Abstract
SH2B1 is an SH2 domain-containing adaptor protein that plays a key role in the regulation of energy and glucose metabolism in both rodents and humans. Genetic deletion of SH2B1 in mice results in obesity and type 2 diabetes. Single-nucleotide polymorphisms in the SH2B1 loci and chromosomal deletions of the SH2B1 loci associate with obesity and insulin resistance in humans. In cultured cells, SH2B1 promotes leptin and insulin signaling by binding via its SH2 domain to phosphorylated tyrosines in Janus kinase 2 and the insulin receptor, respectively. Here we generated three lines of mice to analyze the role of the SH2 domain of SH2B1 in the central nervous system. Transgenic mice expressing wild-type, SH2 domain-defective (R555E), or SH2 domain-alone (ΔN503) forms of SH2B1 specifically in neurons were crossed with SH2B1 knockout mice to generate KO/SH2B1, KO/R555E, or KO/ΔN503 compound mutant mice. R555E had a replacement of Arg555 with Glu within the SH2 domain. ΔN503 contained an intact SH2 domain but lacked amino acids 1-503. Neuron-specific expression of recombinant SH2B1, but not R555E or ΔN503, corrected hyperphagia, obesity, glucose intolerance, and insulin resistance in SH2B1 null mice. Neuron-specific expression of R555E in wild-type mice promoted obesity and insulin resistance. These results indicate that in addition to the SH2 domain, N-terminal regions of neuronal SH2B1 are also required for the maintenance of normal body weight and glucose metabolism. Additionally, mutations in the SH2 domain of SH2B1 may increase the susceptibility to obesity and type 2 diabetes in a dominant-negative manner.
The SH2 domain of SH2B1 is required but not sufficient for the regulation of body weight, insulin sensitivity, and glucose metabolism.
The brain controls energy balance and body weight by sensing and integrating numerous neuronal and metabolic signals (1). Leptin and insulin serve as key metabolic signals that convey information about peripheral energy storage and availability to the brain (1,2). Leptin is secreted by adipocytes in direct proportion to white adipose tissue mass and reduces body weight by suppressing food intake and promoting energy expenditure (1,2). Leptin promotes weight loss mainly by activating its receptor in the hypothalamus (1). Insulin is secreted from pancreatic β-cells in response to glucose. Brain-restricted deletion of the insulin receptor in mice results in obesity, suggesting that insulin signaling in the central nervous system (CNS) is also involved in body weight regulation (3). Paradoxically, the levels of both circulating leptin and insulin are markedly elevated in obese individuals (4,5,6), suggesting that leptin and/or insulin resistance in the CNS may underlie the pathogenesis of obesity. Obesity is the primary risk factor for insulin resistance and type 2 diabetes.
Impaired leptin or insulin signaling is likely to be the primary determinant for leptin or insulin resistance, respectively (1,7). We reported that SH2B1 mediates both leptin and insulin signaling in cultured cells (8,9,10). SH2B1 is a cytoplasmic pleckstrin homology (PH) and SH2 domain-containing adaptor molecule (11). The SH2B family consists of three members: SH2B1 (previously known as SH2-B), SH2B2 (also named adapter protein with a PH and an SH domain, APS), and SH2B3 (Lnk) (1,12). SH2B3 expression is restricted to hematopoietic cells and regulates immune responses (13,14,15). SH2B2/APS has been proposed to mediate insulin stimulation of glucose uptake in adipocytes (16); however, genetic deletion of SH2B2/APS does not alter glucose metabolism or insulin sensitivity in mice (17,18). In contrast, genetic deletion of SH2B1 results in severe leptin resistance, insulin resistance, obesity, and type 2 diabetes in mice (8,9). Moreover, neuron-specific restoration of SH2B1 reverses the obesity and type 2 diabetes phenotypes in SH2B1 null mice (19). Human SH2B1 may similarly regulate body weight and glucose metabolism. Single-nucleotide polymorphisms within the human SH2B1 loci are associated with leptin resistance and obesity (20,21,22,23). Chromosomal deletions that eliminate the SH2B1 gene are also associated with severe obesity and insulin resistance in humans (24,25). Collectively these findings indicate that the SH2B1 gene has a conserved role in the control of both body weight and glucose homeostasis in rodents and humans, presumably by promoting leptin and insulin signaling.
SH2B1 binds directly to Janus kinase (JAK)-2 via its SH2 domain (11). JAK2 is a cytoplasmic tyrosine kinase that mediates leptin signaling (1). Leptin stimulates JAK2 autophosphorylation at tyrosine813, which subsequently binds to the SH2 domain of SH2B1 (10). The SH2B1-JAK2 interaction markedly enhances JAK2 activity, thus enhancing leptin signaling (10,26,27). SH2B1 also directly binds to insulin receptor substrate (IRS)-1 and IRS2, two key upstream activators of the phosphatidylinositol 3-kinase pathway, promoting leptin stimulation of the phosphatidylinositol 3-kinase/Akt pathway (28). Additionally, SH2B1 directly binds via its SH2 domain to the insulin receptor (IR) (29,30). The SH2B1-IR interaction markedly stimulates IR kinase activity to enhance insulin signaling in cultured cells (8,31,32,33). Disruption of the SH2 domain of SH2B1 completely abrogates the ability of SH2B1 to stimulate both leptin and insulin signaling (8,28). Interestingly, the SH2 domain of SH2B1 alone is sufficient to stimulate the kinase activity of both JAK2 and IR in vitro and in cultured cells (33,34). It is likely that the physical interaction of the SH2 domain of SH2B1 with JAK2 or IR induces a conformational change in JAK2 or IR, thus promoting the catalytic activity of JAK2 or IR.
