Abstract
The prognosis for malignant gliomas remains poor, and new treatments are urgently needed. Targeted suicide gene therapy exploits the enzymatic conversion of a prodrug, such as a nucleoside analog, into a cytotoxic compound. Although this therapeutic strategy has been considered a promising regimen for central nervous system (CNS) tumors, several obstacles have been encountered such as inefficient gene transfer to the tumor cells, limited prodrug penetration into the CNS, and inefficient enzymatic activity of the suicide gene. We report here the cloning and successful application of a novel thymidine kinase 1 (TK1) from the tomato plant, with favorable characteristics in vitro and in vivo. This enzyme (toTK1) is highly specific for the nucleoside analog prodrug zidovudine (azidothymidine, AZT), which is known to penetrate the blood-brain barrier. An important feature of toTK1 is that it efficiently phosphorylates its substrate AZT not only to AZT monophosphate, but also to AZT diphosphate, with excellent kinetics. The efficiency of the toTK1/AZT system was confirmed when toTK1-transduced human glioblastoma (GBM) cells displayed a 500-fold increased sensitivity to AZT compared with wild-type cells. In addition, when neural progenitor cells were used as delivery vectors for toTK1 in intracranial GBM xenografts in nude rats, substantial attenuation of tumor growth was achieved in animals exposed to AZT, and survival of the animals was significantly improved compared with controls. The novel toTK1/AZT suicide gene therapy system in combination with stem cell–mediated gene delivery promises new treatment of malignant gliomas.
Keywords: malignant glioma, neural stem cells, plant thymidine kinase 1, suicide gene therapy, zidovudine (azidothymidine)
Suicide gene therapy has been applied for the most common aggressive primary brain tumor malignant gliomas, albeit with limited success.1–6 Combinations of suicide genes, prodrugs, and gene transfer technologies are being investigated to make the therapy more efficient.7–13 There are specific requirements for enzymes used in suicide gene therapy. Deoxyribonucleoside kinases (dNKs) have been the prime suicide gene candidates in cancer gene therapy in combination with nucleoside analog prodrugs.2 dNK catalyzes the first and most often the rate-limiting step in the salvage DNA synthesis pathway, the phosphorylation of dN or their analogs, to the corresponding monophosphates (dNMP).14 An ideal suicide gene candidate would exhibit a highly different and increased selectivity and a higher catalytic turnover for the nucleoside analog, compared with the endogenous enzymes.15 Preferably, the nucleoside analog should not be activated at all by the human dNKs. One inherent limitation of suicide genes is the limited specificity for the nucleoside analog compared with the natural dN, which is reflected in a high Km value. HSV-tk has a 70-fold higher Km value for its prodrug ganciclovir (GCV) than for thymidine (Thd),16 which in vivo results in substrate out-competition of GCV by Thd.15,17 An effective enzyme-prodrug system should have both low Km and high Vmax values for the prodrug, defining a favorable catalytic efficiency kcat/Km. Most of the existing enzyme-prodrug systems do not show these favorable properties.17 Therefore, new suicide genes with better kinetic parameters of the encoding enzymes are needed.
One of the major complicating factors of suicide gene therapy, specific for brain tumors is the presence of the blood-brain barrier (BBB) that restricts passage of systemically delivered prodrug into the brain. Most prodrugs of existing suicide gene therapy systems do not adequately pass the BBB. GCV penetrates the BBB poorly, and once entered into the central nervous system (CNS) becomes eliminated.18 In this context, the antiretroviral agent zidovudine (3-azidothymidine, AZT) could be an ideal prodrug in the suicide gene therapy. AZT exhibits pharmacokinetic properties superior to other nucleoside analogs, with better solubility as well as better BBB penetration.19,20 The cerebrospinal fluid–plasma ratio of AZT increases in a linear fashion with time after drug administration.21 Another favorable characteristic of AZT is its poor phosphorylation into the AZT-diphosphate form (AZT-DP) by human dNKs, which presents an additional limiting step for AZT activation toward a general cytotoxic compound.22,23 However, none of the suicide gene enzymes characterized to date exhibit a strong specificity for AZT. We have cloned a thymidine kinase 1 (TK1)–like enzyme from tomatos, the tomato TK1(toTK1), which can overcome this problem by its efficient phosphorylation of AZT to AZT monophosphate (AZT-MP) as well as AZT-MP to AZT-DP. This property has not previously been described for any TK1-like enzyme.
