Abstract
Human genetics indicate that kisspeptin and neurokinin B (NKB) signaling are necessary for generating pulsatile LH release and therefore for initiation of puberty and maintaining gonadal function. In the present study, male monkeys were employed to examine 1) whether activation of the NKB receptor (NK3R) is associated with GnRH release, and 2) hypothalamic localization of these peptides using immunofluorescence histochemistry. Agonadal juveniles, in which pituitary responsiveness to GnRH was heightened by GnRH priming, were employed to indirectly examine GnRH-releasing actions of NK3R and kisspeptin receptor agonists by tracking LH after their iv injection. Castrated adults were used for immunohistochemistry. Single iv injections of NKB or senktide (an NK3R agonist) elicited robust LH discharges that were abolished by GnRH receptor antagonism (acyline) confirming the ligands’ hypothalamic action. Intermittent infusion of senktide (1-min pulse every hour for 4 h), in contrast to that of kisspeptin, failed to sustain pulsatile GnRH release. Repetitive senktide injections did not compromise the GnRH-releasing action of kisspeptin. NKB and kisspeptin were colocalized in perikarya of the arcuate nucleus and in axonal projections to the median eminence, confirming earlier findings in sheep. These results are consistent with the human genetics, and indicate that although brief activation of NK3R stimulates GnRH release, repetitive stimulation of this pathway, in contrast to that of kisspeptin receptor, fails to sustain pulsatile GnRH release. In addition, the data provide a platform for future elucidation of the interactions between NKB and kisspeptin that are required for generating pulsatile GnRH release in primates.
Neurokinin B is found in the monkey hypothalamus, and iv injections of this peptide activate the hypothalamic-pituitary axis.
The findings reported in 2003 that inactivation of kisspeptin signaling due to mutations of G protein-coupled receptor 54 [kisspeptin receptor (KISS1R)] are associated with hypogonadotropic hypogonadism and absent or delayed puberty in man (1,2) provided the catalyst for the many subsequent studies that together have led to a kisspetinocentric view of the neural control of GnRH release (3,4,5). With this in mind, the recent observations by Topaloglu et al. (6) and Topaloglu and co-workers (7) that mutations of the genes encoding neurokinin B (NKB) (TAC3) and its receptor (TAC3R) are also associated with hypogonadotropic hypogonadism as reported earlier for KISS1R mutations is therefore of considerable interest. What is even more fascinating is a study of sheep indicating that kisspeptin and NKB are coexpressed in neurons in the arcuate nucleus of the mediobasal hypothalamus (MBH) (8). Taken together, these results suggest that coexpression of KISS1, the gene encoding for kisspeptin (9), and TAC3 in arcuate neurons is obligatory for sustained pulsatile release of GnRH that is necessary for initiation of puberty and for maintenance of gonadal function in adult men and women (10).
Although transgenic mice with targeted deletions in Kiss1R possess a phenotype similar to their human counterparts (1), genetic disruption of the NKB pathway in mice does not lead to infertility (11). Thus, elucidation of the cellular and molecular interactions within the hypothalamus that must underlie the requirement for both kisspeptin and NKB signaling to generate pulsatile GnRH release may be facilitated by the study of nonhuman primates. Indeed, studies of rodents, to date, indicate that NKB has an inhibitory or no action on GnRH/LH release (12,13,14), effects which are difficult to readily reconcile with the human genetics (6,7). Similar findings have been reported for ovariectomized and estrogen treated ovariectomized goats (see Ref. 15; also, see Discussion).
For these reasons, a series of experiments designed to examine the action of NKB signaling on GnRH release was initiated with the rhesus monkey, a higher primate that exhibits a similar postnatal pattern of pulsatile GnRH release to that in man (10). The juvenile male was selected, because during this stage of development, the restraint on spontaneous GnRH release is more marked than that in the female (10), and therefore, it was considered that any stimulatory action of NKB on GnRH release might be more readily detected. Animals were castrated to eliminate any confounding effects of testicular feedback on gonadotropin secretion. GnRH was monitored indirectly by using the GnRH primed in situ pituitary as a bioassay for hypothalamic GnRH release, as described by us previously (16,17). The results of these experiments are described here.
Materials and Methods
Animals
Eight male rhesus monkeys (Macaca mulatta) were used. Four were castrated juveniles (13–14 months, 2.3–2.6 kg) obtained from the California National Primate Research Center (Davis, CA). The age of these animals at the end of this study ranged from 21–22 months; the pubertal reactivation of GnRH release in the absence of the testes typically occurs at around 30 months of age (16,18). The remaining four animals were adults (7–14 yr, 6–13 kg) born in Pittsburgh or obtained from U.S. vendors (Three Springs Scientific, Perkasie, PA; and Valley Biosystems, West Sacramento, CA). They had been castrated at least 1 month before this study. One of the four adults had provided hypothalamic sections for a previously described study (19). Monkeys were maintained under controlled photoperiod (lights on 0700–1900 h) and temperature (21 C) in accordance with National Institutes of Health guidelines. The Institutional Animal Care and Use Committee approved the experimental procedures.
Receptor agonists and antagonists
NKB and human kisspeptin-10 (Kp-10) (amino acids 112–121) were synthesized at the Peptide/Protein Core Facility of the Massachusetts General Hospital Endocrine/Reproductive Endocrine Unit (Boston, MA). A stock NKB solution (5 μg/μl) was made in dimethylsulfoxide (DMSO) and a stock Kp-10 solution (1 μg/μl) in 5% DMSO in sterile physiological saline, both were stored at −20 C. Senktide, a selective NKB receptor (NK3R) peptide agonist (20), was obtained from Phoenix Pharmaceuticals, Inc., (Burlingame, CA), and a stock solution (1 μg/μl) in sterile physiological saline was stored at 4 C.
A stock solution (10 μg/μl) of the nonpeptide NK3R antagonist (SB222200; Tocris Bioscience, Ellisville, MO) was prepared in 60% DMSO in sterile physiological saline and stored at 4 C. The NK3R and KISS1R ligands were administered as iv boluses or brief infusions (1 min) in 1 ml of sterile saline (Kp-10 and senktide) or 60% DMSO in sterile saline (SB222200) or in 200 μl DMSO (NKB).