In this study, we examined the role of the SH2 domain of SH2B1 in vivo. We first confirmed our previous findings that neuronal SH2B1 plays a key role in controlling both body weight and glucose metabolism in mice. We then demonstrated that the intact SH2 domain of SH2B1 was required for the maintenance of normal body weight and glucose homeostasis in mice. A SH2 domain-defective variant of SH2B1 functioned in a dominant-negative manner in the CNS to promote obesity and insulin resistance. Furthermore, the SH2 domain of SH2B1 alone was unable to regulate body weight and glucose metabolism in mice. These results suggest that neuronal SH2B1 controls body weight and glucose metabolism by both SH2 domain-dependent and -independent mechanisms.
Materials and Methods
Generation of transgenic and compound mutant mice
SH2B1 knockout (KO) and transgenic (Tg-SH2B1) mice have been described (8,19). Briefly, cDNAs encoding wild-type rat SH2B1β (SH2B1), SH2B1β(R555E) (R555E), or SH2B1β (ΔN503) (ΔN503) were inserted 3′ of the rat neuron-specific enolase (NSE) promoter and GH enhancer sequences as described previously (19,35). R555E is a SH2 domain-defective mutant in which the conserved Arg555 residue within the SH2 domain of SH2B1β is replaced with Glu, whereas ΔN503 is an N-terminally truncated form of SH2B1β containing an intact SH2 domain (amino acids 504-670). A Myc-tag was engineered at the N terminus of SH2B1, R555E, or ΔN503 to facilitate the detection of recombinant proteins. These cDNA constructs were used to generate recombinant SH2B1, R555E, and ΔN503 transgenic mice, respectively, at the University of Michigan Transgenic Animal Model Core facilities. Ten R555E and 11 ΔN503 founders were obtained. One line of SH2B1, two independent lines of R555E mice (no. 205 and 244), and two lines of ΔN503 mice (no. 301 and 315) (C57BL/6 × SJL background) were characterized in this study.
SH2B1, R555E, or ΔN503 transgenic mice were crossed with SH2B1 KO mice to generate KO/SH2B1, KO/R555E, or KO/ΔN503 compound mutant mice (C57BL/6 × SJL background). Mice were housed on a 14-h light, 10-h dark cycle in the Unit for Laboratory Animal Medicine at the University of Michigan. Mice were fed a normal rodent diet (9% fat; Lab Diet; Purina Mills, St. Louis, MO) ad libitum with free access to water. Animal experiments were conducted following protocols approved by the University Committee on the Use and Care of Animals.
Immunoprecipitation and immunoblotting
Mice (fasted 16 h) were anesthetized with Avertin (0.5 g tribromoethanol and 0.25 g tert-amyl alcohol in 39.5 ml water; 0.02 ml/g body weight) and treated with PBS or insulin (3 U/kg body weight; Eli Lilly, Indianapolis, IN) via inferior vena cava injection. Five minutes after stimulation, gastrocnemius muscles and liver were dissected, frozen in liquid nitrogen, and stored at −80 C until analysis. Tissues were homogenized in lysis buffer (50 mm Tris, pH 7.5; 1% Nonidet P-40; 150 mm NaCl; 2 mm EGTA; 1 mm Na3VO4; 100 mm NaF; 10 mm Na4P2O7; 1 mm phenylmethylsulfonyl fluoride; 10 g/ml aprotinin; and 10 g/ml leupeptin). Immunoprecipitation and immunoblotting were conducted as described previously (8). Proteins were visualized using ECL reagents (Amersham, Indianapolis, IN) or Odyssey infrared imaging system (Li-COR Biosciences, Lincoln, NE). SH2B1 antibodies have been described (8,19). Akt, phospho-specific Akt (Thr308), and Myc antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho-specific Akt (Ser473) antibody was from BioSource (Camarillo, CA).
Growth and body composition
Body weight was recorded weekly beginning at 4 wk of age. Mice were briefly anesthetized (2–3% isoflurane), and body composition was determined by dual-energy x-ray absorptiometry (PIXImus2 Dexa scanner; GE Lunar Corp., Madison, WI).
Measurements of food intake and energy expenditure
Mice were individually housed and food intake was recorded daily for 5–7 d. Metabolic rates were measured by indirect calorimetry (Oxymax Equal Flow system; Columbus Instruments, Columbus, OH). Mice were individually housed and acclimated for 24 h in metabolic cages. After acclimation, exhaust air was sampled for a 24-h period. Samples were recorded for 1 min at 27-min intervals and O2 and CO2 content was determined. Oxygen consumption and carbon dioxide production were normalized to lean body mass.
Serum analysis
Blood samples were collected from the tail vein. Blood glucose concentrations were determined using a glucometer (Glucometer Elite XL; Bayer Corp., Elkhart, IN). Plasma insulin levels were determined using a rat insulin ELISA kit (Crystal Chem, Inc., Chicago, IL).
Glucose and insulin tolerance tests (ITTs)
For glucose tolerance tests (GTTs), mice were fasted 16 h and d-glucose (2 g/kg body weight) was injected ip. Blood glucose was measured 0, 15, 30, 60, and 120 min after injection. For ITTs, mice were fasted for 6 h and human insulin (1 U/kg) was injected ip. Blood glucose was measured 0, 15, 30, and 60 min after injection.
Statistical analysis
Data are presented as means ± sem. Differences between groups were determined by two-tailed Student’s t tests or ANOVA. P < 0.05 was considered significant.
Results
Generation of transgenic mice expressing various forms of SH2B1 in the CNS
We previously reported that neuronal SH2B1 controls energy balance and body weight in mice (19). To define the role of the SH2 domain of neuronal SH2B1 in vivo, we generated SH2B1 transgenic mice in which wild-type or two mutant forms of SH2B1 were expressed specifically in neurons under the control of the neuron-specific enolase promoter (Fig. 1A). An N-terminal Myc tag was engineered to facilitate the detection of recombinant SH2B1. We previously showed that recombinant SH2B1 is expressed specifically in the brain, including the hypothalamus, but is not detectable in muscle, liver, adipose depots, and other peripheral tissues in SH2B1 transgenic mice (19).
