Abstract
Synaptic vesicle protein 2A (SV2A) has been identified as the binding site for the antiepileptic drug levetiracetam and is thought to decrease neuronal excitability. Since knockout of SV2A in mice leads to seizures, we hypothesized that a reduction in SV2A expression promotes seizure generation in epilepsy-associated brain tumors. We compared the SV2A expression and distribution in surgically removed tumor tissue (n = 63) and peritumoral cortex (n = 31) of patients with glial and glioneuronal tumors to normal control cortex obtained at autopsy in nonbrain tumor patients (n = 6). Additionally, we compared the SV2A expression and distribution in tumor patients with epilepsy (n = 39) with SV2A expression in tumor patients without epilepsy (n = 24). Immunohistochemistry in control cortex demonstrated strong and diffuse SV2A immunoreactivity (IR) throughout all cortical layers. Similar strong SV2A IR (with the same diffuse distribution pattern) was observed in the peritumoral cortical specimens in both patients with and without epilepsy. Modest SV2A IR was observed within the tumor area. The SV2A-positive cells detected within the tumor area were mainly entrapped neurons. Oligodendrogliomas and glioneuronal tumors displayed variable SV2A neuropil staining. In ganglioglioma (GG), strong SV2A IR was present along the dysplastic neuronal cell borders and processes. In both GG and dysembryoplastic neuroepithelial tumors, SV2A IR was occasionally observed within the neuronal perikarya. We found no differences in SV2A expression in the peritumoral cortex between the patients with and without epilepsy, which suggests that the role of SV2A in epileptogenesis in patients with glial tumors is questionable. The distinct pattern of SV2A IR in glioneuronal tumors suggests a redistribution of SV2A.
Keywords: brain tumors, epilepsy, human, peritumoral cortex, synaptic vesicle protein
Epilepsy is a frequent symptom in patients with brain tumors. Particularly, low-grade gliomas (eg, WHO grade II astrocytomas and oligodendrogliomas) and glioneuronal tumors (eg, ganglioglioma [GG] and dysembryoplastic neuroepithelial tumors [DNTs]) are common causes of focal epilepsy.1 The clinical significance and impact of epilepsy on the quality of life of patients with glial and glioneuronal tumors is high, especially as patients with these tumors are very often resistant to treatment with a broad range of antiepileptic drugs (AEDs).1–4 Moreover, patients with brain tumors tend to be more sensitive to side-effects of AEDs than those without brain tumors.5,6 Furthermore, AEDs often interact with the therapeutic regimen of these patients, such as chemotherapeutic agents.
Given that the clinical significance is high and a more effective therapy is urgently needed, it is important to get more insight into epileptogenesis in patients with glial and glioneuronal tumors. However, the cellular mechanisms underlying the epileptogenicity of these tumors are largely unknown. Several mechanisms are possibly involved. Much attention has been paid to alterations of the balance between excitation and inhibition in the cortex adjacent to the tumor.1,7 In the large majority of brain tumors, including gliomas, the epileptiform activity is thought not to arise in the tumor itself, but in the peritumoral tissue.8,9 However, in patients with glioneuronal tumors, the cellular composition and neurochemical profile of the tumors themselves, containing both glial and neuronal cells, also appear to be relevant for the generation and propagation of seizure activity.3,10,11
Recently, synaptic vesicle protein 2A (SV2A) has been identified as the binding site for the AED levetiracetam (LEV) and its analogs brivaracetam and selectracetam, suggesting that SV2A is involved in neuronal (hyper-)excitability.12–16 LEV is a relatively new drug with relatively little side-effects and without well-known pharmacokinetic interactions that most traditional AEDs have. Several studies have found that LEV is both effective and safe as a therapy for epilepsy in patients with brain tumors.1,17,18
Given the effectiveness of LEV in patients with glial and glioneuronal tumors, the underlying mechanism of action of LEV could be a lead to elucidate the pathophysiology of seizures in this patient group. SV2A is a membrane glycoprotein present in synaptic vesicles of neurons and endocrine cells and is the most widely distributed isoform of a family of three related synaptic vesicle proteins (SV2A, SV2B, and SV2C).19 The functions of these SV2 proteins are not completely understood, but experimental studies using SV2A and/or SV2A/SV2B knockout mice suggest that these proteins play a role as calcium regulators in neurotransmitter release and modulate synaptic networks.20,21 Knockout of the SV2A protein in mice leads to abnormal neurotransmission and development of severe seizures.20,22 Moreover, reduced gene and protein expression of SV2A has recently been observed in the hippocampus after status epilepticus (SE) in a rat model of temporal lobe epilepsy.23,24 These observations suggest that a reduction in SV2A expression or activity may promote seizure generation, although such a reduction might not necessarily influence the efficacy of LEV. LEV binding enhances an SV2A function that inhibits abnormal neuronal activity that is not automatically related to SV2A quantity.25 The expression of SV2A has not been previously studied in primary brain tumors. Assessment of SV2A expression in brain tumors and in the peritumoral cortex may contribute to understanding the epileptogenesis in brain tumors. We hypothesize that a reduction in SV2A expression in the peritumoral cortex correlates with the epileptogenesis in brain tumors.
