcripts. MAF2–Col variant 1 is a transcript that encodes the full-length protein (Figure S5). Even though some of the predicted proteins encoded by the MAF2 insertion alleles appear to contain both MADS-box and K-box conserved domains, there is either a truncation or change in the sequence within the K-box domain that may affect the functionality of these proteins even if they are synthesized and stably retained in the cell. Other MAF2 insertion transcripts encode proteins identical or similar to MAF2–Col variants 2, 3, and 4 that encode a truncated protein containing only MADS-box domain. The function of these proteins is currently unknown.
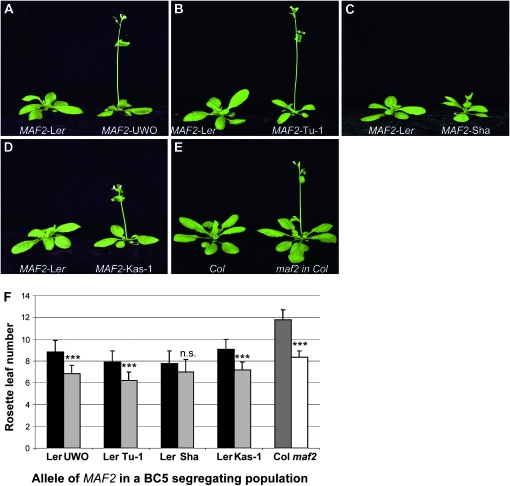
MAF2 insertion alleles confer an early flowering phenotype in BC5 lines:
Given the transcript and predicted protein sequence variation in MAF2 insertion alleles, and its known association with flowering time, we asked whether insertion alleles are linked to variation in flowering time. To assess the effects of these alleles in a common background, we examined the flowering time of BC5 lines, in which the MAF2 alleles of UWO, Tu-1, Sha, and Kas-1 lines had been introgressed into Ler. Plants homozygous for MAF2 insertion alleles flowered with about two leaves less than MAF2–Ler homozygote siblings (Figure 8). Thus, despite differences between the insertion alleles, all BC5 lines displayed an early flowering phenotype under long days. This early flowering phenotype is similar to that observed in the maf2 loss-of-function allele in Col, indicating that MAF2 insertion alleles may not support the normal function of MAF2.
Figure 8.—
Flowering-time comparison of BC5 homozygous lines. Each MAF2 insertion allele line is compared to a MAF2–Ler derived from the same BC5 F2 population. All photos were taken at an equivalent age. (A) MAF2–Ler, MAF2–UWO; (B) MAF2–Ler, MAF2–Tu-1; (C) MAF2–Ler, MAF2–Sha; (D) MAF2–Ler, MAF2–Kas-1; (E) Col, maf2 in Col; (F) flowering time of homozygous plants derived from the BC5 segregating population. Plants were scored for flowering time by counting leaves produced prior to bolting and subsequently genotyped. Homozygous MAF2–Ler progeny, solid bars; MAF2 insertion allele homozygous progeny, light shaded bars; homozygous MAF2–Col plants, dark shaded bars; maf2 in Col plants, open bars. Statistical difference in flowering time of MAF2 insertion allele homozygotes compared to MAF2–Ler homozygotes is indicated at ***P < 0.01 with ± SD.