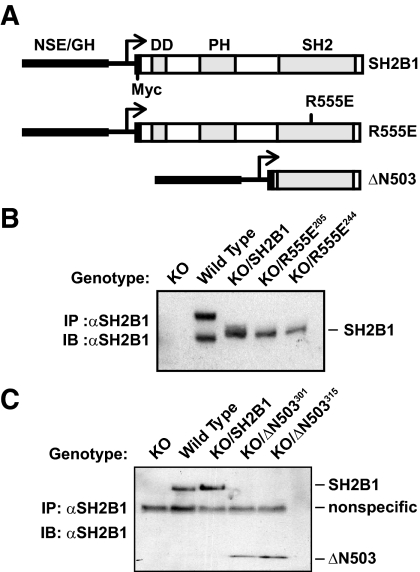
Figure 1.
Generation and verification of KO/SH2B1, KO/R555E, and KO/ΔN503 mice. A, Schematic representation of transgenic constructs used to generate SH2B1, R555E, and ΔN503 transgenic mice. The rat NSE promoter and GH enhancer sequences were fused to cDNA encoding Myc epitope tagged rat SH2B1β (SH2B1), dominant-negative SH2B1 (R555E), or amino-terminally truncated SH2B1 (ΔN503). The NSE promoter, GH enhancer, Myc epitope, DD, PH, and SH2 domains are indicated. B and C, SH2B1, R555E, and ΔN503 transgenic mice were crossed with SH2B1 KO mice to generate KO/SH2B1, KO/R555E (lines 205 and 244), and KO/ΔN503 (lines 301 and 315) mice. Protein extracts were prepared from the brains of males. Extracts (2 mg) were immunoprecipitated (IP) with anti-SH2B1 (αSH2B1) antibody and immunoblotted (IB) with αSH2B1. B, αSH2B1 was raised against full-length SH2B1. C, αSH2B1 was raised against ΔN503. Each lane represents a sample from one mouse.
To disrupt the SH2 domain, the conserved Arg555 residue within the SH2 domain of SH2B1 was replaced with Glu (designated R555E). In cultured cells, R555E acts as a dominant-negative mutant to inhibit the ability of endogenous SH2B1 to promote leptin and insulin signaling (8,28). To determine whether the SH2 domain alone is sufficient to mediate SH2B1 regulation of body weight, an N-terminally truncated mutant of SH2B1 lacking amino acids 1-503 was prepared and used to generate transgenic mice (ΔN503) (Fig. 1A). ΔN503 contains an intact SH2 domain and a short C terminus but lacks the PH and dimerization domains (DDs). In cultured cells, ΔN503 is sufficient to stimulate JAK2 activity and to enhance insulin signaling (27,33,34).
To introduce SH2B1 transgenes into the CNS of SH2B1 KO mice, SH2B1, R555E, or ΔΝ503 transgenic mice were crossed with KO mice to generate KO/SH2B1, KO/R555E, and KO/ΔN503 compound mutant mice, respectively. To verify neuron-specific expression of these SH2B1 transgenes, brain extracts were immunoprecipitated with anti-SH2B1 antibody (αSH2B1, raised against full length rat SH2B1β) and immunoblotted with αSH2B1. Two forms of endogenous SH2B1 were detected in wild-type (WT) but not KO mice (Fig. 1B). Importantly, only one form, corresponding to recombinant SH2B1 or R555E, was detected in the brains from KO/SH2B1 or KO/R555E mice (Fig. 1B). Recombinant R555E was detected in two independent lines of KO/R555E mice (no. 205 and 244), and R555E levels in both lines were comparable with SH2B1 levels in KO/SH2B1 mice (Fig. 1B). In agreement with our previous results, recombinant SH2B1 and R555E were specifically expressed in the brain, but not in skeletal muscle, the liver, or white and brown adipose tissue, of transgenic mice (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) (19). Because the αSH2B1 antibody used in Fig. 1B poorly recognized ΔN503 (data not shown), a second anti-SH2B1β antibody raised against amino acids 504–670 of rat SH2B1β was used to detect ΔN503 in immunoprecipitation and immunoblotting assays. ΔN503 was detected in the brains of two independent lines of KO/ΔN503 mice (no. 301 and 315) but not in WT, KO, and KO/R555E mice; ΔN503 levels were similar to SH2B1 levels in KO/SH2B1 (Fig. 1C).
The SH2 domain of SH2B1 is required for the maintenance of energy balance and body weight
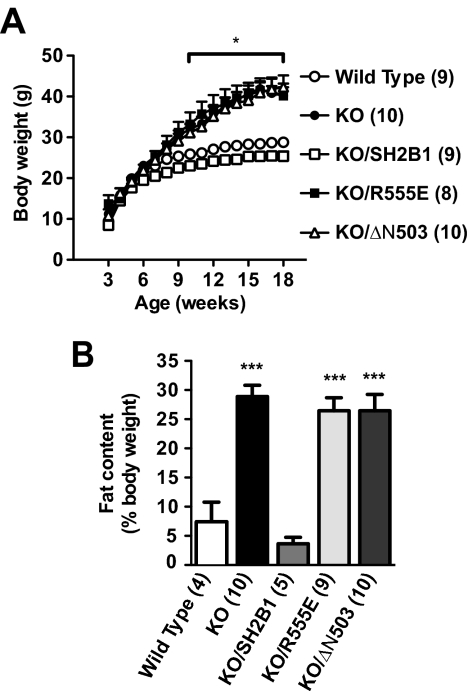
WT, KO, KO/SH2B1, and KO/R555E mice were fed a standard diet, and body weight was monitored weekly. KO males were significantly heavier than WT mice at 10 wk of age and continued to gain weight thereafter (Fig. 2A). KO mice were nearly 1.3 times heavier than WT mice at 16 wk of age. Neuron-specific restoration of SH2B1 prevented obesity in KO/SH2B1 mice (Fig. 2A), consistent with our previous observations (19). However, neuron-specific expression of R555E in KO/R555E males failed to reverse the obesity phenotypes observed in SH2B1 null mice (Fig. 2A). Body weight was similar between KO and KO/R555E mice (KO: 41.9 ± 1.8 g, n = 10; KO/R555E: 39.5 ± 2.2 g, n = 6; 16 wk). Moreover, both the onset and rate of weight gain were similar between KO and KO/R555E mice.