GnRH was obtained from the National Hormone and Peptide Program, Harbor-University of California, Los Angeles Medical Center (Torrance, CA); stock (1 mg/ml) and working (0.3–0.6 μg/ml) dilutions were prepared as previously described (21). A stock solution (300 μg/ml) of the GnRH receptor (GnRH-R) antagonist acyline (Bioqual, Rockville, MD) in 5% aqueous mannitol (AMVET Scientific Products, Yaphank, NY) was stored at 4 C.
Antibodies
The NKB antibody (IS-681) was raised in a rabbit against h prepro-NKB (the hapten was a 28-amino acid-long carboxyterminal peptide coupled to bovine thyroglobulin by glutaraldehyde) and used at 1:6000. For preabsorption control, the immunogen (hapten-glutaraldehyde-carrier) was used, and abolition of labeling was obtained at a dilution of 1:400 corresponding to a virtual concentration of 1.7 × 10−7 m of hapten (see Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
The kisspeptin antibody (GQ2), raised in sheep against synthetic human Kp-54 and kindly provided by Stephen Bloom (Imperial College London, Hammersmith Hospital, London, UK), has been validated for immunohistochemical analysis of monkey hypothalamus (19). GQ2 was again used at a dilution of 1:120,000.
The GnRH antibody (HU4H), a mouse monoclonal antibody (22), was kindly provided by Henryk Urbanski (Oregon National Primate Research Center, Beaverton, OR) and used at 1:4000. Preabsorption of HU4H with synthetic GnRH (10 μg/ml) eliminated GnRH labeling (Supplemental Fig. 2).
Alexa Fluor 488 donkey antisheep IgGs (Invitrogen Corp., Carlsbad, CA), Cy3-conjugated AffiniPure donkey antirabbit IgGs (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and Alexa Fluor 488 donkey antimouse IgGs were used as secondary antibodies at 1:200 dilution to detect kisspeptin, NKB, and GnRH immunoactivity, respectively.
Surgical procedures
Surgeries were performed under sterile conditions after the animals had been sedated with ketamine hydrochloride (10–20 mg/kg, im, Ketaject; Phoenix Scientific, Inc., St Joseph, MO) and anesthetized with isoflurane inhalation (1–2%, in oxygen; Abbott Animal House, North Chicago, IL) as previously described (16,23). On the day of surgery, animals received an im injection of penicillin (Pen-G, 40,000 U/kg; Phoenix Scientific, Inc.). After castration, the animals continued to receive daily injections of penicillin and twice daily im injection of Ketofen (ketoprofen, 2 mg/kg; Apothecon, Princeton, NJ) for 4 d. For animals with iv catheters, twice daily iv injections of a broad-spectrum antibiotic (Kefzol, 25 mg/kg; Apothecon) were given.
For access to the venous circulation, two indwelling catheters (inner diameter, 0.040 inches; and outer diameter, 0.085 inches, Stuart Bio-Sil; Sil-Med Corporation, Taunton, MA) were implanted: one in an internal jugular vein and the other in a femoral vein. Catheterization was performed 2–3 months after castration.
Remote iv sampling/infusion
A jacket and tether system was employed for remote collection of blood and infusion of receptor ligands, as previously described (23). Blood samples (1–2 ml) were collected under sterile conditions and during periods of frequent blood sampling, packed blood cells were resuspended in sterile saline and returned to the respective animal. Plasma was stored at −20 C.
RIA
Plasma LH levels were measured using a homologous RIA as described previously (24). The sensitivity of the LH assay ranged between 0.08 and 0.34 ng/ml, and the mean intra- and interassay coefficient of variation at approximately 50% binding was less than 5 and 9%, respectively. LH concentrations below detection were assigned a value equivalent to the minimum detectable concentration.
In situ GnRH bioassay
To use the pituitary of the juvenile monkey as an in situ bioassay to track endogenous GnRH release, pituitary responsiveness to GnRH was enhanced with an intermittent iv infusion of synthetic GnRH (0.3–0.6 μg over 2 min every hour) as previously described (16,17). GnRH priming was initiated on the day of catheterization and maintained for several weeks. During this period, pituitary LH response to GnRH was monitored weekly until robust LH discharges were observed.
Transcardial perfusion and hypothalamus sectioning
Animals were sedated with ketamine hydrochloride (10–20 mg/kg) and deeply anesthetized with sodium pentobarbital (∼30 mg/kg, iv, nembutal sodium solution; Abbott Laboratories, North Chicago, IL) as previously described (19). Brains were perfused with approximately 1 liter of saline containing 2% sodium nitrite and 5000 U heparin/liter followed by approximately 2–3 liter of 4% paraformaldehyde in 0.1 m PBS (pH 7.2). The hypothalamus was immersed in 30% sucrose (in 0.1 m PBS) for at least 24 h before serial sectioning at 25 μm on a freezing microtome. Sections were stored in an cryoprotectant at −20 C (25).
Immunohistochemistry
Dual immunofluorescence histochemistry to localize NKB and kisspeptin, and NKB and GnRH, in the hypothalamus was performed as described previously (19). Sections were rinsed at room temperature in 50 mm potassium PBS (KPBS) (pH 7.3) (eight times 15 min), incubated for 30 min in 3% hydrogen peroxide (CVS Pharmacy, Inc., Woonsocket, RI), rinsed again in 50 mm KPBS (six times 5 min), and incubated overnight at 4 C in KPBS buffer containing 5% horse serum (Vector Laboratories, Inc., Burlingame, CA), 0.05% Triton X-100, and 5% BSA (Sigma Chemical Co., St. Louis, MO) to block nonspecific binding. Sections were incubated for 48 h at 4 C in a cocktail of primary antibodies in KPBS-horse serum buffer and washed in 50 mm KPBS (four times 5 min). Sections were incubated for 1 h in the dark in a cocktail of the appropriate fluorescent secondary antibodies in KPBS-horse serum buffer, both at a dilution of 1:200. After washing in 50 mm KPBS (four times 5 min), sections were mounted on slides (Fisherbrand Superfrost Plus; Fisher Scientific, Pittsburgh, PA) and coverslipped using GEL/MOUNT aqueous mounting medium with antifading agents (Biomeda Corp., Foster City, CA).