For this purpose, we compared the expression of SV2A protein in tumor tissue and peritumoral cortex of patients with glial and glioneuronal tumors with the expression in control cortex and we evaluated the difference between the expression of SV2A in tumor patients with and without epilepsy. In order to do this, we defined not only the SV2A expression, but also the distribution of SV2A expression in tumor tissue, peritumoral cortex, and control cortex.
Materials and Methods
Subjects
We examined surgical specimens of 51 brain tumor patients with a glial tumor (30 glioblastoma multiforme [GBM], 5 WHO grade III astrocytoma, 8 WHO grade III oligodendroglioma, 4 WHO grade II astrocytoma, and 4 WHO grade II oligodendroglioma) and 12 patients with glioneuronal tumors (6 GGs and 6 DNTs). In 31 patients, a significant amount of peritumoral tissue (cortex or white matter adjacent to the lesion that did not include the visible microscopical infiltration zone or significant reactive gliosis; range, 0.7–2.3 cm; mean, 12 cm) was resected as well. On exposure to the same seizure activity, drugs, fixation time, and obviously the same age and gender, this material represents a good disease-reference tissue. In addition, normal-appearing control cortex/white matter was obtained at autopsy from 6 adult control patients without a history of seizures or other neurological diseases. The causes of death of these 6 patients were: 1 sudden cardiac death, 2 bronchopneumonias, 2 acute myocardial infarctions in combination with chronic heart disease, and 1 acute myocardial infarction with bronchopneumonia. All autopsies were performed within 12 hours after death. A chart review was conducted of all patients. Epilepsy was defined as the experience of one or more seizures, and data regarding seizure frequency and seizure type were obtained from patient histories. We collected additional data including age, gender, tumor location, and use of AED. Patient data and specimens were obtained from the databases of the departments of Neuropathology of the Academic Medical Center (University of Amsterdam; UVA) in Amsterdam and the University Medical Center in Utrecht (UMCU), both situated in the Netherlands. Informed consent was obtained for the use of brain tissue and for access to medical records for research purposes. Tissue was obtained and used in a manner compliant with the Declaration of Helsinki. Two neuropathologists (E.A., W.G.M.S.) reviewed all cases independently, and the diagnosis was confirmed according to the revised WHO classification of tumors of the central nervous system.26
Tissue Preparation
Tissue was fixed in 10% buffered formalin and embedded in paraffin. Paraffin-embedded tissue was sectioned at 6 µm, mounted on organosilane-coated slides (SIGMA, St. Louis, MO) and used for immunohistochemical staining as described below.
Antibodies
Antibodies specific for glial fibrillary acidic protein (GFAP; polyclonal rabbit, DAKO, Glostrup, Denmark; 1:4000; monoclonal mouse; DAKO; 1:50), vimentin (mouse clone V9; DAKO; 1:1000), neuronal nuclear protein (NeuN; mouse clone MAB377; Chemicon, Temecula, California; 1:2000), and synaptophysin (mouse clone Sy38; DAKO; 1:200; rabbit anti-synaptophysin; DAKO; 1:200) were used in the routine immunohistochemical analysis of glial and glioneuronal tumors. For the detection of SV2A, we used the mouse anti-SV2A (15E11; 1:50, Abcam, Cambridge, UK).
Immunohistochemistry
For single-label immunohistochemistry, paraffin-embedded sections were deparaffinized, re-hydrated, and incubated for 20 minutes in 0.3% H2O2 diluted in methanol to quench the endogenous peroxidase activity. Antigen retrieval was performed by incubation for 10 minutes at 121°C in citrate buffer (0.01 M, pH 6.0), and sections were washed with phosphate-buffered saline (PBS) and incubated for 30 minutes in 10% normal goat serum (Harlan Sera-Lab, Loughborough, UK). Sections were incubated with the primary antibodies overnight at 4°C. Hereafter, sections were washed in PBS and we used the ready-for-use Powervision peroxidase system (Immunologic, Duiven, The Netherlands) and 3,3′-diaminobenzidine (Sigma) as chromogen. Sections were counterstained with haematoxylin, dehydrated, and coverslipped. Sections incubated without the primary antibody were essentially blank.