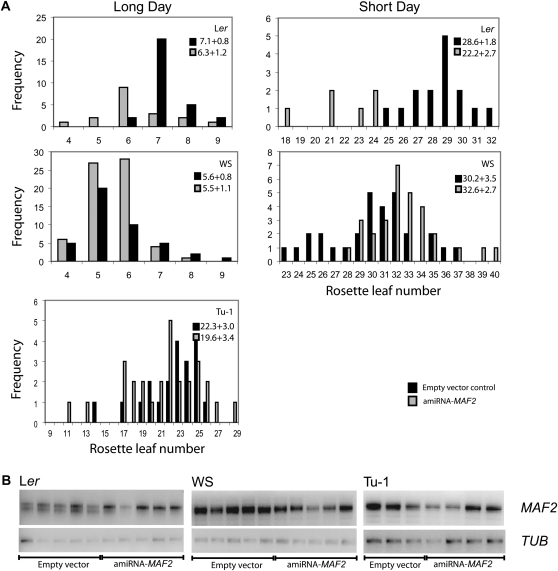
Association of MAF2 insertion alleles with early flowering indicates that they are either loss-of-function alleles or that they are linked to another locus that confers early flowering phenotype. To distinguish between these possibilities we used the accessions Wassilewskija (WS), bearing the MAF2–WS insertion allele belonging to the MAF2–UWO subclass, Tu-1, bearing the MAF2–Tu-1 allele, and Ler, bearing the MAF2–Ler allele, as representatives of the MAF2 insertion and wild-type alleles. Accessions were transformed with an amiRNA construct designed to specifically knock down MAF2 expression. The amiRNAs targeted a cDNA region present in the full-length MAF2–Ler transcript and all transcripts detected in MAF2 insertion allele accessions (see material and methods for details). All T1 amiRNA lines were grown under long- and short-day conditions (Figure 9). The Ler transformants expressing the amiRNA–MAF2 construct had reduced expression of the full-length MAF2 transcript and flowered earlier than the empty vector control line under both LD and SD conditions (LD, P = 0.005**; SD, P = 0.0001***). However, in the MAF2–WS amiRNA or MAF2–Tu-1 amiRNA lines, despite the demonstrated ability of the amiRNA to downregulate MAF2 expression (Figure 9B), the flowering time was indistinguishable from the control plants expressing an empty vector under both LD and SD conditions (WS—LD, P = 0.986 NS; SD, P = 0.140 NS; Tu-1—LD, P = 0.44 NS). Therefore, the MAF2–WS and MAF2–Tu-1 are loss-of-function alleles.
Figure 9.—
MAF2 artificial miRNA (MAF2 amiRNA) does not reduce time to flowering in the WS or Tu-1 accessions. (A) The accession Ler, which bears an active allele of MAF2, and WS and Tu-1, which bear MAF2 insertion alleles, were transformed with an empty vector control construct (solid bars) or the MAF2 amiRNA construct (shaded bars). Flowering time was recorded under long- and short-day conditions as rosette leaf number at flowering. (B) RT–PCR of MAF2 expression, using primers 44 and 45 in Ler, WS, and Tu-1 lines transformed with the empty vector or the MAF2 amiRNA construct.
Segregation distortion at MAF2:
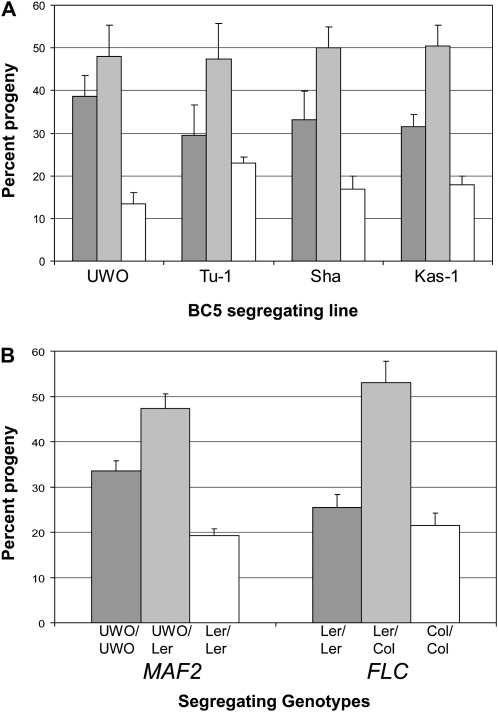
Several studies have reported a flowering-time QTL in the vicinity of the MAF locus, as well as a segregation distortion favoring the early flowering allele (Loudet et al. 2002; El-Lithy et al. 2004, 2006; Simon et al. 2008). To determine the frequency of the insertion alleles relative to MAF2–Ler, we analyzed the BC5 F2 segregating populations. All populations had a higher frequency of insertion alleles than the MAF2–Ler allele (Figure 10A and Table S4). Significant deviations from the predicted 1:2:1 ratio was observed in lines segregating for MAF2–UWO, MAF2–Sha, and MAF2–Kas-1. Segregation distortion at MAF2 was also observed in an independent F2 population derived from the cross MAF2–UWO/MAF2–UWO, FLC–Ler/FLC–Ler in Ler × MAF2–Ler/MAF2–Ler, FLC–Col in Ler /FLC–Col in Ler. The degree of segregation distortion was similar to that previously observed for the MAF2–UWO allele (Figure 10B). However, the segregation ratio for FLC–Col/FLC–Ler in this progeny did not deviate from the expected 1:2:1 ratio (Figure 10B, Table S5), suggesting that MAF2 and FLC segregate independently, as expected, and that segregation distortion is associated with the bottom of chromosome V and not the entire chromosome.