Confocal microscopy
Imaging of fluorescence labeling for NKB, kisspeptin, and GnRH was performed using an Olympus FV1000 confocal microscope (Olympus America, Inc., Melville, NY) as previously described (19). For low (×10–×20) and high magnification (×40–×100) profiles, optical sections along the z-axis were collected at 1-μm intervals. Composite digital images were then converted to tagged image file format and imported into Adobe Photoshop (Adobe Photoshop CS2, version 9.0; Adobe Systems, Inc., San Jose, CA), and color balance was generally adjusted for presentation.
Statistical analyses
Significance differences between mean hormone concentrations were determined using Student’s t test or ANOVA with repeated measure followed by Student-Newman-Keuls multiple range test using SigmaStat (version 2.0; SPSS, San Rafael, CA). Significance was accepted at P ≤ 0.05. Values are expressed as mean ± sem.
Experimental design
Experiments 1–6 were conducted in GnRH primed juveniles. For the first five experiments, GnRH priming was stopped at approximately 1500 h on the day before the peptide challenges and reinitiated upon completion of the experiments, unless otherwise stated.
Experiment 1: effects of increasing doses of iv bolus injections of senktide on LH secretion (n = 3)
On the day after termination of GnRH priming and for the next 2 d, the animals received an iv bolus injection of one of three doses of senktide (5, 15, and 50 μg or 6, 18, and 59 nmol, respectively). A dose of senktide was given on each day with the three animals receiving a different dose on a given day. GnRH priming was restored for approximately 6 h between each senktide challenge. Changes in LH levels were monitored in blood samples collected 10 min before and at 10, 20, 30, and 50 min after each dose of peptide injected. On separate occasions, 100 μg (119 nmol, three animals) and 500 μg (593 nmol, one animal) of senktide were also administered.
Experiment 2: effect of single bolus iv injections of senktide or NKB on LH release (n = 3)
Animals received bolus iv injections of senktide (50 μg), NKB (100 μg or 83 nmol), or GnRH (0.3 μg or 250 pmol) at 2 h interval. A GnRH dose of 0.3 μg was selected, because previous studies indicate that this dose provides the gonadotrophs with a hypophysiotropic signal comparable with that generated spontaneously in postpubertal males (21). The order in which the peptides were administered was adjusted so that each animal received the three agonists in a unique sequence. Changes in LH levels were monitored in blood samples collected 10 min before and at 10, 20, 30, 50, 70, 90, and 110 min after each injection.
Using a similar protocol on a different occasion after GnRH repriming, a control experiment was conducted to determine whether DMSO (200 μl) influenced either spontaneous or senktide (50 μg)-induced LH release.
Experiment 3: effect of pretreatment with a GnRH-R antagonist (acyline) on senktide or NKB-induced LH release (n = 4)
On the day of the experiment, animals first received an iv bolus of GnRH (0.3 μg). Thirty minutes later, a sc injection of acyline (60 μg/kg) was administered. Three hours later, a bolus iv injection of senktide (50 μg) was administered followed, at 2-h intervals, by injections of NKB (100 μg) and GnRH (0.3 μg). Changes in LH levels were monitored in blood samples collected 10 min before the first GnRH challenge and at frequent intervals (10–45 min) thereafter. After an interval of 2–3 wk of GnRH repriming and confirmation that the action of the GnRH-R antagonist had dissipated, the experiment was repeated with the acyline vehicle (5% mannitol). This time, however, LH responses to NKB and the second GnRH challenge were not examined.
Experiment 4: comparison of the LH-releasing action of single bolus iv injections of senktide or Kp-10 (n = 3)
Animals received bolus iv injections of senktide (50 μg), Kp-10 (2 μg, 1.5 nmol), and GnRH (0.3 μg) with an interdose interval of 2 h. The Kp-10 dose of 2 μg was selected, because previous studies showed that it mimicked the LH-releasing action of a “physiological” dose of GnRH (26). The order in which the peptides were administered was adjusted so that each animal received the three agonists in a unique sequence. Changes in LH levels were monitored in blood samples collected 10 min before and at 10, 20, 30, 50, 70, 90, and 110 min after each injection.
Experiment 5: effect of pretreatment with a selective NK3R antagonist (SB222200) on senktide or Kp-10 induced LH release (n = 3)
Animals received a bolus iv injection of the NK3R antagonist (500 μg or 1.3 μmol) followed 15 min later by a bolus iv injection of senktide (50 μg). Changes in LH levels were monitored in blood samples collected 30 and 15 min before and at 10, 20, 30, 50, 70, 90, and 110 min after the senktide injection. After GnRH repriming, the experiment was repeated with the NK3R antagonist vehicle. Finally, after further repriming, the effect of the NK3R antagonist on Kp-10 (2 μg)-induced LH release was examined.
Experiment 6: effect of repetitive intermittent iv injections of senktide or Kp-10 on LH release (n = 3)
In this experiment, GnRH priming was not stopped until the day of the experiment. The LH discharge to the last GnRH-priming pulse was monitored before the GnRH infusion was terminated and immediately replaced with four bolus iv injections, at 1-h intervals, of senktide (50 μg/pulse). One hour after the last senktide pulse, a bolus iv injection of Kp-10 (2 μg) was administered. Changes in LH levels were monitored in blood samples collected 10 min before and at 10, 20, 30, and 55 min after each pulse infusion of peptide. Priming was reinitiated, and the protocol repeated, but this time, four pulses of vehicle were administered instead of senktide. Finally, the response to five pulses of Kp-10 (2 μg/pulse) was examined again after repriming.