For double-labeling studies, after incubation of SV2A combined with GFAP or synaptophysin overnight at 4°C, sections were incubated for 2 hours at room temperature (RT) with Alexa Fluor® 568-conjugated anti-rabbit IgG and Alexa Fluor® 488 anti-mouse IgG (1:100, Molecular Probes, Leiden, The Netherlands). Sections were then analyzed by means of a laser scanning confocal microscope (Leica TCS Sp2, Wetzlar, Germany) equipped with an argon-ion laser.
Evaluation of Immunostaining
Semiquantitative evaluation of immunoreactivity (IR) was performed as previously reported,27,28 using an Olympus microscope and examining in each section high-power non-overlapping fields (of 0.0655 × 0.0655 mm width; each corresponding to 4.290 µm2; using a square grid inserted into the eyepiece). The staining intensity was evaluated using the intensity score: a semiquantitative three-point scale where IR is defined as: 0, absent; 1, weak; 2, moderate; 3, strong staining. This score represents the predominant staining intensity found in each group (tumor, peritumoral, and control) as averaged from the selected fields and the different sections per group (as previously described).27,28 The evaluation of the IR in tumor specimens was performed in the center of the lesion, and the infiltration zone was disregarded.
We also performed optical density (OD) measurements in peritumoral and control cortex as previously reported.7 Sections were digitized using an Olympus microscope equipped with a DP-10 digital camera (Olympus, Tokyo, Japan). Images from consecutive, nonoverlapping fields (magnification, ×10) were collected using image acquisition and analysis software (Phase 3 Image System integrated with Image Pro Plus; Media Cybernetics, Silver Spring, Maryland). The absolute pixel staining density and the background from fields lacking immunoreactive profiles was determined. A mean OD value for peritumoral cortex was calculated, expressed as a ratio (ODR) of the mean OD of the background, and compared with control brain tissue.
Western Blot Analysis
For immunoblot analysis, freshly frozen human histologically normal cortex (n = 3), WHO grade II astrocytomas (n = 3), GBM (n = 3), and GG (n = 3) samples were homogenized in lysis buffer containing 10 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 1% NP-40, 0.4 mg/mL sodium orthovanadate, 5 mM EDTA (pH 8.0), 5 mM NaF, and protease inhibitor cocktail (Boehringer, Mannheim, Germany). Protein content was determined using the bicinchoninic acid method.29
For electrophoresis, equal amounts of proteins (50 µg/lane) were separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide). Separated proteins were transferred to nitrocellulose paper by electroblotting for 1 hour and 30 minutes (BioRad, Transblot SD, Hercules, California). After blocking for 1 hour in TBST (20 mM Tris, 150 mM NaCl, 1% Tween, pH 7.5)/5% nonfat dry milk, blots were incubated overnight with mouse anti-SV2A (15E11; 1:500) or mouse anti-β-actin (clone AC-15, Sigma; 1:50 000), used as reference protein. After several washes in TBST, the membranes were incubated in TBST/5% nonfat dry milk, containing horseradish peroxidase (HRP)-labeled goat anti-mouse (DAKO; 1:2500) for 1 hour at RT. After washes in TBST, IR was visualized using Lumi–light PLUS western blotting substrate (Roche Diagnostics, Mannheim, Germany) and digitized using a Luminescent Image Analyzer (LAS-3000, Fuji Film, Tokyo, Japan). The OD of each sample was measured using Image (Scion Corporation, release beta 3b, Frederick, Maryland) software. For each sample, the OD of the SV2A was calculated relative to the OD of β-actin.
Statistical Analysis
Statistical analysis was performed with SPSS for Windows. Data were analyzed with one-way analysis of variance and Fisher's post hoc test to assess the difference between groups (tumor, peritumoral, and control). Correlations between immunostaining and different clinical variables were assessed with the Spearman's rank correlation test. P < .05 was defined as statistically significant.
Results
Case Material
The clinical features of the cases included in this study are summarized in Table 1. The mean age of tumor patients was 46.1 (SEM, 1.6) years. The mean age of control patients was 49.5 (SEM, 6.2) years. Of the 63 tumor patients, 39 patients had epilepsy. Of the 31 patients of whom peritumoral tissue was resected as well, 23 patients had epilepsy. In glioneuronal tumors, the predominant seizure type was complex partial seizures with or without secondary generalization. All 12 patients had seizures despite maximal tolerated doses of AEDs (carbamazepine, valproic acid, phenytoin, oxcarbazepine, and in 4 patients [2 GG and 2 DNT] LEV as add-on therapy). In glial tumors the predominant seizure type was simple partial seizures with or without secondary generalization. Of patients with glial tumors, 13 (48%) patients had seizures despite maximal tolerated AEDs (carbamazepine, valproic acid, phenytoin, clonazepam, lamotrigine, and LEV).
Table 1.