Established suicide gene therapies use viral or nonviral vectors for gene transfer. These vectors, armed with suicide genes, do not penetrate glioma tissue well. Packaging cell lines transplanted directly into animal brain-bearing glioblastoma (GBM) did not show tropism for the tumor cells.4,24,25 In contrast, neural stem/progenitor cells implanted intracranially contralateral to GBM xenografts, or administered intravenously in a GBM animal model, infiltrated the tumor bed and tracked down the disseminated tumor cells.26,27 Therefore, genetically engineered neural progenitor cells (NPCs) can be used to deliver therapeutic molecules to malignant glioma cells in vivo.13,28,29 We have used the human NPC line NGC-407 as delivery vehicle of toTK1 in a human GBM xenograft model. For this purpose, we have specifically studied this cell line for its phenotypic characteristics in vitro and in vivo, including expression of the gap junction protein connexin43 and gap junction coupling with GBM cells.30,31 We show here that toTK1 is a novel candidate for efficient stem cell–mediated suicide gene therapy of malignant gliomas.
Materials and Methods
Materials
3H-labeled Thd was obtained from Amersham Corp., and 3H-labeled AZT (methyl-3H AZT), thymidine monophosphate (TMP), thymidine diphosphate, AZT-MP (methyl-3H AZT-MP), and AZT-DP (methyl-3H AZT-DP) were from Moravek Biochemicals Inc. Unlabeled nucleosides and nucleotides were from Sigma. AZT was purchased from Sigma (A2169). For the treatment of animals, AZT was dissolved at 40 mg/mL in 15% methyl-β-cyclodextrin (Kleptose CRYSMEB, Roquette).
Novel Kinase Genes and Plasmids
Several sequences containing open-reading frames (ORFs) encoding a kinase were subcloned from genomic DNA and cDNA libraries or plasmids obtained from the originator laboratories, and inserted into the BamHI/EcoRI site of pGEX-2T (Amersham-Pharmacia). The corresponding original plasmid for toTK1 was obtained from the Clemson University Genomic Institute, and the insert complete sequence was determined giving an ORF of 234 amino acid residues (GenBank accession number AF514775). The plasmid was used as a template in PCR-mediated subcloning into the BamHI/EcoRI site of pGEX-2T (Amersham-Pharmacia). The resulting expression plasmid was named P579. The TK-deficient Escherichia coli strain KY895 (F−, tdk-1, ilv) was transformed with P579. Growth of colonies was tested with logarithmic dilutions of various nucleoside analogs including AZT to determine LD100.
Recombinant Expression and Purification
The TK-negative E. coli strain KY895 was transformed with P579, the expression induced and recombinant protein isolated as described.15
Enzyme Assay
dN kinase activities were determined by initial velocity measurements based on 4 time samples by the DE-81 filter paper assay using tritium-labeled nucleoside substrates and liquid scintillation. The standard assay conditions used ATP and the 3H-labeled acceptor substrate at different concentrations.15 The dNMP kinase activities were determined by initial velocity measurements based on 4 time samples. The products of the monophosphate kinase reaction were analyzed by thin-layer chromatography on polyethyleneimine–cellulose plates. The separation took place in 0.25 M LiCl as the developing solvent. Spots were identified under UV-light and were then cut out. Radioactivity in the spots were extracted with 0.2 M KCl/0.1 M HCl and quantified by liquid scintillation. One Unit (U) of kinase activity is defined as 1 µmol of the corresponding monophosphate or diphosphate product formed per minute.