Experiment 7: immunohistochemical localization of NKB, kisspeptin, and GnRH in the MBH of castrated adult male monkeys (n = 4)
A series of coronal sections taken every 250 μm throughout the MBH of each of four castrated adult male monkeys were stained for NKB and kisspeptin as described above and examined for double labeled somata and fibers. In two monkeys, sections through the arcuate nucleus median eminence at an anterio- posterior level, established to contain double labeled (NKB and kisspeptin) somata and axons, were stained for NKB and GnRH. Hypothalamic sections from castrated adults were used here, because intensely immunopositive kisspeptin neurons are detected in abundance in the arcuate nucleus of postpubertal males in the absence of testicular feedback (19). Moreover, these sections were available in our tissue bank.
Results
Experiment 1: effects of increasing doses of iv bolus injections of senktide on LH secretion (n = 3)
The 15- and 50-μg bolus injections of senktide induced unequivocal discharges of LH. At these doses, basal LH concentrations of 0.56 ± 0.13 ng/ml (mean ± sem) increased rapidly after the senktide challenge, and at 10 min after injection, LH levels had significantly increased to 1.32 ± 0.22 and 2.52 ± 0.42 ng/ml, respectively. Changes in mean LH concentrations after the 5 μg dose of senktide were not significant (0.81 ± 0.43 and 0.98 ± 0.52 ng/ml, basal and 10 min post senktide, respectively).
On another occasion, a senktide dose of 100 μg was administered and produced peak LH levels of approximately 3.5 ng/ml, whereas 500 μg of the NK3R agonist given to one monkey produced a peak LH level of 7 ng/ml (data not shown).
Experiment 2: effect of single bolus iv injections of senktide and NKB on LH release (n = 3)
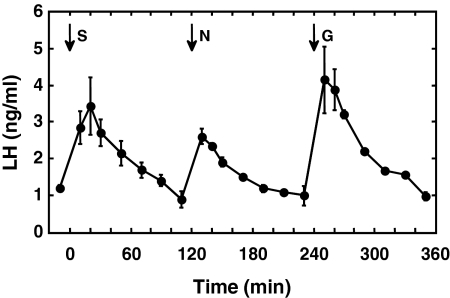
Senktide at the 50-μg dose again elicited a robust LH discharge (Fig. 1) of similar magnitude to that observed in the first experiment. Plasma LH concentrations were tracked for 2 h after the senktide challenge, and at this time, LH levels had returned to basal values (Fig. 1). A bolus injection of 100 μg NKB also elicited a marked discharge of LH, as did that of GnRH (0.3 μg) (Fig. 1). The magnitude of the senktide-induced LH discharge was comparable with that observed in response to GnRH. Injection of DMSO (NKB vehicle) was without effect on plasma LH levels and did not interfere with the LH discharge induced by senktide administered 2 h later (data not shown).
Figure 1.
Circulating concentrations of LH (mean ± sem) after bolus iv injections (arrows) of senktide (S) (50 μg), NKB (N) (100 μg), and GnRH (G) (0.3 μg) given at 2-h intervals to agonadal juvenile male monkeys. Note that the order of treatment with the three peptides was different for each of the three animals (see experiment 2 under experimental design in Materials and Methods), and for presentation, the responses of two animals have been reordered to match those for the animal that received the sequence S, N, and G. Also, note that LH values for the last four time points after GnRH represent data from two animals only. n = 3.
Experiment 3: effect of pretreatment with a GnRH-R antagonist (acyline) on senktide or NKB-induced LH release (n = 4)
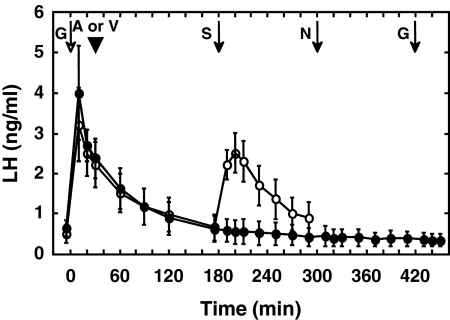
Administration of a GnRH-R antagonist (acyline) abolished the LH response to a senktide, NKB, and GnRH challenge administered 2.5, 4.5, and 6.5 h later, respectively (Fig. 2). Injection of vehicle used for the GnRH-R antagonist was without effect on the senktide-induced LH discharge (Fig. 2).
Figure 2.
Circulating concentrations of LH (mean ± sem) after the last GnRH (G) pulse of the priming infusion (open arrow) and sequential bolus iv injections (arrows) of senktide (S) (50 μg), NKB (N) (100 μg), and GnRH (0.3 μg) (black arrows) given at 2-h intervals to agonadal juvenile male monkeys when a GnRH-R antagonist, acyline (A) (arrowhead), was given 30 min after the first GnRH challenge (closed data points). Note the absence of LH discharges in response to the three peptides after administration of acyline. Treatment with the vehicle for the GnRH-R antagonist (V) (5% mannitol in saline; arrowhead) did not interrupt the LH discharge induced by a bolus iv injection of senktide (open data points). The response to NKB and the second GnRH challenge was not studied after vehicle administration. n = 4.
Experiment 4: comparison of the LH-releasing action of single bolus iv injections of senktide or Kp-10 (n = 3)
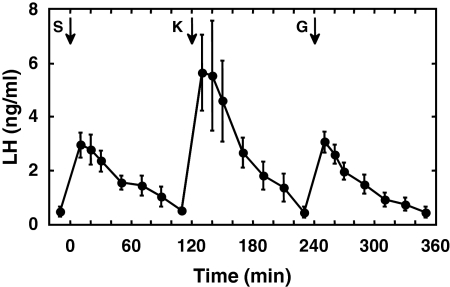
The time course of the LH discharge in response to a 2-μg iv bolus injection of Kp-10 was similar to that induced by senktide (50 μg). The magnitude of the LH response to Kp-10, however, was greater than that to senktide (Fig. 3). GnRH (0.3 μg) again elicited a LH discharge comparable with that produced in response to senktide (Fig. 3).
Figure 3.
Circulating concentrations of LH (mean ± sem) after bolus iv injections (arrows) of senktide (S) (50 μg), Kp-10 (K) (2 μg), and GnRH (G) (0.3 μg) given at 2-h intervals to agonadal juvenile male monkeys. Note that the order of treatment with the three peptides was different for each of the three animals (see experiment 4 under experimental design in Materials and Methods), and for presentation, the responses of two animals have been reordered to match those for the animal that received the sequence S, K, and G. n = 3.