Figure 2.
The SH2 domain of SH2B1 in the CNS is required but not sufficient for the maintenance of normal body weight and adiposity in mice. A, Growth curves for WT, KO, KO/SH2B1, KO/R555E, and KO/ΔN503 male mice. B, Whole-body fat content in WT, KO, KO/SH2B1, KO/R555E, and KO/ΔN503 male mice (15–17 wk of age). The number in parentheses indicates the number of mice per group. *, P < 0.05; ***, P < 0.001.
Systemic deletion of SH2B1 markedly increased adiposity. Whole-body fat content was 3.5 times higher in KO males than WT males (Fig. 2B). Neuron-specific restoration of SH2B1 dramatically reduced adiposity in KO/SH2B1 mice (Fig. 2B), consistent with our previous findings (19). By contrast, neuron-specific expression of R555E failed to reduce adiposity in SH2B1 null mice, and fat content was similar between KO and KO/R555E mice (Fig. 2B). Deletion of SH2B1 also resulted in severe obesity in female mice, and neuron-specific expression of SH2B1, but not R555E, fully reversed the obesity phenotype in SH2B1 null females (data not shown).
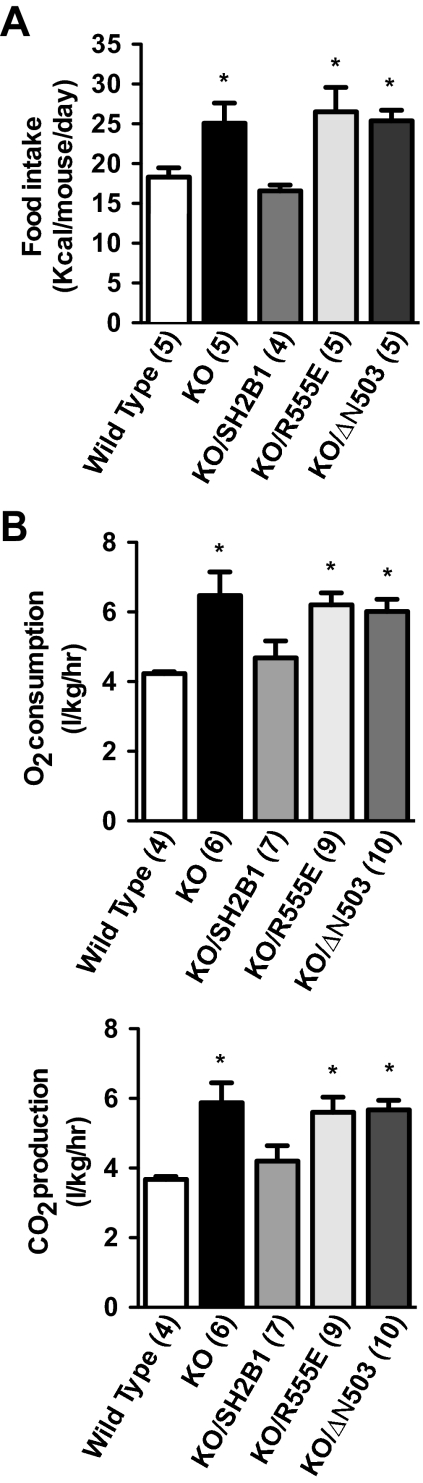
To gain insight into the underlying physiological mechanisms of SH2B1 regulation of body weight, we analyzed energy metabolism in male mice. Systemic deletion of SH2B1 significantly increased food intake in KO mice, and neuron-specific restoration of SH2B1 fully corrected hyperphagia in KO/SH2B1 mice (Fig. 3A). By contrast, neuron-specific expression of R555E was unable to suppress hyperphagia in KO/R555E mice (Fig. 3A). Previously we reported that energy expenditure is increased in KO mice (9); however, energy intake still exceeds expenditure in KO mice, resulting in positive energy imbalance and obesity (9). In agreement with these observations, deletion of SH2B1 increased oxygen consumption and carbon dioxide production in KO mice (Fig. 3B). Neuron-specific expression of SH2B1 normalized energy expenditure in KO/SH2B1 mice; in contrast, neuron-specific expression of R555E failed to normalize energy expenditure in KO/R555E mice (Fig. 3B). Two independent KO/R555E lines (no. 205 and 244) exhibited similar metabolic phenotypes (data not shown). Collectively these data indicate that the SH2 domain of SH2B1 is required for the maintenance of normal energy balance and body weight in mice.
Figure 3.
The SH2 domain of SH2B1 in the CNS is required but not sufficient for the maintenance of energy balance. A, Average daily food intake for WT, KO, KO/SH2B1, KO/R555E, and KO/ΔN503 male mice (13–14 wk of age). B, Oxygen consumption (VO2) and carbon dioxide production (VCO2) for WT, KO, KO/SH2B1, KO/R555E, and KO/ΔN503 male mice (15–17 wk of age). Energy expenditure was normalized to lean body mass. The number in parentheses indicates the number of mice per group. *, P < 0.05.