Figure 10.—
Segregation distortion increases the frequency of MAF2 insertion alleles in the progeny ratio. (A) Segregation distortion observed in BC5 F2 populations segregating for various MAF2 insertion alleles and MAF2–Ler. MAF2 insertion allele homozygotes are dark shaded bars, plants heterozygous for a MAF2 insertion allele and MAF2–Ler are light shaded bars, and MAF2–Ler homozygotes are shown as open bars. (B) Segregation distortion of MAF2 and FLC alleles observed in the BC4 F2 progeny of MAF2–UWO in Ler × FLC-Col in Ler. MAF2–UWO homozygotes are dark shaded bars, MAF2–Ler homozygotes are shown are open bars, and heterozygous plants are light shaded bars. FLC–Ler homozygotes are dark shaded bars, FLC-Col homozygotes are open shaded bars, and heterozygous plants are light shaded bars.
DISCUSSION
Common allelic variation underlies flowering-time variation in A. thaliana:
Although several genes that are associated with flowering-time variation have been described for A. thaliana, common allelic variation has been reported only for FRI, FLC, and PHYC (Johanson et al. 2000; Gazzani et al. 2003; Le Corre 2005; Lempe et al. 2005; Balasubramanian et al. 2006a). Most other naturally occurring alleles of flowering-time genes have been found only in single accessions (Aukerman et al. 1997; El-Din El-Assal et al. 2001; Werner et al. 2005; Wang et al. 2007). We found that the MAF2 locus harbors great genetic variability resulting in the generation of transcript complements that differ from the canonical patterns present in Col. Of 147 MAF2 alleles initially tested, 52 displayed alteration in MAF2 transcript sizes. The chimeric alleles at the MAF2 locus were the most numerous. These alleles are associated with early flowering and are present in at least 41% of the strains, providing another example for common alleles contributing to flowering-time variation in A. thaliana. Similar to FRI and FLC, we find extensive heterogeneity at the insertion alleles of MAF2. Although several MAF2 insertion alleles were identified by Caicedo et al. (2009), the unique set of PCR primers used for screening in this study resulted in the identification of additional MAF2 insertion alleles, showing that variation at this locus is more extensive than previously reported. In the case of the MAF2 insertion alleles, similar to allelic effects of FRI and FLC, a common phenotypic effect is observed in spite of the heterogeneity.
The MAF locus on chromosome V has been suggested as a cause for natural variation in flowering time in several QTL studies that used parents falling into the MAF2–Sha allele subclass. QTL were found in the following populations: Ler × Kas-2, Ler × Kondara (El-Lithy et al. 2006), Sorbo × Gy-0 (O'neill et al. 2008), Col × Sha (Simon et al. 2008), and Bay-0 × Sha (Botto and Coluccio 2007). Our BC5 data confirm the association of a flowering-time QTL in this region. However, the discovery of putative loss-of-function polymorphisms at the MAF3 gene linked to MAF2 insertion alleles, or a possibility of mutation in another locus linked to the MAF region, confounds the assignment of this effect to MAF2 insertion alleles. To unequivocally associate the early flowering phenotype to changes at the MAF2 locus, we expressed an amiRNA construct designed to specifically knock down MAF2 transcripts. While MAF2 amiRNA constructs showed capability of downregulating MAF2 gene expression, it conferred early flowering phenotype in Ler but not in the WS accession carrying a MAF2 insertion allele. Failure of the MAF2 amiRNA construct to alter flowering time in the WS and Tu-1 amiRNA transgenic lines shows that these insertion alleles are loss-of-function, as was previously suggested in the BC5 lines. The loss-of-function in these alleles could be due to the MAF3 insertion or to the lower abundance of individual transcript splice variants.
Segregation distortion in A. thaliana:
In addition to the association of the early flowering-time phenotype to MAF2 insertion alleles, we observed segregation distortion favoring the MAF2 insertion alleles in BC5 F2 segregating lines. Segregation distortion has been widely reported in A. thaliana (Loudet et al. 2002; Bikard et al. 2009) and other plant species (Lu et al. 2002; Park et al. 2005; Koide et al. 2008). In A. thaliana, chimeric genes, as well as duplicated genes that undergo divergent evolution, have been shown to lead to genetic incompatibilities (Bomblies et al. 2007; Alcazar et al. 2009; Bikard et al. 2009). We have repeatedly observed that segregation distortion favors the MAF2 insertion allele in the progeny of a single, self-fertilized BC5 heterozygote. Therefore, segregation distortion is not caused by inadvertent selection during the generation of BC5 lines, but must have been induced by some other mechanisms that would result in segregation distortion in the subsequent generation.