Experiment 5: effect of pretreatment with a selective NK3R antagonist (SB222200) on senktide or Kp-10 induced LH release (n = 3)
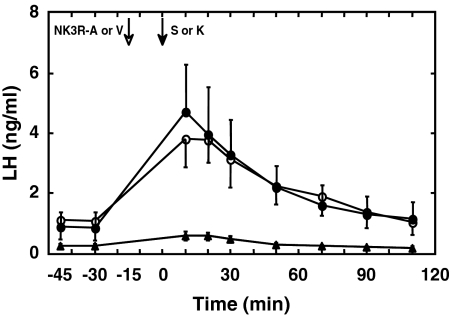
Pretreatment with the NK3R antagonist 15 min before a bolus injection of senktide abolished the LH response to the NK3R agonist but did not interrupt the LH discharge induced by Kp-10 (Fig. 4).
Figure 4.
Administration of a nonpeptide NK3R antagonist (SB222200; white arrow) at −15 min abolished the LH response to an iv bolus injection of senktide (S) (50 μg; black arrow, triangle data points) given at time 0 but did not interfere with LH release when an iv bolus of KP-10 (K) (2 μg; black arrow, closed circle data points) was given at time 0. Administration of vehicle (V) at −15 min did not interfere with senktide induced LH release (open data points). Mean LH concentrations (±sem) are shown. n = 3.
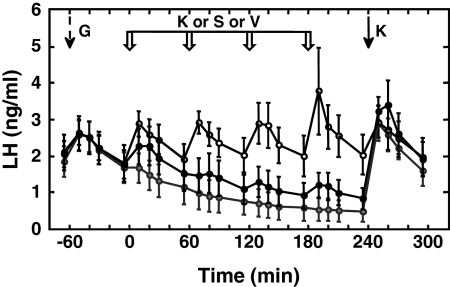
Experiment 6: effect of repetitive intermittent iv injections of senktide or Kp-10 on LH release (n = 3)
As anticipated, repetitive iv injection of Kp-10 at hourly intervals resulted in a sustained train of LH discharges with a magnitude similar to that produced by the intermittent GnRH-priming infusion (Fig. 5). Although the first injection of a similar regimen of repetitive 50 μg of senktide challenges elicited an LH discharge, responses to subsequent senktide injection were markedly blunted, and LH concentrations fell in parallel with those observed during repetitive vehicle administration (Fig. 5). The mean LH concentration during the gonadotropin discharge induced by the 4th pulse of Kp-10 (2.63 ± 0.69 ng/ml) was significantly greater than that induced by the corresponding pulse of either senktide (1.02 ± 0.32 ng/ml) or vehicle (0.60 ± 0.28 ng/ml). The difference between senktide and vehicle was not significant. The apparent down-regulation of the LH response to repetitive senktide administration did not compromise the action of Kp-10 to stimulate LH secretion 1 h after the last injection of the NK3R agonist (Fig. 5).
Figure 5.
Discharges of LH, as reflected by circulating concentrations of the gonadotropin (mean ± sem), during the last priming pulse of GnRH (G) (broken black arrow) in agonadal male monkeys were sustained without decrement by an intermittent iv infusion of 2 μg Kp-10 administered as a 1-min pulse every hour for 5 h and initiated at time 0 (open data points) after concomitantly terminating the GnRH-priming infusion. In striking contrast, a similar intermittent iv infusion of senktide (50 μg/pulse) after termination of GnRH priming failed to mimic the action of repetitive Kp-10 administration. Instead, LH levels (closed data points) fell in parallel with those observed during intermittent iv infusion of vehicle (gray data points). The down-regulation of the LH response to repetitive senktide administration, however, did not compromise the GnRH-releasing action of an iv bolus of Kp-10 (K) (black arrow) administered after the last of four pulses of senktide. White block arrows indicate time of hourly intermittent infusions of Kp-10 (K) or senktide (S) or vehicle (V). Black arrow at 240 min indicates time of the last of 5 Kp-10 pulses or of the Kp-10 challenge after the intermittent infusion of either senktide or vehicle. n = 3.
Experiment 7: immunohistochemical localization of NKB, kisspeptin, and GnRH in MBH of castrated adult male monkeys (n = 4)
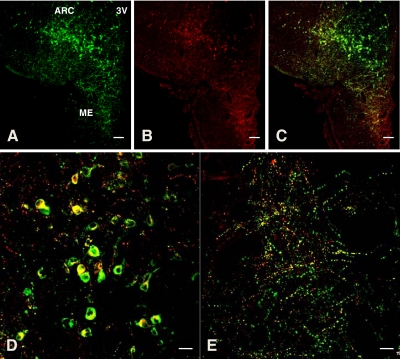
As anticipated, heavily immunolabeled kisspeptin perikarya were found throughout the rostro-caudal extent of the arcuate nucleus in the MBH of castrated adult monkeys. Although NKB staining of perikarya throughout the arcuate nucleus was also robust, only 40–60% of kisspeptin immunopositive cell bodies were costained for NKB (Figs. 6 and 7). Cell bodies immunopositive for only NKB were not observed in the arcuate nucleus. The median eminence, and particularly the internal zone, was richly innervated with both double labeled and kisspeptin labeled fibers (Fig. 6E). Also as anticipated, dense networks of GnRH axonal fibers were found throughout the median eminence, and in the internal zone, these were frequently associated with beaded NKB axons (Fig. 8).
Figure 6.
Confocal immunofluorescence projections showing colocalization of NKB (red fluorescence, Cy3) and kisspeptin (green fluorescence, Alexa 488) positive perikarya and fiber networks in the arcuate nucleus (ARC) and median eminence (ME), respectively, of a castrated adult male rhesus monkey. A–C, Confocal projections (magnification, ×10; 1-μm optical sections) showing immunopositive kisspeptin (A) and NKB (B) profiles and their merged image (C) in a coronal hemisection taken through the ARC. The third ventricle (3V) is seen on the right hand side of the half section. Note that the colocalization of NKB with kisspeptin (yellow) in both ARC and ME. D and E, Higher magnification (×40, 1-μm optical sections) confocal projections taken from the ARC (D) and the ME (E) of the section shown in A–C. Note that the colocalization of NKB and kisspeptin in many of the ARC kisspeptin positive perikarya (D) and in kisspeptin axonal projections in the ME (E). Scale bar, 100 μm (A–C) and 20 μm (D and E).