Clinical and histopathological features
| A II (n = 4) | A III (n = 5) | GBM (n = 30) | O II (n = 4) | O III (n = 8) | GG (n = 6) | DNT (n = 6) | Control (n = 6) | |
|---|---|---|---|---|---|---|---|---|
| Male gender | 2 (50) | 2 (40) | 23 (76.7) | 3 (75) | 5 (62.5) | 3 (50) | 3 (50) | 3 (50) |
| Age, yearsa | 38 (31–45) | 40 (32–56) | 57 (41–76) | 40 (31–51) | 46 (41–57) | 35 (19–46) | 31 (18–38) | 50 (21–63) |
| Location | ||||||||
| Frontal | 2 (50) | 1 (20) | 9 (30) | — | 6 (75) | — | — | 1 (17) |
| Parietal | 2 (50) | — | 2 (7) | 1 (25) | — | — | — | 1 (17) |
| Temporal | — | — | 11 (37) | 1 (25) | — | 6 (100) | 6 (100) | 3 (50) |
| Occipital | — | 1 (20) | 1 (3) | 1 (25) | — | — | — | 1 (17) |
| Thalamus | — | — | 1 (3) | — | — | — | — | — |
| Parieto-occipital | — | 2 (40) | 1 (3) | — | — | — | — | — |
| Temporo-parietal | — | 1 (20) | — | — | — | — | — | — |
| Fronto-temporal | — | — | 1 (3) | — | — | — | — | — |
| Fronto-parietal | — | — | 4 (13) | 1 (25) | 2 (25) | — | — | — |
| Peritumoral tissue | 2 (50) | 3 (60) | 10 (33) | 3 (75) | 3 (38) | 5 (83) | 5 (83) | — |
| Epilepsy | 2 (50) | 1 (25) | 14 (47) | 3 (75) | 7 (88) | 6 (100) | 6 (100) | — |
| Duration epilepsya | 13.6 (4–24) mo | 2 (2–2) mo | 13.9 (0.5–120) mo | 31.5 (1–108) mo | 22.3 (1–70) mo | 16.5 (3–25) y | 15.6 (2–22) y | — |
| AED use | — | |||||||
| LEV monotherapy | — | — | 1 | — | 1 | — | — | — |
| Seizure free | — | — | 0 | — | 1 | — | — | — |
| LEV add-on | 1 | — | 1 | 1 | — | 2 | 2 | — |
| Seizure free | 1 | — | 0 | 0 | — | 0 | 0 | — |
| Other AED | 1 | 1 | 12 | 2 | 6 | 4 | 4 | — |
| Seizure free | 1 | 1 | 5 | 2 | 3 | 0 | 0 | — |
All data in number of patients (percentages) or as indicated.
A II, astrocytoma WHO II; A III, astrocytoma WHO III; GBM, glioblastoma multiforme; O II, oligodendroglioma WHO II; O III, oligodendroglioma, WHO III; GG, ganglioglioma; DNT, dysembryoplastic neuroepithelial tumor; AED, antiepileptic drug; LEV, levetiracetam.
aMean (range).
SV2A IR in Human Control, Glial, and Glioneuronal Tumors
Control Cortex and Peritumoral Cortex
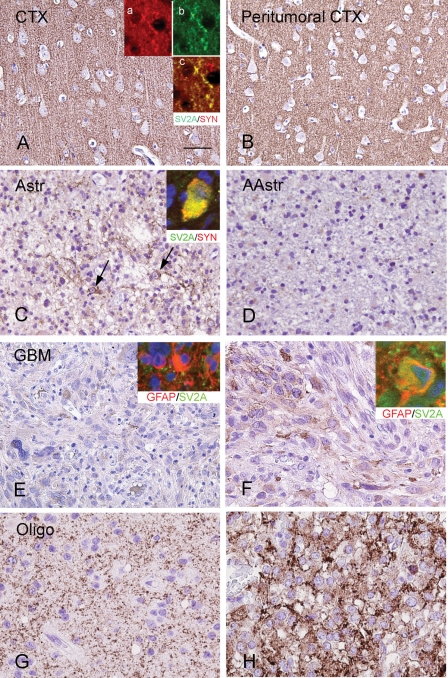
In control cortex, SV2A IR was present in all cortical layers with a diffuse neuropil staining (Fig. 1A). The neuronal somata were devoid of staining; however, perikaryal punctate labeling was detected in pyramidal neurons. SV2A was co-localized with the presynaptic marker synaptophysin (inserts in Fig. 1A). The monoclonal SV2A antibody stained a protein of approximately 90 kDa (Fig. 2A), which is similar to the molecular weight of SV2A.12
Fig. 1.