Since BC5 lines are expected to be greater than 90% isogenic, the factor disrupting segregation likely resides at or close to the MAF locus. Segregation distortion has been identified in several RILs studies. It coincided with the QTL for early flowering in Ler × Kas-2 RILs, and favored the Kas-2 allele (El-Lithy et al. 2006), as it did with Kas-1 in this study. In the Sha × Bay-0 study, a significant segregation distortion favoring the Sha genomic region was observed in the region of the MAF locus at the end of chromosome V (Loudet et al. 2002). Significant linkage disequilibrium between a QTL at the MAF locus and another region on chromosome IV was observed in a Sha × Col RIL population (Simon et al. 2008). These studies support our assertion that a factor capable of distorting the expected Mendelian ratio is linked to the MAF locus in accessions with MAF2 insertion alleles. Although the identity and precise location of this factor is unknown, linkage of the MAF2 insertion alleles to a segregation distortion factor may have contributed to the broad geographical distribution of the MAF2 insertion alleles regardless of the phenotypic effect of these alleles on flowering time.
Evolution of tandemly duplicated genes:
Discovery of novel chimeric alleles at the MAF2 locus is consistent with the mode of TAG sequence evolution observed in other organisms. Our analysis revealed that all MAF2 alleles with MAF3 insertions produced chimeric transcripts that were usually not observed in Col. However, alternative splicing of exons 3 and 4 in the MAF2 pre-mRNA created some transcripts that are lacking the MAF3 insertion sequences. Similar conditional penetrance of chimeric exons through alternative splicing has been observed previously (Cusack and Wolfe 2007a,b), demonstrating that gene rearrangements coupled with changes in alternative splicing provide a good substrate for functional evolution of genes.
Geographic clustering of MAF2 alleles:
Most chimeric MAF2 allele forms have a clustered geographical distribution, suggesting that each allele spread from the site of its origin. Distinct population structure at a single locus in A. thaliana is unusual (Sharbel et al. 2000; Hoffmann et al. 2003; Schmuths et al. 2004), but has been occasionally observed in multilocus studies (Nordborg et al. 2005; Schmid et al. 2005; Beck et al. 2008; Francois et al. 2008). For example, Schmuths et al. (2004) found distinct population structure at only 2 of 67 CAPS polymorphisms. Like in this study, the identified markers displayed an east–west dichotomy. The east–west dichotomy is considered to be the result of postglacial expansion from eastern and western refugial populations after the last glaciation, possibly assisted by the expansion of Neolithic agriculture (Beck et al. 2008; Francois et al. 2008). Population expansion may partially explain the distribution and fixation of the MAF2 insertion alleles in eastern Eurasia.
It is possible that segregation distortion also contributed to numerical prominence of these alleles in eastern Eurasia during population expansion. A haplotype that dominates the segregation ratio will have a biased representation in progeny. Segregation bias may ultimately lead to the segregation distortion of linked genes in a population. This “genic meiotic drive” is considered a form of selection (Lyttle 1991). If segregation distortion acted in the natural population in the same way as it operated in the lab (this study), then it could have contributed to the fixation of these alleles in eastern Eurasia (Schierup et al. 2008; Schierup and Vekemans 2008). Coupling a segregation distorter to a population expansion would provide a potent evolutionary force (Morita et al. 1992).
Acknowledgments
We thank Josip Perkovic for help with plant work and Richard Clark, Kirsten Bomblies, Daniel Ortiz-Barrientos, and Patrice Salomé for comments and critical reading of the manuscript. We thank the National Science Foundation-supported Arabidopsis Biological Resource Centre (ABRC), the European Arabidopsis Stock Centres (NASC) for seeds, and Oliver Ratcliffe for the gift of maf2 seeds. This work was supported by an European Molecular Biology Organization (EMBO) Long Term fellowship (S.B.), a Gottfried Wilhelm Leibniz Award of the DFG and the Max Planck Society (D.W.), and the Natural Sciences and Engineering Research Council of Canada (NSERC) (V.G.). Sequence data from this article have been deposited with the GenBank Data Libraries under consecutive accession numbers from HM487066 to HM487102.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.116392/DC1.
Available freely online through the author-supported open access option.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession nos. HM487066–HM487102.