Kinetic and Statistical Analyses
Kinetic data were evaluated by nonlinear regression analysis using the Michaelis–Menten equation v = Vmax × [S]/(Km + [S]). The equations were fitted to all available data in a global fit. One unit of enzyme activity is defined as 1 µmol of substrate phosphorylated per minute. Statistical significance between means of the tumor volumes in different experimental groups was assessed by Student's t-test for unpaired values. The Kaplan–Meier survival analysis with log-rank (Mantel–Cox) test (including test for trend) was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software).
Construction of a Retrovirus Vector Expressing toTK1
The cDNA of toTK1 was cloned into a retrovirus vector based on the Moloney murine leukemia virus to generate a replication-deficient recombinant retrovirus containing the toTK1. DNA fragments were amplified with Pfu polymerase (Stratagene) using primers with designed flanking restriction enzyme sites. toTK1 constructs were cut with BglII/XhoI and cloned into the BglII/XhoI site of the pLCXSN plasmid vector (Clontech) under the control of the CMV promoter. The construct was named ZG59. The construct was verified by DNA sequencing. pLCXSN alone and the vector containing HSV-tk (cloned into BamHI/XhoI site) were used as controls.
Cell Cultures and Establishment of toTK1-Expressing Cells
The human GBM cell line U87MG and human NPC line NGC-407 were cultured as described.30 Transduction of U87MG cells with toTK1 and HSV-tk was performed using the retroviral vectors. NGC-407 cells were transfected with the toTK1-containing pCI plasmid (Promega) using FuGENE 6 Transfection Reagent (Roche). Positive clones were then selected with hygromycin. The recombinant cells were also tested for sensitivity to AZT. To investigate the NGC-407 cell tropism toward xenografted human GBM, transfection with the pCI-EGFP plasmid expressing green fluorescent protein (GFP) was done using Nucleofector™ (Amaxa Biosystems). The cells were selected for their resistance to hygromycin.
Cell Proliferation Assay
The cells were plated in equal number (1500–2500 cells/well) in 96-well plates coated with poly-l-lysine. AZT treatment was started 24 hours after the plating. Cell survival was assayed by the XTT assay (Roche) after 5 days of drug exposure (Fig. 1). To investigate the bystander effect, mixed cell cultures of parental U87MG and toTK1-expressing U87MG cells were seeded and pretreated with 65 µM of 18α-glycyrrhetinic acid (AGA, dissolved in DMSO; Sigma) for 6 hours followed by 5 mM AZT treatment for 5 days. Plates were read in a microplate reader (absorbance [A] 450 nm with a 690 nm reference). Each experiment was performed in 4 replicates. The IC50 value of the investigated compounds was calculated as the mean value of these experiments using SigmaPlot® and the formula A = Amax/(1 + [I/IC50]). Parental NGC-407 and NGC-407-toTK1 cells were treated with a logarithmic range concentration of AZT for 6 days. The IC50 values were calculated from each of the 6 head-to-head independent experiments using SigmaPlot® and the formula mentioned above.
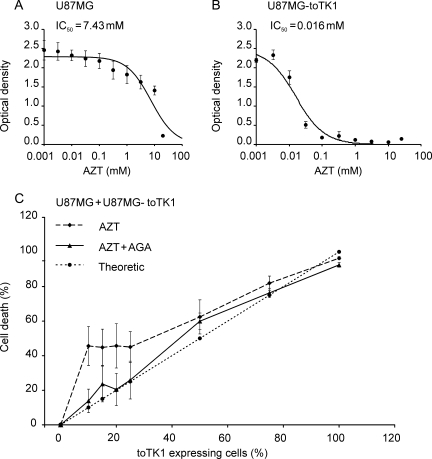
Fig. 1.
GBM cell killing effect of toTK1 in vitro. (A) Logarithmic graph showing the IC50 of AZT in U87MG cells not transduced with toTK1. (B) Logarithmic graph showing the IC50 of AZT in toTK1-transduced U87MG cells. (C) Graphs showing cell death in the coculture of the indicated proportion of toTK1-transduced and nontransduced U87MG cells upon exposure to AZT and treated with AGA to investigate the gap junction–mediated bystander cell killing effect. The theoretic line represents the cell death in the case where the only toTK1-expressing U87MG cells are killed. Note that the inhibition by AGA of the bystander killing is more pronounced at 10%–20% toTK1-expressing U87MG cells. Values are the means of 3 independent experiments. Error bars represent the standard errors.