SV2A IR in control, peritumoral cortex, and glial tumors. (A) and (B) Histologically normal cortex (CTX) and peritumoral CTX showing diffuse and strong neuropil SV2A IR. Inserts in (A) show colocalization of synaptophysin (red; a) with SV2A (green; b); c, merged image. (C) and (D) SV2A IR in astrocytomas (astrocytomas WHO grade II and anaplastic astrocytomas, WHO grade III) showing low SV2A IR within the tumor area, with occasional expression in few residual neurons in the infiltration zone of the lesion (arrows in C); insert in (C) shows colocalization (yellow; merged image) of synaptophysin (red) with SV2A (green). (E) and (F) SV2A IR in GBM showing low SV2A IR within the tumor area, with occasionally focal areas of immunostained tumor cells. Insert in (E), merged image, shows GFAP-positive cells (red) without SV2A (green) IR. Insert in (F), merged image, shows occasional colocalization of SV2A (green) with GFAP (red) in a tumor cell. (G) and (H) SV2A IR in oligodendrogliomas showing variable matrix IR for SV2A in two anaplastic oligodendroglioma (Oligo, WHO grade III). Scale bar in (A). (A) and (B) 70 µm; (C) and (E) 80 µm; (F)–(H) 40 µm.
Fig. 3.
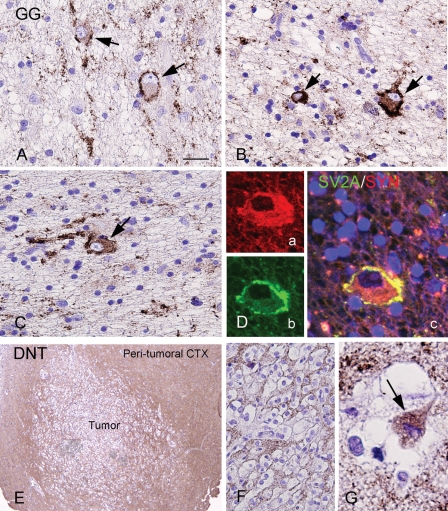
SV2A IR in glioneuronal tumors GGs, gangliogliomas; DNT, dysembryoplastic neuroepithelial tumors. (A)–(D) SV2A IR in GG showing variable matrix SV2A IR within the tumor area with strong SV2A IR along the dysplastic neuronal cell borders and processes (arrows in [A]–[C]). SV2A IR is occasionally observed within the neuronal perikarya (arrows in [B] and [C]). (D) Coexpression of synaptophysin (a, SYN; red) with SV2A (b; green) in a dysplastic neuronal cell (c, merged image). (E)–(G) SV2A IR in DNT showing low SV2A IR within the tumor area ([E]–[F]), but diffuse and strong neuropil IR in the peritumoral cortex (Peritumoral CTX [E]). SV2A IR is occasionally observed within the neuronal perikarya within the tumor (arrow in [G]); the surrounding oligodendrocyte-like cells are negative. Scale bar: (A)–(C), (F) 40 µm; (D) and (G) 20 µm; (E) 286 µm.
Similar to the observations found in control cortex, a diffuse SV2A IR was observed in all cortical layers in the peritumoral cortex of all 31 cases in which peritumoral cortex was obtained (Figs 1B and 3E). Accordingly, the mean intensity score and ODR in the peritumoral cortex were not significantly different from control cortex (Table 2; P > .05). No significant differences in SV2A IR existed between peritumoral specimens of patients with and without epilepsy and no significant correlations were found between SV2A IR and other clinical variables such as age at surgery, age at seizure onset, duration of epilepsy, and AED regimens.
Fig. 2.
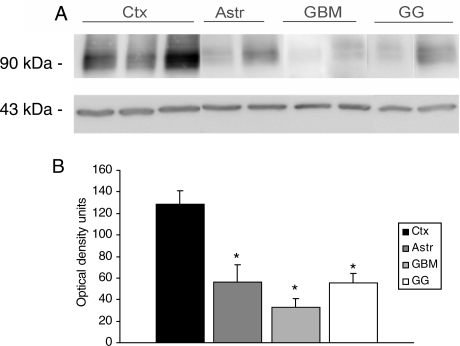
Expression of SV2A in glial and glioneuronal tumors (A). Representative immunoblot of SV2A in total homogenates from 3 cortex (Ctx), 3 astrocytomas grade II (Astr), 3 glioblastoma multiforme (GBM), and 3 ganglioglioma (GG). (B) Densitometric analysis of Western blots. Values (Optical density (OD) units) are mean ± SEM of 3 control cortex (Ctx), 3 Astr II, 3 GBM, and 3 GG relative to the OD of β-actin; all tumor specimens exhibited lower SV2A expression compared with control cortex. *P < .05.
Table 2.