References
- Alcazar, R., A. V. Garcia, J. E. Parker and M. Reymond, 2009. Incremental steps toward incompatibility revealed by Arabidopsis epistatic interactions modulating salicylic acid pathway activation. Proc. Natl. Acad. Sci. USA 106 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armisen, D., A. Lecharny and S. Aubourg, 2008. Unique genes in plants: specificities and conserved features throughout evolution. BMC Evol. Biol. 8 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman, M. J., M. Hirschfeld, L. Wester, M. Weaver, T. Clack et al., 1997. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, E. G., C. Toomajian, M. Kreitman and J. Bergelson, 2006. A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 18 1803–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, S., S. Sureshkumar, M. Agrawal, T. P. Michael, C. Wessinger et al., 2006. a The PHYTOCHROME C photoreceptor gene mediates natural variation in flowering and growth responses of Arabidopsis thaliana. Nat. Genet. 38 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, S., S. Sureshkumar, J. Lempe and D. Weigel, 2006. b Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2 e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, J. B., H. Schmuths and B. A. Schaal, 2008. Native range genetic variation in Arabidopsis thaliana is strongly geographically structured and reflects Pleistocene glacial dynamics. Mol. Ecol. 17 902–915. [DOI] [PubMed] [Google Scholar]
- Becker, A., and G. Theissen, 2003. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 29 464–489. [DOI] [PubMed] [Google Scholar]
- Bikard, D., D. Patel, C. Le Mette, V. Giorgi, C. Camilleri et al., 2009. Divergent evolution of duplicate genes leads to genetic incompatibilities within A. thaliana. Science 323 623–626. [DOI] [PubMed] [Google Scholar]
- Blanc, G., K. Hokamp and K. H. Wolfe, 2003. A recent polyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Res. 13 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies, K., J. Lempe, P. Epple, N. Warthmann, C. Lanz et al., 2007. Autoimmune response as a mechanism for a Dobzhansky–Muller-type incompatibility syndrome in plants. PLoS Biol. 5 e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto, J. F., and M. P. Coluccio, 2007. Seasonal and plant-density dependency for quantitative trait loci affecting flowering time in multiple populations of Arabidopsis thaliana. Plant Cell Environ. 30 1465–1479. [DOI] [PubMed] [Google Scholar]
- Caicedo, A. L., J. R. Stinchcombe, K. M. Olsen, J. Schmitt and M. D. Purugganan, 2004. Epistatic interaction between Arabidopsis FRI and FLC flowering time genes generates a latitudinal cline in a life history trait. Proc. Natl. Acad. Sci. USA 101 15670–15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo, A. L., C. Richards, I. M. Ehrenreich and M. D. Purugganan, 2009. Complex rearrangements lead to novel chimeric gene fusion polymorphisms at the Arabidopsis thaliana MAF2–5 flowering time gene cluster. Mol. Biol. Evol. 26 699–711. [DOI] [PubMed] [Google Scholar]
- Clark, R. M., G. Schweikert, C. Toomajian, S. Ossowski, G. Zeller et al., 2007. Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science 317 338–342. [DOI] [PubMed] [Google Scholar]
- Cusack, B. P., and K. H. Wolfe, 2007. a Not born equal: increased rate asymmetry in relocated and retrotransposed rodent gene duplicates. Mol. Biol. Evol. 24 679–686. [DOI] [PubMed] [Google Scholar]
- Cusack, B. P., and K. H. Wolfe, 2007. b When gene marriages don't work out: divorce by subfunctionalization. Trends Genet. 23 270–272. [DOI] [PubMed] [Google Scholar]
- Cutler, G., L. A. Marshall, N. Chin, H. Baribault and P. D. Kassner, 2007. Significant gene content variation characterizes the genomes of inbred mouse strains. Genome Res. 17 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret, L., E. Gasteiger and G. Perriere, 1996. LALNVIEW: a graphical viewer for pairwise sequence alignments. Comput. Appl. Biosci. 12 507–510. [DOI] [PubMed] [Google Scholar]
- Edwards, K., C. Johnstone and C. Thompson, 1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Din El-Assal, S., C. Alonso-Blanco, A. J. Peeters, V. Raz and M. Koornneef, 2001. A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet. 29 435–440. [DOI] [PubMed] [Google Scholar]
- El-Lithy, M. E., E. J. Clerkx, G. J. Ruys, M. Koornneef and D. Vreugdenhil, 2004. Quantitative trait locus analysis of growth-related traits in a new Arabidopsis recombinant inbred population. Plant Physiol. 135 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Lithy, M. E., L. Bentsink, C. J. Hanhart, G. J. Ruys, D. Rovito et al., 2006. New Arabidopsis recombinant inbred line populations genotyped using SNPWave and their use for mapping flowering-time quantitative trait loci. Genetics 172 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J., 2005. PHYLIP (Phylogeny Inference Package), version 3.6. Department of Genome Sciences, University of Washington, Seattle.