The SH2 domain of SH2B1 alone is not sufficient to regulate energy balance and body weight
The SH2 domain of SH2B1 alone is sufficient to stimulate the catalytic activity of both JAK2 and the insulin receptor in cultured cells (27,33,34). To determine whether the SH2 domain of SH2B1 alone is also sufficient to regulate body weight in mice, we measured body weight, adiposity, food intake, and energy expenditure in KO/ΔN503 mice. In KO/ΔN503 mice, ΔN503, an N-terminally truncated mutant of SH2B1 that contains the entire SH2 domain, was expressed specifically in the brain under the control of the neuron-specific enolase promoter in SH2B1 null mice (Fig. 1, A and C). As described above, neuron-specific restoration of SH2B1 fully reversed metabolic abnormalities in SH2B1 null mice. By contrast, neuron-specific expression of ΔN503 failed to reverse the hyperphagia and obesity phenotypes in KO/ΔN503 mice. Body weight (Fig. 2A), fat content (Fig. 2B), food intake (Fig. 3A), and energy expenditure (Fig. 3B) were similar between KO and KO/ΔN503 mice. Two independent lines of KO/ΔN503 mice (no. 301 and 315) exhibited similar metabolic disorders (data no shown). Thus, the SH2 domain of SH2B1 is required but not sufficient for the regulation of energy balance and body weight in mice.
Neuronal SH2B1 regulates glucose metabolism by SH2 domain-dependent and -independent mechanisms
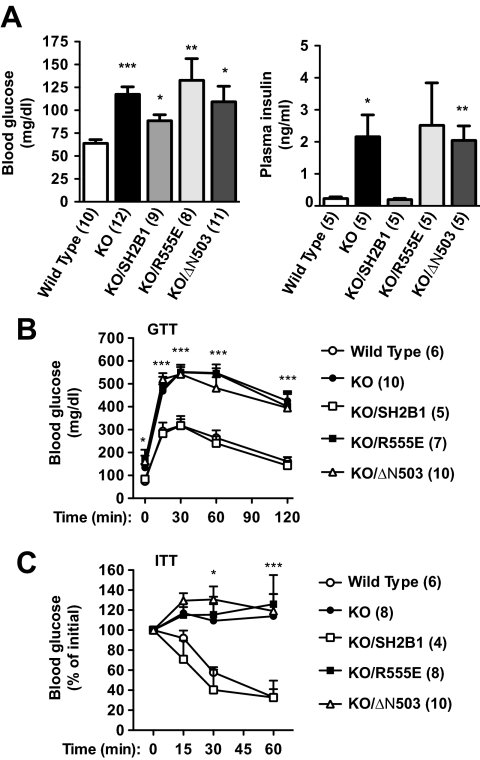
We previously reported that systemic deletion of SH2B1 resulted in severe insulin resistance, glucose intolerance, and type 2 diabetes in mice (8) and that neuron-specific restoration of SH2B1 rescues insulin resistance and glucose intolerance in SH2B1 null mice (19). To determine whether neuron-specific expression of R555E or ΔN503 also ameliorates insulin resistance and glucose intolerance, insulin sensitivity, and glucose homeostasis were examined in KO/R555E and KO/ΔN503 mice. Deletion of SH2B1 significantly increased both blood glucose and plasma insulin levels in KO mice, and neuron-specific restoration of SH2B1 rescued both hyperglycemia and hyperinsulinemia in KO/SH2B1 mice (Fig. 4A). By contrast, neuron-specific expression of neither R555E (in KO/R555E mice) nor ΔN503 (in KO/ΔN503 mice) ameliorated hyperglycemia and hyperinsulinemia in SH2B1 null mice (Fig. 4A).
Figure 4.
Neuron-specific expression of SH2B1, but not R555E or ΔN503, improves glucose homeostasis and insulin sensitivity in SH2B1 KO mice. A, Fasting (16 h) blood glucose and plasma insulin levels in WT, KO, KO/SH2B1, KO/R555E, and KO/ΔN503 male mice (17–18 wk of age). B, GTTs performed on WT, KO, KO/SH2B1, KO/R555E, and KO/ΔN503 male mice (18–19 wk of age). Mice were fasted overnight (16 h) and d-glucose (2 g/kg body weight) was administered ip. Blood glucose levels were measured 0, 15, 30, 60, and 120 min after injection. C, ITTs in male mice (18–19 wk of age). Mice were fasted for 6 h and human insulin (1 U/kg body weight) was administered ip. Blood glucose was measured 0, 15, 30, and 60 min after injection. Values are expressed as a percentage of initial glucose concentration (time 0). The number in parentheses indicates the number of mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further examine insulin sensitivity and glucose metabolism, GTTs and ITTs were performed. Glucose injection increased blood glucose to much higher levels at each time point in KO mice than wild-type littermates during the GTTs (Fig. 4B). Neuron-specific restoration of SH2B1 largely corrected glucose intolerance in SH2B1 null mice (Fig. 4B). By contrast, neuron-specific expression of neither R555E nor ΔN503 improved glucose intolerance, and glucose injection increased blood glucose to similar levels in KO, KO/R555E, and KO/ΔN503 mice (Fig. 4B). In ITTs, exogenous insulin reduced blood glucose levels in WT but not KO mice; neuron-specific restoration of SH2B1 restored insulin sensitivity in KO/SH2B1 mice (Fig. 4C). In contrast, neither R555E nor ΔN503 was able to restore insulin sensitivity in SH2B1 null mice, and exogenous insulin failed to reduce blood glucose levels in KO, KO/R555E, and KO/ΔN503 mice (Fig. 4C). Together these data indicate that the SH2 domain of SH2B1 in the CNS is required but not sufficient for the maintenance of normal insulin sensitivity and glucose homeostasis in vivo. Because the SH2 domain of SH2B1 is sufficient to stimulate JAK2 and the IR, the above observations further suggest that neuronal SH2B1 regulates body weight and glucose metabolism in vivo by new mechanisms in addition to enhancing the catalytic activity of JAK2 and the IR.