SV2A immunoreactivity in control and peritumoral cortex
| Control (n = 6) | Peritumoral cortex |
P Value | ||
|---|---|---|---|---|
| With epilepsy (n = 23) | Without epilepsy (n = 8) | |||
| Intensity score | 2.8 ± 0.13 | 2.5 ± 0.11 | 2.6 ± 0.15 | >.05 |
| ODR | 68.5 ± 2.8 | 68.5 ± 1.5 | 66.6 ± 2.3 | >.05 |
Values represent the mean ± SEM of the number of samples indicated in parentheses. ODR, relative optical density ratio of SV2A-IR.
Tumor Tissue
Regarding the 63 tumor specimens, the following results were found. Modest SV2A IR was observed within the tumor area of both glial and glioneuronal tumors. The mean intensity score showed no significant differences between different tumor types (Table 3; P > .05). There was little evidence of SV2A expression in tumor cells of astrocytomas grade II, grade III, and GBM (Fig. 1C–F). The SV2A-positive cells detected in these glial tumors are mainly entrapped neurons (Fig. 1C). Only occasionally SV2A was observed in GFAP-positive tumor cells in GBM (inserts in Fig. 1E and F). Oligodendrogliomas showed a variable SV2A matrix IR with intermittently SV2A IR in the neuropil (Fig. 1G and H). Glioneuronal tumors displayed variable SV2A neuropil staining (Fig. 3). In GG, strong SV2A IR was present along the dysplastic neuronal cell borders and processes (perisomatic synapses; Fig 3A–C). This epiperikaryal IR colocalized with the characteristic strong perineuronal synaptophysin staining was observed in dysplastic neuronal cells of GG (Fig. 3D). In both GG and DNT, cytoplasmic SV2A IR was occasionally observed within the neuronal component of the tumor (Fig. 3A–C and G).
Table 3.
SV2A immunoreactivity in glial and glioneuronal tumors
| A II (n = 4) | A III (n = 5) | GBM (n = 30) | O II (n = 4) | O III (n = 8) | GG (n = 6) | DNT (n = 6) | |
|---|---|---|---|---|---|---|---|
| Intensity score | 1.5 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.3 | 1.2 ± 0.3 | 1.5 ± 0.4 | 1.3 ± 0.4 |
A II, astrocytoma WHO II; A III, astrocytoma WHO III; GBM, glioblastoma multiforme; O II, oligodendroglioma WHO II; O III, Oligodendroglioma, WHO III; GG, ganglioglioma; DNT, dysembryoplastic neuroepithelial tumor.
On western blot analysis, homogenates from astrocytomas, GBM, and GG cases displayed a less dense band than that observed in control cortex (Fig. 2A and B). Accordingly, the mean intensity scores of IR in the tumors are lower than the intensity scores of IR in control cortex (Tables 2 and 3). Hence, glial and glioneuronal tumors generally show little SV2A expression within the tumor cells, except for the strong IR within neuronal components of the tumors.
Correlation Between SV2A Expression and Distribution and Epilepsy
With this uncovered information we compared the expression and distribution of SV2A in tumor tissue of the 39 tumor patients with epilepsy with that of the 24 tumor patients without epilepsy. In both glial and glioneuronal tumors, no differences in IR were observed between the specimens of patients with epilepsy and without epilepsy. Both the tumor cells of patients with and without epilepsy showed modest IR. The peritumoral cortex did not show any differences between the two groups in IR as well. In addition, the mean intensity score and ODR in the peritumoral cortex of the 23 tumor patients with epilepsy were not significantly different from peritumoral cortex of the 8 tumor patients without epilepsy (Table 2).
Discussion
The histologically normal peritumoral cortex in 31 of the 63 brain tumor patients did not show significant differences in neuropil expression for SV2A compared with cortical tissue of 6 controls, and we found no differences in neuropil expression for SV2A in tumor patients with and without epilepsy.
In contrast to our results, reduced protein expression for SV2A has been detected in rat hippocampus after SE, as well as in specimens of hippocampal sclerosis in patients.24 Reduced expression of SV2A has also been observed within surgical neocortical specimens of patients with focal malformations of cortical development (ie, focal cortical dysplasia).30 According to these studies, reduction in SV2A expression might have been expected in the epileptogenic zone of the lesions of our patient group, which is the peritumoral cortex in case of glial tumors and the tumor itself in case of glioneuronal tumors. The absence of such a reduction (also in patients with low-grade tumors and long duration of epileptic activity) suggests that different mechanisms of regulation of SV2A expression play a role in specific pathologies.
Low SV2A expression was expected in astroglial and oligodendroglial tumor cells, since SV2A is a neuronal and neuroendocrine cell marker. Indeed, SV2A IR was not observed at all in GFAP-positive tumor cells of low-grade astrocytomas, and only occasionally in high-grade astrocytomas, expression of neuronal antigens has previously been shown in high-grade astrocytomas.31 Oligodendrogliomas displayed variable SV2A IR in the neuropil. Although expression of neuronal markers has been reported in oligodendroglial tumors more often than in their astrocytic counterparts,32,33 the SV2A matrix IR in oligodendrogliomas most likely represents the preexisting neuronal structures. The presence of a true intracellular expression of SV2A in oligodendroglial cells requires further investigation, considering the lack of specific oligodendroglial markers and the coexpression of neuronal markers in these tumor cells.