- Florea, L., G. Hartzell, Z. Zhang, G. M. Rubin and W. Miller, 1998. A computer program for aligning a cDNA sequence with a genomic DNA sequence. Genome Res. 8 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois, O., M. G. Blum, M. Jakobsson and N. A. Rosenberg, 2008. Demographic history of european populations of Arabidopsis thaliana. PLoS Genet. 4 e1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling, M., E. Lyons, B. Pedersen, M. Alam, R. Ming et al., 2008. Many or most genes in Arabidopsis transposed after the origin of the order Brassicales. Genome Res. 18 1924–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley, A. R., and T. Kobayashi, 2007. Highly efficient concerted evolution in the ribosomal DNA repeats: total rDNA repeat variation revealed by whole-genome shotgun sequence data. Genome Res. 17 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, L. Z., and H. Innan, 2004. Very low gene duplication rate in the yeast genome. Science 306 1367–1370. [DOI] [PubMed] [Google Scholar]
- Gaut, B. S., S. I. Wright, C. Rizzon, J. Dvorak and L. K. Anderson, 2007. Recombination: an underappreciated factor in the evolution of plant genomes. Nat. Rev. Genet. 8 77–84. [DOI] [PubMed] [Google Scholar]
- Gazzani, S., A. R. Gendall, C. Lister and C. Dean, 2003. Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol. 132 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J., and X. Gu, 2003. Natural history and functional divergence of protein tyrosine kinases. Gene 317 49–57. [DOI] [PubMed] [Google Scholar]
- Harrison, P. M., and M. Gerstein, 2002. Studying genomes through the aeons: protein families, pseudogenes and proteome evolution. J. Mol. Biol. 318 1155–1174. [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. H., A. S. Glass, J. Tomiuk, H. Schmuths, R. M. Fritsch et al., 2003. Analysis of molecular data of Arabidopsis thaliana (L.) Heynh. (Brassicaceae) with Geographical Information Systems (GIS). Mol. Ecol. 12 1007–1019. [DOI] [PubMed] [Google Scholar]
- Jelesko, J. G., R. Harper, M. Furuya and W. Gruissem, 1999. Rare germinal unequal crossing-over leading to recombinant gene formation and gene duplication in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 96 10302–10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelesko, J. G., K. Carter, W. Thompson, Y. Kinoshita and W. Gruissem, 2004. Meiotic recombination between paralogous RBCSB genes on sister chromatids of Arabidopsis thaliana. Genetics 166 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson, U., J. West, C. Lister, S. Michaels, R. Amasino et al., 2000. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290 344–347. [DOI] [PubMed] [Google Scholar]
- Kofuji, R., N. Sumikawa, M. Yamasaki, K. Kondo, K. Ueda et al., 2003. Evolution and divergence of the MADS-box gene family based on genome-wide expression analyses. Mol. Biol. Evol. 20 1963–1977. [DOI] [PubMed] [Google Scholar]
- Koide, Y., M. Ikenaga, N. Sawamura, D. Nishimoto, K. Matsubara et al., 2008. The evolution of sex-independent transmission ratio distortion involving multiple allelic interactions at a single locus in rice. Genetics 180 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, H., L. L. Landherr, M. W. Frohlich, J. Leebens-Mack, H. Ma et al., 2007. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J. 50 873–885. [DOI] [PubMed] [Google Scholar]
- Korbel, J. O., P. M. Kim, X. Chen, A. E. Urban, S. Weissman et al., 2008. The current excitement about copy-number variation: how it relates to gene duplications and protein families. Curr. Opin. Struct. Biol. 18 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang, H., S. S. Woo, B. C. Meyers, E. Nevo and R. W. Michelmore, 2004. Multiple genetic processes result in heterogeneous rates of evolution within the major cluster disease resistance genes in lettuce. Plant Cell 16 2870–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang, H., O. E. Ochoa, E. Nevo and R. W. Michelmore, 2006. The disease resistance gene Dm3 is infrequent in natural populations of Lactuca serriola due to deletions and frequent gene conversions at the RGC2 locus. Plant J. 47 38–48. [DOI] [PubMed] [Google Scholar]
- Kuang, H., K. S. Caldwell, B. C. Meyers and R. W. Michelmore, 2008. Frequent sequence exchanges between homologs of RPP8 in Arabidopsis are not necessarily associated with genomic proximity. Plant J. 54 69–80. [DOI] [PubMed] [Google Scholar]
- Labarga, A., F. Valentin, M. Anderson and R. Lopez, 2007. Web services at the European bioinformatics institute. Nucleic Acids Res. 35 W6–W11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander, E. S., P. Green, J. Abrahamsom, A. Barlow, M. J. Daley et al., 1987. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1 174–181. [DOI] [PubMed] [Google Scholar]
- Le Corre, V., 2005. Variation at two flowering time genes within and among populations of Arabidopsis thaliana: comparison with markers and traits. Mol. Ecol. 14 4181–4192. [DOI] [PubMed] [Google Scholar]
- Leister, D., 2004. Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance genes. Trends Genet. 20 116–122. [DOI] [PubMed] [Google Scholar]
- Lempe, J., S. Balasubramanian, S. Sureshkumar, A. Singh, M. Schmid et al., 2005. Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet. 1 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln, S., M. Daly and E. Lander, 1992. Constructing genetic maps with MAPMAKER/EXP, version 3.0. Whitehead Institute Technical Report, Whitehead Institute, Cambridge, MA.