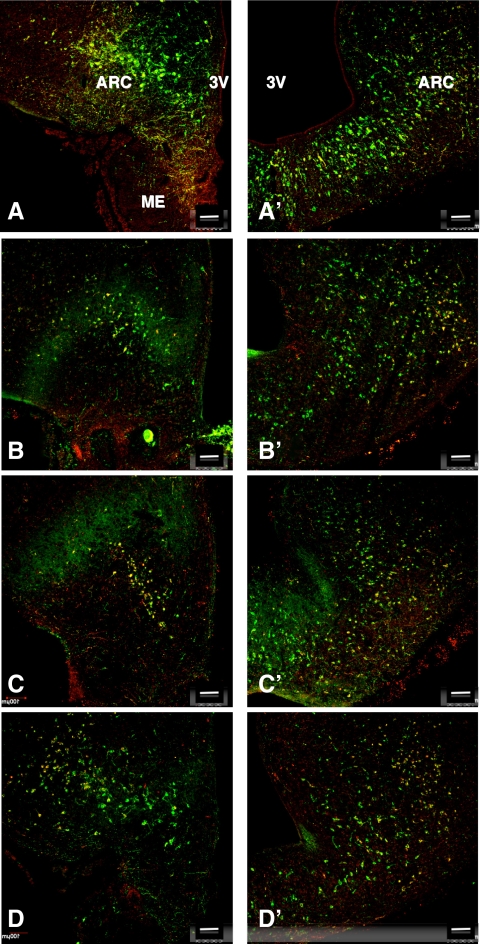
Figure 7.
Confocal dual immunofluorescence projections (magnification, ×10; 1-μm optical sections) of a pair of coronal hemi-MBH sections taken at the level of the arcuate nucleus (ARC)-median eminence (ME) region from each of four castrated adult male monkeys stained for NKB (red fluorescence, Cy3) and kisspeptin (green fluorescence, Alexa 488). The left hand section of each pair (A–D) is anterior with respect to the more caudal section shown on the right (A′–D′). Note that colocalization of the two peptides (yellow) in approximately 40–60% of kisspeptin positive neurons of the ARC. 3V, Third ventricle. Scale bar, 100 μm.
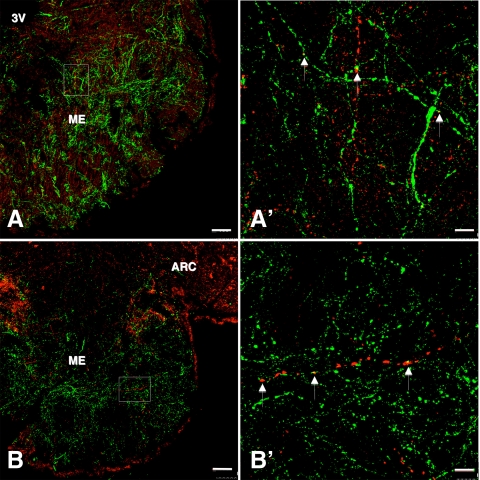
Figure 8.
A and B, Confocal immunofluorescence projections showing the distribution of NKB fibers (red fluorescence, Cy3) in relation to GnRH axons (green fluorescence, Alexa 488) in coronal hemisections of the median eminence (ME) of two castrated adult male monkeys (magnification, ×10; 1-μm optical sections). A′ and B′, Confocal projections (magnification, ×100; 1-μm optical sections) of NKB-GnRH interactions in regions of the internal zone of the median eminence shown in the area outlined by the rectangles in A and B, respectively. Contacts between NKB and GnRH fibers (white arrows) were confirmed by examination of individual optical sections. 3V, Third ventricle; ARC, lateral arcuate nucleus. Scale bars, 100 μm (A and B) and 10 μm (A′ and B′).
Discussion
The present study was prompted by the recent findings that inactivating mutations of the genes that encode for either the ligand or receptor in the NKB-NK3R signaling pathway is associated with hypogonadotropic-hypogonadism in man (6,7). Because the human genetics indicates that NKB is likely to be involved in a pathway stimulatory to GnRH and LH release, this possibility was examined in the monkey employing an experimental paradigm, where endogenous GnRH release is minimal to enhance the detection of any stimulatory action of NK3R activation. As for analogous earlier studies of the GnRH-releasing action of kisspeptin (26), the juvenile male rhesus monkey was again selected for this purpose, because at this stage of development, administration of GnRH secretogogues readily elicits robust discharges of the releasing factor in the face of minimal spontaneous secretion. The monkeys were again first castrated to eliminate the potential confounding effects of gonadal steroid feedback to the hypothalamus and pituitary. GnRH release was monitored indirectly by tracking LH secretion after the pituitary of the juvenile males had been sensitized to the releasing action of GnRH with a chronic intermittent infusion of the synthetic decapeptide, as previously described on numerous occasions (16,17,26).
Although NKB injected as a 100 μg iv bolus elicited a robust discharge of LH from the GnRH-primed pituitary of the juvenile monkey, we chose not to use the native NK3R ligand routinely, because concentrated DMSO was required as vehicle to keep the peptide in solution. Instead, most experiments were conducted with senktide, a synthetic peptide agonist of NK3R (20) soluble in saline. Single iv boluses of senktide elicited a dose-related increase in the amplitude of the LH discharge. Moreover, senktide (and also NKB)-induced LH release was abolished by pretreatment with a GnRH-R antagonist (acyline), establishing the GnRH dependency of the induced LH release, and indicating that the site of action of the NK3R agonists, as previously established for kisspeptin (26), was at the level of the hypothalamus. Senktide administered as an iv bolus of 50 μg elicited a discharge of LH comparable with that observed in response to 0.3 μg of GnRH. This challenge of GnRH elicits an LH discharge with a magnitude similar to that released spontaneously in adult males in response to endogenous pulsatile GnRH release (21), and therefore, it seems reasonable to propose that the GnRH discharge from the hypothalamus of the juvenile in response to a single 50-μg dose of senktide administered iv is roughly equivalent to that produced spontaneously in pubertal and postpubertal animals. Previous studies had established that the corresponding dose of Kp-10 that induces a physiological discharge of LH was 2 μg (26), and in the present study, this dose of Kp-10 produced an LH discharge with an amplitude marginally greater than that observed in response to 50 μg of senktide. If assumptions are made that the clearance of injected Kp-10 (decapeptide) and senktide (hexapeptide) from the circulation is similar and the distribution of the peptides to the hypothalamus is comparable, it would be concluded that Kp-10 is the most potent releaser of GnRH.