SH2B1 variants with a defective SH2 domain promote obesity, insulin resistance, and glucose intolerance in mice
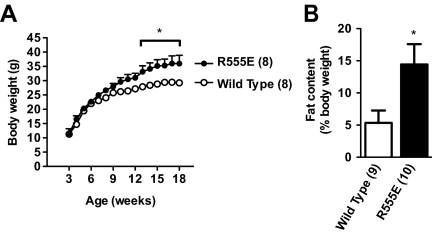
To determine whether SH2B1 variants with a defective SH2 domain act as dominant-negative mutants in vivo, R555E was expressed specifically in the brains of WT mice under the control of the neuron-specific enolase promoter (Fig. 1, A and B). Neuron-specific expression of R555E increased body weight and adiposity in R555E transgenic mice. R555E mice were significantly heavier than WT littermates after 13 wk of age (Fig. 5A). Whole-body fat content was significantly higher in R555E mice than WT littermates (Fig. 5B). By contrast, neuron-specific expression of Δ503 did not alter body weight or adiposity in WT mice (Supplemental Fig. 2, A and B). These data suggest that SH2 domain-defective variants of SH2B1 function in a dominant-negative manner to promote obesity by inhibiting endogenous SH2B1 in the brain.
Figure 5.
Neuron-specific expression of SH2 domain-defective R555E results in obesity in WT mice. A, Growth curves for WT and R555E transgenic male littermates. B, Whole-body fat content in WT and R555E male mice (15–17 wk of age). The number in parentheses indicates the number of mice per group. *, P < 0.05.
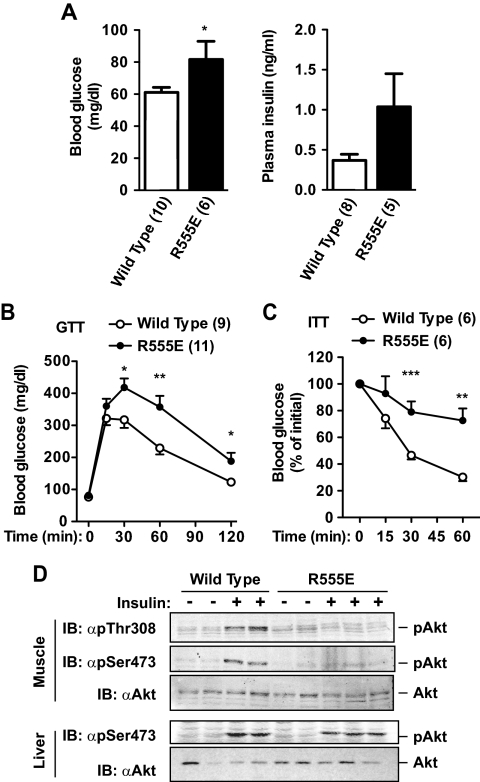
Neuron-specific overexpression of R555E markedly increased the levels of both blood glucose and plasma insulin in R555E transgenic mice (Fig. 6A). During GTTs, glucose injection increased blood glucose levels to higher levels in R555E mice than WT littermates (Fig. 6B). Neuron-specific overexpression of R555E also markedly reduced the ability of exogenous insulin to decrease blood glucose in ITTs (Fig. 6C). Both lines of R555E (no. 205 and 244) exhibited similar metabolic phenotypes (data not shown), indicating that these phenotypes are independent of the transgene integration sites. In contrast, neuron-specific expression of ΔN503 did not significantly alter insulin sensitivity and glucose metabolism in ΔN503 transgenic mice (Supplemental Fig. 2, C–E).
Figure 6.
Neuron-specific expression of R555E impairs systemic insulin sensitivity and glucose metabolism. A, Fasting (16 h) blood glucose and plasma insulin levels in WT and R555E transgenic males (17–18 wk of age). B, GTTs performed on WT and R555E male mice (18–19 wk of age; 2 g/kg body weight). C, ITTs in male mice (18–19 wk of age; 1 U/kg body weight). The number of mice in each group is indicated in parentheses. D, Male mice (20–21 wk) were fasted for 16 h and treated with PBS or insulin (3 U/kg body weight). Tissue extracts were prepared 5 min after stimulation. Liver and muscle extracts were immunoblotted with phospho-specific Akt antibodies against phospho-Thr308 (αp Thr308) or phospho-Ser473 (αp Ser473) and αAkt. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further confirm insulin resistance in R555E mice, insulin signaling was examined in skeletal muscle and liver isolated from WT and R555E transgenic mice treated with vehicle or insulin. Insulin stimulated Akt phosphorylation in both liver and muscle in WT mice; however, insulin-stimulated Akt phosphorylation was attenuated in these tissues from R555E mice (Fig. 6D). Taken together, these data indicate that SH2 domain-defective variants of SH2B1 can act as dominant-negative mutants in the CNS to impair SH2B1 function, resulting in systemic insulin resistance and glucose intolerance.
Discussion
SH2B1 is a newly identified key regulator of body weight and glucose metabolism (1,8,9). We previously reported that genetic deletion of SH2B1 results in severe leptin resistance, insulin resistance, obesity, and type 2 diabetes in mice (8,9). Recent studies confirm that SH2B1 is also a potential obesity gene in humans. Several groups independently reported that single-nucleotide polymorphisms within the human SH2B1 loci genetically link to leptin resistance and obesity (20,21,22,23,36). Chromosomal deletions that eliminate the SH2B1 gene are also associated with severe obesity and insulin resistance in humans (24,25). Interestingly, disruption of the Drosophila SH2B1 homolog also increases lipid accumulation and fat content in flies (45). Therefore, SH2B1 plays a key and conserved role in the control of energy and glucose metabolism in insects, rodents, and humans.
In this study, we observed that neuron-specific restoration of SH2B1 corrected hyperphagia, obesity, and type 2 diabetes in SH2B1 null mice, confirming our previous observations that neuronal SH2B1 is essential in regulating energy balance and body weight (19). Interestingly, neuron-specific expression of R555E, a SH2B1 variant with a defective SH2 domain, failed to correct obesity and type 2 diabetes in SH2B1 null mice, even though recombinant SH2B1 and R555E were expressed at similar levels in the brain. These data indicate that a functional SH2 domain of SH2B1 is required for the regulation of energy and glucose homeostasis. Furthermore, neuron-specific expression of R555E promoted the development of age-dependent obesity, insulin resistance, and glucose intolerance in mice with the intact SH2B1 gene. These findings suggest that SH2B1 variants with a defective SH2 domain can increase the susceptibility to obesity and type 2 diabetes by acting as dominant-negative mutants in the CNS to block normal SH2B1 action. In the future, it will be important to identify mutations within the SH2 domain of SH2B1 that genetically link to obesity and type 2 diabetes in at-risk human populations.