- Loudet, O., S. Chaillou, C. Camilleri, D. Bouchez and F. Daniel-Vedele, 2002. Bay-0 x Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis. Theor. Appl. Genet. 104 1173–1184. [DOI] [PubMed] [Google Scholar]
- Lyttle, T.W., 1991. Segregation distorters. Annu. Rev. Genet. 25 511–557. [DOI] [PubMed] [Google Scholar]
- Michaels, S. D., and R. M. Amasino, 1999. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. P., 2005. Alleles in space (AIS): computer software for the joint analysis of interindividual spatial and genetic information. J. Hered. 96 722–724. [DOI] [PubMed] [Google Scholar]
- Mondragon-Palomino, M., and B. S. Gaut, 2005. Gene conversion and the evolution of three leucine-rich repeat gene families in Arabidopsis thaliana. Mol. Biol. Evol. 22 2444–2456. [DOI] [PubMed] [Google Scholar]
- Mondragon-Palomino, M., B. C. Meyers, R. W. Michelmore and B. S. Gaut, 2002. Patterns of positive selection in the complete NBS-LRR gene family of Arabidopsis thaliana. Genome Res. 12 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, T., H. Kubota, K. Murata, M. Nozaki, C. Delarbre et al., 1992. Evolution of the mouse t haplotype: recent and worldwide introgression to Mus musculus. Proc. Natl. Acad. Sci. USA 89 6851–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, V., P. A. Mieczkowski, H. M. Kim, T. D. Petes and K. S. Lobachev, 2006. The pattern of gene amplification is determined by the chromosomal location of hairpin-capped breaks. Cell 125 1283–1296. [DOI] [PubMed] [Google Scholar]
- Nei, M., and A. P. Rooney, 2005. Concerted and birth-and-death evolution of multigene families. Annu. Rev. Genet. 39 121–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg, M., T. T. Hu, Y. Ishino, J. Jhaveri, C. Toomajian et al., 2005. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 3 e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, C. M., C. Morgan, J. Kirby, H. Tschoep, P. X. Deng et al., 2008. Six new recombinant inbred populations for the study of quantitative traits in Arabidopsis thaliana. Theor. Appl. Genet. 116 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenicova, L., S. de Folter, M. Kieffer, D. S. Horner, C. Favalli et al., 2003. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell 15 1538–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y.-H., M. Alabady, M. Ulloa, B. Sickler, T. Wilkins et al., 2005. Genetic mapping of new cotton fiber loci using EST-derived microsatellites in an interspecific recombinant inbred line cotton population. Mol. Genet. Genomics 274 428–441. [DOI] [PubMed] [Google Scholar]
- Ratcliffe, O. J., R. W. Kumimoto, B. J. Wong and J. L. Riechmann, 2003. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redon, R., S. Ishikawa, K. R. Fitch, L. Feuk, G. H. Perry et al., 2006. Global variation in copy number in the human genome. Nature 444 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, P., I. Longden and A. Bleasby, 2000. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 16 276–277. [DOI] [PubMed] [Google Scholar]
- Sakurai, T., G. Plata, F. Rodriguez-Zapata, M. Seki, A. Salcedo et al., 2007. Sequencing analysis of 20,000 full-length cDNA clones from cassava reveals lineage specific expansions in gene families related to stress response. BMC Plant Biol. 7 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierup, M. H., and X. Vekemans, 2008. Genomic consequences of selection on self-incompatibility genes. Curr. Opin. Plant Biol. 11 116–122. [DOI] [PubMed] [Google Scholar]