Animal Studies
Animal experiments were conducted with ethical permission from North Stockholm Animal Ethics Committee. Male nude rats (Hsd:RH-rnu, Harlan, Germany), aged 8–9 weeks, weighing 150–200 g were used. They were anesthetized by isoflurane inhalation, and in a stereotactic platform under microscopic guidance, a burr hole of <1 mm diameter was made in the skull, 2 mm right lateral to the bregma. About 1.5 × 105 U87MG cells in a 3-µL volume were injected, 3.5 mm deep from the skull surface over approximately 5 minutes. Tumors were allowed to grow for 7 days. Then, 5 × 105 NGC-407-GFP cells were injected contralateral to the tumor implant as shown in Fig 2. Fourteen days after stem cell implantation, animals were sacrificed and the brain tissue was processed and analyzed to detect NGC-407 cell migration and integration.
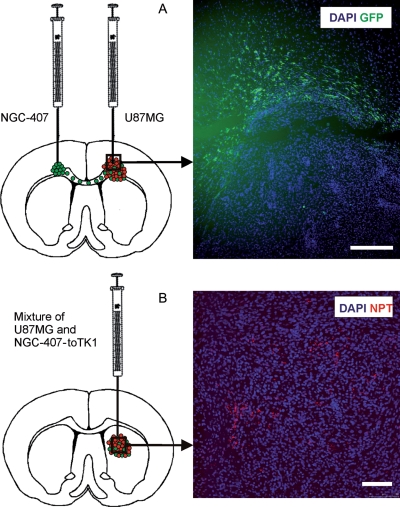
Fig. 2.
(A) NGC-407 cells display tropism toward xenografted GBM. U87MG GBM cells were injected into the right hemisphere and NGC-407-GFP cells into the left hemisphere, both at the level of the corpus callosum. The cartoon shows the green NGC-407 cells migrating through the corpus callosum toward the GBM xenograft. Immunohistochemistry showing GFP-positive NGC-407 cells infiltrating the tumor bed. High cell density identified by DAPI staining localizes the tumor area. Scale bar is 100 µM. (B) NGC-407-toTK1 cells stained red with NPT II are dispersed throughout the U87MG xenograft and intermingling with the tumor cells. The cartoon is showing the coimplantation of NGC-407-toTK1 and U87MG cells into the right hemisphere. NPT was introduced into the NGC-407 cells for selection purposes during the myc immortalization phase. Scale bar is 100 µM.
To investigate the in vivo bystander effect, 3 × 105 NGC-407-toTK1 and U87MG cells were mixed at the indicated ratios in a 4 µL volume and injected through a burr hole, 2 mm right lateral and 3 mm posterior to the bregma and 5 mm deep from the skull surface. Starting 24 hours after implantation, the rats were treated intraperitoneally with AZT at 400 mg/kg of body weight per day in 2 divided doses for up to 21 days. The control groups received an equal volume of vehicle (15% methyl-β-cyclodextrin). Tumor growth was followed by MRI on days 12–13 and 22–23 postimplantation. Animals were observed twice daily for weight loss (>10%) and abnormal behavior, including food intake, mobility, and other neurological symptoms, which were set as end points of the experiment. After decapitation, the brains were quickly collected and immediately frozen in 2-methyl butane cooled by dry ice. The brains were then transferred into a –75°C freezer until they were sectioned by a cryostat onto SuperFrost Plus glass slides (Menzel-Gläser) in a 14-µm thickness.