Neuropil staining was also observed in glioneuronal tumors (GG and DNT), but was clearly restricted to the neuronal component of the lesions. The high SV2A expression in dysplastic neurons within GG specimens could be explained by the disturbance of normal synaptic connection or by a compensatory mechanism in areas of hyperexcitability. Accordingly, SV2A protein has been shown to be implicated in the control of exocytosis, modulating synaptic networks.20–22 A similar pattern of IR outlining the border of dysplastic cells has been reported in GG for synaptophysin.34–36 Colocalization of these two presynaptic vesicle proteins has been corroborated in our study.
Surprisingly, no reduction of SV2A expression was observed in the peritumoral cortex of glial tumors of patients with epilepsy when compared with specimens of patients without epilepsy. Although the mechanisms of SV2A function are far from completely understood, reduction in SV2A expression appears to lead to abnormal neurotransmission and development of seizures.20,22 Since no reduction in SV2A expression was found in peritumoral tissue, the role of SV2A in epileptogenesis in patients with glial tumors remains questionable.
Nevertheless, the high efficacy of LEV in tumor-related epilepsy, combined with the fact that SV2A is identified as the binding site for LEV, suggests that SV2A contributes to the mechanism of seizure generation and maintenance in these patients. It is imaginable that not the reduction of SV2A expression but the loss of SV2A function initiates the loss of control of synaptic vesicle exocytosis and consequently alters neurotransmission and eventually contributes to the epileptogenesis in patients with brain tumors. LEV might reduce excessive neuronal activity by modulating SV2A function and restoring the ability of a neuron to regulate its neurotransmitter release.25
The relationship between SV2A expression and the efficacy of LEV is not clear either. No evidence exists that a reduced expression of SV2A interferes with the effectiveness of LEV (ie, in focal cortical dysplasia)30 and it is not known if a high expression of SV2A promotes the efficacy of LEV. Additional prospective clinical studies are required to further address this issue.
This study provides a large population of tumor patients with and without epilepsy. However, the data remain retrospective. Hence, it is interesting to investigate the expression and cellular distribution of SV2A in patients who were effectively treated with LEV and who were ineffectively treated with LEV in a prospective fashion.
Conflict of interest statement. None declared.
Funding
This work was supported by the National Epilepsy Fund (NEF 05-11, E. Aronica and K. Boer) and an unrestricted grant of UCB Pharma (S.T. Toering, M. de Groot).
References
- 1.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 2.Aronica E, Leenstra S, van Veelen CW, et al. Glioneuronal tumors and medically intractable epilepsy: a clinical study with long-term follow-up of seizure outcome after surgery. Epilepsy Res. 2001;43(3):179–191. doi: 10.1016/s0920-1211(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 3.Blumcke I, Wiestler OD. Gangliogliomas: an intriguing tumor entity associated with focal epilepsies. J Neuropathol Exp Neurol. 2002;61(7):575–584. doi: 10.1093/jnen/61.7.575. [DOI] [PubMed] [Google Scholar]
- 4.Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life 3. Ann Neurol. 2003;54(4):514–520. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- 5.Wen PY, Marks PW. Medical management of patients with brain tumors. Curr Opin Oncol. 2002;14(3):299–307. doi: 10.1097/00001622-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Maschio M, Dinapoli L, Zarabia A, Jandolo B. Issues related to the pharmacological management of patients with brain tumours and epilepsy. Funct Neurol. 2006;21(1):15–19. [PubMed] [Google Scholar]
- 7.Aronica E, Redeker S, Boer K, et al. Inhibitory networks in epilepsy-associated gangliogliomas and in the perilesional epileptic cortex. Epilepsy Res. 2007;74(1):33–44. doi: 10.1016/j.eplepsyres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Baayen JC, de Jongh A, Stam CJ, et al. Localization of slow wave activity in patients with tumor-associated epilepsy. Brain Topogr. 2003;16(2):85–93. doi: 10.1023/b:brat.0000006332.71345.b7. [DOI] [PubMed] [Google Scholar]
- 9.Kohling R, Senner V, Paulus W, Speckmann EJ. Epileptiform activity preferentially arises outside tumor invasion zone in glioma xenotransplants. Neurobiol. Dis. 2006;22(1):64–75. doi: 10.1016/j.nbd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Wolf HK, Birkholz T, Wellmer J, Blumcke I, Pietsch T, Wiestler OD. Neurochemical profile of glioneuronal lesions from patients with pharmacoresistant focal epilepsies. J Neuropathol Exp Neurol. 1995;54(5):689–697. doi: 10.1097/00005072-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Aronica E, Yankaya B, Jansen GH, et al. Ionotropic and metabotropic glutamate receptor protein expression in glioneuronal tumors from patients with intractable epilepsy. Neuropathol. Appl. Neurobiol. 2001;27:1–16. doi: 10.1046/j.0305-1846.2001.00314.x. [DOI] [PubMed] [Google Scholar]