- Schierup, M. H., J. S. Bechsgaard and F. B. Christiansen, 2008. Selection at work in self-incompatible Arabidopsis lyrata. II. Spatial distribution of S haplotypes in Iceland. Genetics 180 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, K. J., S. Ramos-Onsins, H. Ringys-Beckstein, B. Weisshaar and T. Mitchell-Olds, 2005. A multilocus sequence survey in Arabidopsis thaliana reveals a genome-wide departure from a neutral model of DNA sequence polymorphism. Genetics 169 1601–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuths, H., M. H. Hoffmann and K. Bachmann, 2004. Geographic distribution and recombination of genomic fragments on the short arm of chromosome 2 of Arabidopsis thaliana. Plant Biol. 6 128–139. [DOI] [PubMed] [Google Scholar]
- Schwab, R., S. Ossowski, M. Riester, N. Warthmann and D. Weigel, 2006. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortecci, K., S. D. Michaels and R. M. Amasino, 2003. Genetic interactions between FLM and other flowering-time genes in Arabidopsis thaliana. Plant Mol. Biol. 52 915–922. [DOI] [PubMed] [Google Scholar]
- Shakhnovich, B. E., and E. V. Koonin, 2006. Origins and impact of constraints in evolution of gene families. Genome Res. 16 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharbel, T. F., B. Haubold and T. Mitchell-Olds, 2000. Genetic isolation by distance in Arabidopsis thaliana: biogeography and postglacial colonization of Europe. Mol. Ecol. 9 2109–2118. [DOI] [PubMed] [Google Scholar]
- Shindo, C., M. J. Aranzana, C. Lister, C. Baxter, C. Nicholls et al., 2005. Role of FRIGIDA and FLOWERING LOCUS C in determining variation in flowering time of Arabidopsis. Plant Physiol. 138 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, M., O. Loudet, S. Durand, A. Berard, D. Brunel et al., 2008. Quantitative trait loci mapping in five new large recombinant inbred line populations of Arabidopsis thaliana genotyped with consensus single-nucleotide polymorphism markers. Genetics 178 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack, A., P. C. Thornton, D. B. Magner, S. M. Rosenberg and P. J. Hastings, 2006. On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet. 2 e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis, D. E., and P. S. Soltis, 2003. The role of phylogenetics in comparative genetics. Plant Physiol. 132 1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, E. A., G. Dwyer, R. Mauricio, M. Kreitman and J. Bergelson, 1999. Dynamics of disease resistance polymorphism at the RPM1 locus of Arabidopsis. Nature 400 667–671. [DOI] [PubMed] [Google Scholar]
- Sturtevant, A. H., 1925. The effects of unequal crossing over at the bar locus in Drosophila. Genetics 10 117–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, S., Y. He, T. W. Eshoo, Y. Tamada, L. Johnson et al., 2006. Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat. Genet. 38 706–710. [DOI] [PubMed] [Google Scholar]
- Wagner, A., 2008. Gene duplications, robustness and evolutionary innovations. Bioessays 30 367–373. [DOI] [PubMed] [Google Scholar]
- Wang, Q., U. Sajja, S. Rosloski, T. Humphrey, M. C. Kim et al., 2007. HUA2 caused natural variation in shoot morphology of A. thaliana. Curr. Biol. 17 1513–1519. [DOI] [PubMed] [Google Scholar]
- Werner, J. D., J. O. Borevitz, N. Warthmann, G. T. Trainer, J. R. Ecker et al., 2005. Quantitative trait locus mapping and DNA array hybridization identify an FLM deletion as a cause for natural flowering-time variation. Proc. Natl. Acad. Sci. USA 102 2460–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, S., T. Clark, H. Zheng, S. Vang, R. Li et al., 2008. Gene conversion in the rice genome. BMC Genomics 9 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandeau-Nelson, M. D., Y. Xia, J. Li, M. G. Neuffer and P. S. Schnable, 2006. Unequal sister chromatid and homolog recombination at a tandem duplication of the a1 locus in maize. Genetics 173 2211–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., and B. S. Gaut, 2003. Does recombination shape the distribution and evolution of tandemly arrayed genes (TAGs) in the Arabidopsis thaliana genome? Genome Res. 13 2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]