The SH2 domain of SH2B1 binds to phosphorylated tyrosines in many signaling molecules, including JAK2, IRS1, IRS2, and the IR (1,11,26,28,29,30,33). These SH2B1 binding partners mediate leptin (e.g. JAK2, IRS1, and IRS2) and insulin (e.g. IRS1, IRS2, and IR) signaling (1,7). Disruption of the SH2 domain is expected to block the physical interaction and coupling of SH2B1 with these signaling molecules, thus impairing leptin and insulin sensitivity. Additionally, SH2B1 homodimerizes or multimeric oligomerizes via its N-terminal DD (37,38). SH2B1 dimers or multimeric oligomers are likely to assemble signaling complexes containing multiple JAK2, IRS1, and/or IRS2 molecules. SH2B1 variants with a defective SH2 domain are predicted to be capable of dimerizing or oligomerizing with SH2B1 via their DDs but incapable of binding to phosphorylated tyrosines in JAK2 and IRS proteins. In this manner, SH2 domain-defective mutants function as dominant negatives to disrupt the formation of SH2B1 signaling complexes. Consistent with this idea, SH2B2β, an isoform of SH2B2 that has an intact DD but lacks the SH2 domain, binds to SH2B1 and antagonizes the ability of SH2B1 to promote leptin signaling in cultured cells (39). Therefore, SH2B1 variants with a defective SH2 domain, including R555E, are likely to increase the susceptibility to obesity and type 2 diabetes at least in part by disrupting the formation of SH2B1 signaling complexes.
The SH2 domain of SH2B1 alone is sufficient to stimulate the kinase activity of both JAK2 and insulin receptors both in vitro and in cultured cells (26,33,34). However, an N-terminally truncated SH2B1 containing the entire SH2 domain was unable to regulate body weight and glucose metabolism in vivo. Neuron-specific expression of SH2B1, but not ΔN503 that contains the intact SH2 domain, corrected hyperphagia, obesity, and type 2 diabetes in SH2B1 null mice. Therefore, neuronal SH2B1 regulates body weight and glucose metabolism in vivo by multiple mechanisms in addition to enhancing the catalytic activity of JAK2 and the IR. These results also suggest that the N-terminal regions of SH2B1 (amino acids 1–503) play a key role in assembling the aforementioned SH2B1 signaling complexes. These SH2B1 signaling complexes likely play an essential role in the maintenance of normal body weight and glucose homeostasis in animals.
SH2B1 is primarily cytoplasmic. However, SH2B1 contains both a nuclear localization sequence and a nuclear export sequence in its N terminus. A fraction of SH2B1 shuttles between the cytoplasmic and nuclear compartments via these nuclear localization sequences and nuclear export sequences (40,41). Additionally, SH2B1 translocates to the plasma membrane upon ligand stimulation (42,43,44). However, the physiological functions of SH2B1 trafficking remain largely unknown. SH2B1 trafficking, which is mediated by its N-terminal regions, may also be involved in nutrient sensing in the CNS. Interestingly, neuronal SH2B1 suppresses energy expenditure, which cannot be explained by SH2B1 regulation of leptin and insulin signaling in the CNS. SH2B1 trafficking may be involved in leptin- and/or insulin-independent regulation of energy expenditure.
In summary, we demonstrated that neuron-specific expression of SH2B1, but not R555E or ΔN503, corrected obesity and type 2 diabetes in SH2B1 null mice. Neuron-specific expression of R555E also increased susceptibility to both obesity and type 2 diabetes in WT mice. These results indicate that the SH2 domain of SH2B1 is required but not sufficient for the maintenance of normal body weight and glucose homoeostasis. Therefore, neuronal SH2B1 controls body weight and glucose metabolism through multiple pathways that are activated by distinct domains of SH2B1 molecules.
Supplementary Material
Acknowledgments
We thank Drs. Liang Sheng, Decheng Ren, Zhiqing Li, Yingjiang Zhou, Minghua Li, and Lin Jiang for their technical support and helpful discussion.
Footnotes
This work was supported by National Institute of Health (NIH) Grants RO1 DK 065122, RO1 DK073601 (to L.R.), and F31NS056575 (to D.L.M.), and American Diabetes Association Award 1-09-RA-156 (to L.R.). This work used the cores supported by the Michigan Diabetes Research and Training Center (funded by NIH Grant 5P60 DK20572), the University of Michigan’s Cancer Center (funded by NIH Grant 5 P30 CA46592), the University of Michigan Nathan Shock Center (funded by NIH Grant P30AG013283), and the University of Michigan Gut Peptide Research Center (funded by NIH Grant DK34933).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 19, 2010
Abbreviations: APS, Adapter protein with a PH and an SH domain; CNS, central nervous system; DD, dimerization domain; GTT, glucose tolerance test; ITT, insulin tolerance test; IR, insulin receptor; IRS, insulin receptor substrate; JAK, Janus kinase; KO, knockout; ΔN503, SH2B1β(ΔN503); NSE, neuron-specific enolase; PH, pleckstrin homology; R555E, SH2B1β(R555E); SH2, Src homology 2; WT, wild type.