MRI and Image Processing
MRI experiments were performed using a 4.7-T horizontal bore animal MR system operating on a Paravision (version 3.0.2) software platform (Bruker Biospec). Animals were anesthetized with and maintained on isofluorane, and body temperature was maintained with a heated air stream. Respiratory activity was monitored continuously throughout the experiments. Multislice T2-weighted images of rat brains were acquired in the coronal, sagittal, and axial planes (TR 2500 ms, TE 95.6 ms, slice thickness 1.5 or 0.5 mm, FOV 3.5 cm, 5–16 slices depending on tumor size). Tumor volumes from stack images of each anatomical plane in each animal were measured by manually outlining tumors using the public domain program ImageJ (National Institutes of Health, http://rsb.info.nih.gov/ij/, 1997–2007). The tumor volume in an animal was then averaged out from individual volumes in all 3 planes.
Immunohistochemistry
Brain sections were fixed with 4% formaldehyde and blocked with 2% BSA in PBS with 0.2% Triton X-100 for 1 hour. Primary antibody diluted in blocking solution was applied to the sections and incubated for 1 hour. Rabbit anti-neomycin phosphotransferase II (NPT; 1:200; Upstate, 06–747) and chicken anti-GFP polyclonal antibodies (1:1000) (Chemicon, AB 16901) were used. Sections were incubated for 1 hour with the corresponding secondary antibody, Cy3-conjugated antirabbit IgG (1:500; Jackson Immunoresearch Lab) or FITC-conjugated antichicken IgY (1:200; Chemicon, AP 194F). Counterstaining was performed with Hoechst 33342 (0.1 µg/mL; Molecular Probes, H3570). The bound antibodies were visualized by a fluorescence microscope (Olympus BX50WI) or by a confocal microscope (Leica TCS SP5) using the Leica Application Suite Advanced Fluorescence software.
Results
Identification of toTK1
Deposited GenBank sequences were searched for human TK1 homologs from plants, and several putative TK1 were obtained. They were subcloned and expressed in E. coli and tested for their ability to sensitize transformed E. coli KY895 toward AZT.15 A TK1 homolog from tomato, Lycopersicon esculentum (toTK1; plasmid P579) was identified and shown to sensitize the cells against AZT by more than 3000-fold (Table 1).
Table 1.
LD100 values for E. coli KY895 exposed to AZT
| KY895 transformed with | LD100 for AZT (μM) |
|---|---|
| pGEX-2T | >100 |
| toTK1 | 0.0316 |
| HSV-tk | 1.000 |
toTK1 or HSV-tk was subcloned into pGEX-2T, and the TK-deficient E. coli strain KY895 was transformed with the resulting plasmids or the empty vector. Growth of colonies was tested with logarithmic dilutions of AZT. The values are the average from 2 to 4 independent experiments.
toTK1 Phosphorylates Both AZT and AZT-MP with Ideal Suicide Gene Kinetics
Plant dNKs have so far not been thoroughly characterized, and therefore, the recombinant toTK1 was subjected to a detailed kinetic analysis. Purified recombinant toTK1 was not able to phosphorylate deoxyadenosine, deoxycytidine, or deoxyguanosine but could efficiently phosphorylate Thd and AZT. The Vmax and Km values were 739 mU/mg and 4.4 µM, respectively, for Thd and 605 mU/mg and 3.1 µM for AZT (Table 2). Km and Vmax values for prodrugs define the specific catalytic efficiency (kcat/Km), and the kcat/Km values of toTK1 were 72 707 and 84 485/M/s for Thd and AZT, respectively. Even more importantly, toTK1 surprisingly also phosphorylates monophosphate nucleosides, such as TMP and AZT-MP (Table 2). Such unique properties of toTK1 could offer a novel suicide candidate in brain-tumor gene therapy, and therefore, we evaluated and compared the effectiveness of toTK1 as a suicide gene in GBM cells in vitro and in vivo.
Table 2.