The present finding that activation of hypothalamic NK3R in the juvenile monkey is stimulatory to GnRH release is entirely consistent with the human genetics (6,7) but is at variance with recent studies of male mice, in which ip and intracerebroventricular (icv) administration of NKB was not associated with LH release (14). The foregoing difference is unlikely to be related to agonist dose, because in the study by Corander et al. (14), doses for ip injection of NKB ranged from 5–50 nmol, i.e. equivalent to 6–60 μg/mouse or approximately 30–300 μg/kg body weight, which compares with an iv dose of 25 μg/kg body weight in the present study. Similarly, addition of NKB to hypothalamic explants from male rats did not influence GnRH release (14). Studies of the effect on LH release of activating NK3R signaling in the female are inconsistent in nonprimates. Senktide administered icv to ewes in the follicular phase of the ovarian cycle and NKB to steroid (estradiol plus progesterone)-treated goats elicited robust LH secretion (15,27), and recent experiments with intact and estradiol-treated ovariectomized adult rats have also revealed a stimulatory action of icv senktide on LH release (Navarro, V., M. Tena-Sempere, and R. A. Steiner, unpublished data). On the other hand, icv injection of senktide in ovariectomized, estradiol-treated rats and in intact female mice (12,13) and to ovariectomized goats with and without estradiol replacement (15) resulted in an overall decrease in circulating LH concentrations. A reconciliation of these puzzling results may now be close at hand, because in the foregoing study of the ovariectomized goat, NKB administration consistently increased multiunit electrophysiological activity recorded in the region of the arcuate nucleus, but unequivocal evidence of concomitant LH release was only observed in the presence of progesterone when spontaneous GnRH pulse frequency was low (15). Thus, it seems reasonable to propose that the ability to demonstrate NK3R ligand-induced GnRH release, as monitored indirectly by LH secretion, is dependent in part on spontaneous GnRH pulse frequency. This would explain the robust LH responses observed in the present study of a prepubertal primate, where GnRH pulsatility is developmentally restrained (10). It should be noted that the present finding of NKB-induced GnRH release in the juvenile monkey does not address the relative importance of this tachykinin in driving GnRH release at those stages of primate development when GnRH pulsatility is robust, i.e. during fetal, infantile, pubertal, and postpubertal development (10). That the contribution of NKB signaling to GnRH pulse generation may vary throughout primate development has been proposed based on the recent finding that the hypogonadotropism associated with TAC3/TACR3 mutations in pubertal-aged children was attenuated in several subjects after treatment for delayed pubertal development (usually sex steroids) had been discontinued (28).
The action of iv administered NKB or senktide on GnRH release is most likely to be exerted directly on GnRH neurons. Elegant immunohistochemical studies of the rat hypothalamus by Rance and co-workers (29) have demonstrated prominent punctate colocalization of NK3R on GnRH axons innervating the internal and external layers of the median eminence. In addition, because GnRH perikarya in the rat hypothalamus were only occasionally found to express NK3R (29), it is reasonable to propose that the site of the stimulatory action of iv-administered NK3R agonists may be at the level of GnRH fibers in the median eminence, a suggestion consistent with the finding of intimate interactions between NKB and GnRH fibers in the median eminence, as first reported for the rat (29), and confirmed in the present study for the monkey. A similar argument has been put forward for a major site of the stimulatory action of kisspeptin on GnRH release residing at the level of the median eminence (19,30), although other sites of action on the GnRH neuron are not excluded.
The ability of intermittent iv bolus injections of Kp-10 at hourly intervals to elicit a corresponding train of sustained GnRH discharges from the hypothalamus of the agonadal juvenile, reported by this laboratory in 2006 (26), was again observed in the present study. In striking contrast, however, repetitive activation of NK3R at a similar hourly frequency with iv injections of 50 μg of senktide failed to sustain robust GnRH pulsatility, and as a result, circulating LH concentrations fell in parallel with those observed during vehicle administration. Although the first injection of senktide elicited an LH discharge, again with an amplitude marginally less than that of Kp-10, as observed in the earlier experiment, responses to subsequent injections of the NK3R agonist were blunted. The apparent down-regulation of NK3R to repetitive stimulation did not compromise the activity of KISS1R, as reflected by the robust GnRH discharge observed in response to Kp-10, 1 h after the last injection of senktide. The latter finding also eliminated the possibility that frequent injection of senktide compromised the pituitary response to GnRH.
As was anticipated, pretreatment with the NK3R antagonist, SB222200, abolished senktide-induced GnRH release. Because SB222200 did not interfere with the stimulatory action of Kp-10, it seems reasonable to propose that NK3R and KISS1R signaling pathways are independent or that NK3R is upstream from KISS1R, a suggestion consistent with the finding that down-regulation of the NKB pathway did not compromise KP-10-induced GnRH release. The effect of blocking KISS1R on the NKB pathway has not been studied to date, because kisspeptin antagonists (31) are not available in quantities sufficient for iv administration to large animals such as the monkey.