References
- Morris DL, Rui L 2009 Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 297:E1247–E1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Porte Jr D 2005 Diabetes, obesity, and the brain. Science 307:375–379 [DOI] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF 1996 Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM 1995 Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161 [DOI] [PubMed] [Google Scholar]
- Hamilton BS, Paglia D, Kwan AY, Deitel M 1995 Increased obese mRNA expression in omental fat cells from massively obese humans. Nat Med 1:953–956 [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR 2001 Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806 [DOI] [PubMed] [Google Scholar]
- Duan C, Yang H, White MF, Rui L 2004 Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol 24:7435–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Li M, Duan C, Rui L 2005 Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2:95–104 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhou Y, Carter-Su C, Myers Jr MG, Rui L 2007 SH2B1 Enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol 21:2270–2281 [DOI] [PubMed] [Google Scholar]
- Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C 1997 Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol 17:6633–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures TJ, Kurzer JH, Carter-Su C 2007 SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab 18:38–45 [DOI] [PubMed] [Google Scholar]
- Takaki S, Sauer K, Iritani BM, Chien S, Ebihara Y, Tsuji K, Takatsu K, Perlmutter RM 2000 Control of B cell production by the adaptor protein lnk. Definition of a conserved family of signal-modulating proteins. Immunity 13:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T 2002 Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med 195:1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Zhang J, Lodish HF 2005 Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood 105:4604–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kimura A, Baumann CA, Saltiel AR 2002 APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol Cell Biol 22:3599–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ren D, Iseki M, Takaki S, Rui L 2006 Differential role of SH2-B and APS in regulating energy and glucose homeostasis. Endocrinology 147:2163–2170 [DOI] [PubMed] [Google Scholar]
- Minami A, Iseki M, Kishi K, Wang M, Ogura M, Furukawa N, Hayashi S, Yamada M, Obata T, Takeshita Y, Nakaya Y, Bando Y, Izumi K, Moodie SA, Kajiura F, Matsumoto M, Takatsu K, Takaki S, Ebina Y 2003 Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 52:2657–2665 [DOI] [PubMed] [Google Scholar]
- Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L 2007 Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest 117:397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, et al. 2009 Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K 2009 Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41:18–24 [DOI] [PubMed] [Google Scholar]
- Renström F, Payne F, Nordström A, Brito EC, Rolandsson O, Hallmans G, Barroso I, Nordström P, Franks PW 2009 Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet 18:1489–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, van Vliet-Ostaptchouk JV, Wijmenga C, van der Schouw YT 2009 Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr 90:951–959 [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S, Hamilton-Shield J, Clayton-Smith J, O'Rahilly S, Hurles ME, Farooqi IS 2010 Large, rare chromosomal deletions associated with severe early-onset obesity. Nature 463:666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, Falchi M, Chen F, Andrieux J, Lobbens S, Delobel B, Stutzmann F, El-Sayed Moustafa JS, Chèvre JC, Lecoeur C, Vatin V, Bouquillon S, Buxton JL, Boute O, Holder-Espinasse M, Cuisset JM, Lemaitre MP, Ambresin AE, Brioschi A, Gaillard M, et al. 2010 A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature 463:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzer JH, Saharinen P, Silvennoinen O, Carter-Su C 2006 Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol Cell Biol 26:6381–6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Carter-Su C 1999 Identification of SH2-bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA 96:7172–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Li M, Rui L 2004 SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem 279:43684–43691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H, Wang J, Hansen H, Yousaf N 1997 PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J Biochem (Tokyo) 122:1105–1113 [DOI] [PubMed] [Google Scholar]
- Kotani K, Wilden P, Pillay TS 1998 SH2-Bα is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem J 335(Pt 1):103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Pillay TS 2003 Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem J 371:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Deng Y, Tandon R, Bai C, Riedel H 2008 Essential role of PSM/SH2-B variants in insulin receptor catalytic activation and the resulting cellular responses. J Cell Biochem 103:162–181 [DOI] [PubMed] [Google Scholar]
- Morris DL, Cho KW, Zhou Y, Rui L 2009 SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes 58:2039–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Gunter DR, Herrington J, Carter-Su C 2000 Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-bβ. Mol Cell Biol 20:3168–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski TJ, Liu SM, Leibel RL, Chua Jr SC 2001 Transgenic complementation of leptin-receptor deficiency: I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 50:425–435 [DOI] [PubMed] [Google Scholar]
- Jamshidi Y, Snieder H, Ge D, Spector TD, O'Dell SD 2007 The SH2B gene is associated with serum leptin and body fat in normal female twins. Obesity (Silver Spring) 15:5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Werner ED, Oh BC, Frantz JD, Dhe-Paganon S, Hansen L, Lee J, Shoelson SE 2005 Kinase activation through dimerization by human SH2-B. Mol Cell Biol 25:2607–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Ginty DD 2001 SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol Cell Biol 21:1613–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li Z, Morris DL, Rui L 2007 Identification of SH2B2β as an inhibitor for SH2B1- and SH2B2α-promoted Janus kinase-2 activation and insulin signaling. Endocrinology 148:1615–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Carter-Su C 2004 Adapter protein SH2-Bβ undergoes nucleocytoplasmic shuttling: implications for nerve growth factor induction of neuronal differentiation. Mol Cell Biol 24:3633–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures TJ, Chen L, Carter-Su C 2009 Nucleocytoplasmic shuttling of the adapter protein SH2B1β (SH2-Bβ) is required for nerve growth factor (NGF)-dependent neurite outgrowth and enhancement of expression of a subset of NGF-responsive genes. Mol Endocrinol 23:1077–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakonova M, Helfer E, Seveau S, Swanson JA, Kocks C, Rui L, Carlier MF, Carter-Su C 2007 Adapter protein SH2-Bβ stimulates actin-based motility of Listeria monocytogenes in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion. Infect Immun 75:3581–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J, Diakonova M, Rui L, Gunter DR, Carter-Su C 2000 SH2-B is required for growth hormone-induced actin reorganization. J Biol Chem 275:13126–13133 [DOI] [PubMed] [Google Scholar]
- Diakonova M, Gunter DR, Herrington J, Carter-Su C 2002 SH2-Bβ is a Rac-binding protein that regulates cell motility. J Biol Chem 277:10669–10677 [DOI] [PubMed] [Google Scholar]
- Song W, Ren D, Li W, Jiang L, Cho KW, Huang P, Fan C, Song Y, Liu Y, Rui L 2010 SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab 5:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.