- 12.Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101(26):9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillard M, Chatelain P, Fuks B. Binding characteristics of levetiracetam to synaptic vesicle protein 2A (SV2A) in human brain and in CHO cells expressing the human recombinant protein. Eur J Pharmacol. 2006;536(1–2):102–108. doi: 10.1016/j.ejphar.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Bennett B, Matagne A, Michel P, et al. Seletracetam (UCB 44212) Neurotherapeutics. 2007;4(1):117–122. doi: 10.1016/j.nurt.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Rosenstiel P. Brivaracetam (UCB 34714) Neurotherapeutics. 2007;4(1):84–87. doi: 10.1016/j.nurt.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4(1):18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton HB, Goldlust SA, Pearl D. Retrospective analysis of the efficacy and tolerability of levetiracetam in brain tumor patients. J Neurooncol. 2006;78(1):99–102. doi: 10.1007/s11060-005-9070-4. [DOI] [PubMed] [Google Scholar]
- 18.Wagner GL, Wilms EB, van Donselaar CA, Vecht C. Levetiracetam: preliminary experience in patients with primary brain tumours. Seizure. 2003;12(8):585–586. doi: 10.1016/s1059-1311(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 19.Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14(9):5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24(4):1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 21.Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J. Neurosci. 2006;26(4):1303–1313. doi: 10.1523/JNEUROSCI.2699-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowder KM, Gunther JM, Jones TA, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci USA. 1999;96(26):15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorter JA, Van Vliet E, Aronica E, et al. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci. 2006;26(43):11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia. 2009;50(3):422–433. doi: 10.1111/j.1528-1167.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- 25.Stahl SM. Psychopharmacology of anticonvulsants: levetiracetam as a synaptic vesicle protein modulator. J Clin Psychiatry. 2004;65(9):1162–1163. doi: 10.4088/jcp.v65n0901. [DOI] [PubMed] [Google Scholar]
- 26.Louis DN, Ohgaki H, Wiestler OD, Cavanee WK. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravizza T, Boer K, Redeker S, et al. The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol Dis. 2006;24(1):128–143. doi: 10.1016/j.nbd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Aronica E, Boer K, van Vliet EA, et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis. 2007;26(3):497–511. doi: 10.1016/j.nbd.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid [published erratum appears in Anal Biochem 1987;163(1):279] Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 30.Toering ST, Boer K, de Groot M, et al. Expression patterns of synaptic vesicle protein 2A in focal cortical dysplasia and TSC-cortical tubers [published online ahead of print, 2009] Epilepsia. doi: 10.1111/j.1528-1167.2008.01955.x. [DOI] [PubMed] [Google Scholar]
- 31.Wharton SB, Chan KK, Whittle IR. Microtubule-associated protein 2 (MAP-2) is expressed in low and high grade diffuse astrocytomas. J Clin Neurosci. 2002;9(2):165–169. doi: 10.1054/jocn.2001.1055. [DOI] [PubMed] [Google Scholar]
- 32.Wharton SB, Chan KK, Hamilton FA, Anderson JR. Expression of neuronal markers in oligodendrogliomas: an immunohistochemical study. Neuropathol Appl Neurobiol. 1998;24(4):302–308. doi: 10.1046/j.1365-2990.1998.00132.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolf HK, Buslei R, Blumcke I, Wiestler OD, Pietsch T. Neural antigens in oligodendrogliomas and dysembryoplastic neuroepithelial tumors. Acta Neuropathol (Berl) 1997;94(5):436–443. doi: 10.1007/s004010050730. [DOI] [PubMed] [Google Scholar]
- 34.Patel U, Pinto RS, Miller DC, et al. MR of spinal cord ganglioglioma [see comment] Am J Neuroradiol. 1998;19(5):879–887. [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn B. Synaptophysin staining in normal brain: importance for diagnosis of ganglioglioma. Am J Surg Pathol. 1998;22(5):550–556. doi: 10.1097/00000478-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Miller DC, Koslow M, Budzilovich GN, Burstein DE. Synaptophysin: a sensitive and specific marker for ganglion cells in central nervous system neoplasms. Human Pathol. 1990;21(3):271–276. doi: 10.1016/0046-8177(90)90226-u. [DOI] [PubMed] [Google Scholar]