In addition to examining the neuroendocrine sequelae of activating NK3R signaling in the monkey, we also studied the anatomical relationship between NKB and kisspeptin-expressing neurons in the hypothalamus of this primate using double label fluorescence immunohistochemistry. Earlier studies of the mouse, ewe, and goat had demonstrated that these two neuropeptides or their mRNAs are coexpressed by a population of neurons in the arcuate nucleus (8,13,15), but this intriguing and important observation had not been extended to primates. Our findings in the adult monkey provide compelling evidence that, as in nonprimates, NKB is expressed in arcuate kisspeptin neurons of primates. Although not systematically studied, dual labeled somata appeared to be distributed evenly throughout the kisspeptin positive neurons that delineate the arcuate nucleus, and neurons labeled for only NKB were not observed. A similar situation was recapitulated for axonal labeling in the median eminence. In the sheep, more than 75% of kisspeptin somata in the arcuate nucleus were found to coexpress NKB whereas in the monkey only 40 to 60% of the kisspeptin neurons labeled for NKB. This difference may be more apparent than real and simply reflects differences in the relative abilities of the primary antibodies to detect their respective antigens.
In summary, the present study of the monkey provides the first description of a GnRH-releasing action of NK3R agonists in a primate. Although a single iv bolus of NKB and senktide, like that of kisspeptin, elicited a robust discharge of GnRH, repetitive activation of NK3R, in contrast to that of KISS1R, failed to sustain robust pulsatile GnRH release. Additionally, our immunohistochemical analysis extends to the monkey the finding, made originally in sheep, that NKB and kisspeptin are coexpressed by neurons in the arcuate nucleus and provides an anatomical basis, indicating the median eminence as a likely site for NKB signaling to GnRH neurons. Although the current work does not allow us to determine whether NKB and kisspeptin signaling interact hierarchically or in parallel to achieve pulsatile GnRH release, it does provide a framework upon which to begin to address this and other fascinating questions concerning the action of these peptides in driving the neuroendocrine axis governing reproduction.
Supplementary Material
Acknowledgments
We thank Dr. Robert B. Gibbs (Department of Pharmaceutical Sciences, University of Pittsburgh) for use of his confocal imaging facility; Dr. Stephen R. Bloom (Imperial College London, London, UK) and Dr. Henryk Urbanski (Oregon National Primate Research Center, Beaverton, OR) for the generous gift of primary antisera to kisspeptin and GnRH, respectively; Mr. Karthik Dwarki (University of Pittsburgh undergraduate) for his help with the immunohistochemistry; and to the Primate and Assay Core Staff (Mr. Mike Cicco, Ms. Rachel Roslund, and Ms. Carolyn Phalin) for their expert technical assistance.
Footnotes
The work in Pittsburgh and at Massachusetts General Hospital was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD08610 and U54 HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and National Institutes of Health Grant R01 HD13254 (to T.M.P.). P.C. was supported by Institut National de la Santé et de la Recherche Médicale and the University of Bordeaux.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 23, 2010
For editorial see page 4090
Abbreviations: DMSO, Dimethylsulfoxide; GnRH-R, GnRH receptor; icv, intracerebroventricular; KISS1R, kisspeptin receptor; Kp-10, kisspeptin-10; KPBS, potassium PBS; MBH, mediobasal hypothalamus; NK3R, NKB receptor; NKB, neurokinin B.
References
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Apaicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS-1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2008 New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69 [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S 2009 Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta). Peptides 30:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA 2009 Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK 2009 Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab 94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin neurons in the arcuate nucleus of the ewe express both dynormphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR 1996 KiSS1, a novel human malignant melanoma metastis-suppressor gene. J Natl Cancer Inst 88:1731–1737 [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF 2006 Puberty in non-human primates and humans. In: Challis JRG, de Kretser DM, Neill JD, Pfaff DW, Plant TM, Richards JS, Wassarman PM, eds. Knobil and Neill’s Physiology of Reproduction. 3rd ed. San Diego, CA: Elsevier; 2177–2230 [Google Scholar]
- Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW 2004 Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacol Res 50:611–615 [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE 2004 Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA 2009 Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP 2010 A study of the effects of neurokinin B upon gonadotrophin release in male rodents. J Neuroendocrinol 22:181–187 [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H 2010 Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillations of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Pohl CR, Plant TM 1998 The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta). Endocrinology 139:2774–2783 [DOI] [PubMed] [Google Scholar]
- Shahab M, Balasubramaniam A, Sahu A, Plant TM 2003 Central nervous system receptors involved in mediating the inhibitory action of neuropeptide Y on luteinizing hormone secretion in the male rhesus monkey (Macaca mulatta). J Neuroendocrinol 15:965–970 [DOI] [PubMed] [Google Scholar]
- Plant TM 1985 A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology 116:1341–1350 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM 2008 Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, Candenas ML 2004 Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 11:2045–2081 [DOI] [PubMed] [Google Scholar]
- Plant TM, Dubey AK 1984 Evidence from the rhesus monkey (Macaca mulatta) for the view that negative feedback control of luteinizing hormone secretion by the testis is mediated by a deceleration of hypothalamic gonadotropin-releasing hormone pulse frequency. Endocrinology 115:2145–2153 [DOI] [PubMed] [Google Scholar]
- Urbanski HF 1991 Monoclonal antibodies to luteinizing hormone-releasing hormone: production, characterization, and immunocytochemical application. Biol Reprod 44:681–686 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, Pohl CR, Friedman RL, Plant TM 2003 Inhibitory and stimulatory regulation of testicular inhibin B secretion by luteinizing hormone and follicle-stimulating hormone, respectively, in the rhesus monkey Macaca mulatta). Endocrinology 144:1175–1185 [DOI] [PubMed] [Google Scholar]
- El Majdoubi M, Ramaswamy S, Sahu A, Plant TM 2000 Effects of orchidectomy on levels of the mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol 12:167–176 [DOI] [PubMed] [Google Scholar]
- Watson Jr RE, Wiegand SJ, Clough RW, Hoffman GE 1986 Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, DiPietro MJ 2006 Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- McManus CJ, Valent M, Connors JM, Goodman RL, Lehman MN, A neurokinin B agonist stimulates LH secretion in follicular, but not luteal phase, ewes. Program of the 35th Annual Meeting of The Society for Neuroscience, Washington, DC, 2005 (Abstract 760.8) [Google Scholar]
- Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB 2010 TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE 2005 Morphologic evidence that neurokinin B modulates gonadotropin-stimulating hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH 2008 Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP 2009 Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.