Kinetic parameters and substrate specificity of recombinant toTK1 demonstrate that this enzyme is a thymidine/azidothymidine and thymidine/azidothymidine monophosphate kinase
| Substrate | Km (μM) | Vmax (mU/mg) | kcat (per s) | kcat/Km (per M per s) |
|---|---|---|---|---|
| Thymidine | 4.4 | 739 | 0.32 | 72 707 |
| Azidothymidine | 3.1 | 605 | 0.26 | 84 485 |
| Thymidine monophosphate | 16.03 | 0.23 | 9.96 × 10–5 | 6.2 |
| Azidothymidine monophosphate | 26.21 | 0.074 | 3.2 × 10–5 | 1.2 |
Each shown result is an average of 2 independent experiments and the observed variation was less than 10%.
toTK1/AZT Combination is Superior to the HSV-tk/GCV Approach to Kill GBM Cells In Vitro
U87MG cells transduced with toTK1 or HSV-tk were treated with AZT or GCV, respectively. toTK1 dramatically increased the sensitivity of the GBM cells to AZT, whereas HSV-tk increased the sensitivity to GCV modestly. The IC50 for AZT was 7.4 mM in nontransduced cells compared with 16 µM in toTK1-transduced cells, showing an approximately 500-fold increase in AZT sensitivity (Fig. 1A and B). On the other hand, GCV sensitivity was increased only about 60-fold when the cells were carrying HSV-tk. The IC50 for GCV was 1.6 mM in nontransduced U87MG cells and 28.7 µM in HSV-tk-transduced U87MG cells (data not shown).
Efficient Bystander Cell Killing Using toTK1/AZT Combination In Vitro
We then cocultured U87MG-toTK1 cells with nontransduced U87MG cells in different proportions to examine the extent and magnitude of the bystander cell-killing effect. As low as 10%–20% of toTK1-expressing cells in the culture were sufficient to exert a substantial bystander effect on exposure to AZT (Fig. 1C). Since the bystander effect to a great extent is mediated by gap junction communications,32,33 a substantial reduction in the bystander effect was observed when the coculture was treated with the gap junction blocker AGA. AGA had a greater influence in cocultures with fewer toTK1-expressing cells since these are more dependent on the bystander effect (Fig. 1C).
NGC-407 Cells Show Robust Tropism for GBM Xenografts
NGC-407 cells carrying a green fluorescent protein (GFP) reporter gene were implanted into nude rat brain contralateral to the hemisphere bearing a GBM xenograft. NGC-407 cells were traced by their GFP expression at different time points after the stem cell implantation. We found that NGC-407/GFP cells extensively migrated toward the GBM xenografts in the opposite hemisphere through the corpus callosum. They infiltrated the tumor bed and were found intermingling with the tumor cells inside the xenograft 2 weeks after their implantation (Fig. 2A). This also demonstrates that NGC-407 cells can survive in vivo and can efficiently express the foreign gene GFP. NGC-407-toTK1 cells coimplanted with U87MG cells were dispersed throughout the xenograft and also found intermingling with U87MG cells (Fig. 2B).
NGC-407-mediated toTK1/AZT Therapy Substantially Reduces the Size of GBM Xenografts and Improves Survival
First, NGC-407 cells carrying toTK1 were tested in vitro for their sensitivity to AZT. The recombinant NGC-407-toTK1 cells showed a 10–50-fold increased sensitivity toward AZT compared with the nontransduced NGC-407 cells. The IC50 for AZT in the recombinant cells was 4.2 ± 1.7 µM (mean ± SEM, n = 6), whereas it was 138.9 ± 92.6 µM (mean ± SEM, n = 6) in the parental cells (data not shown in figure).
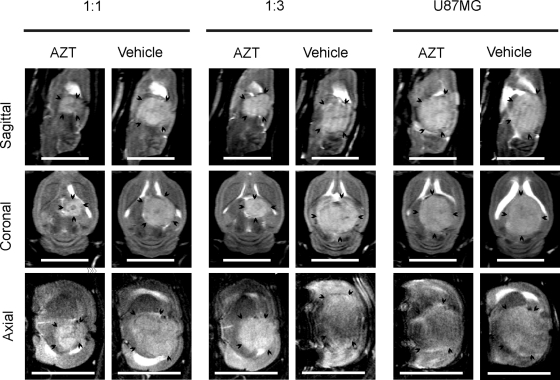
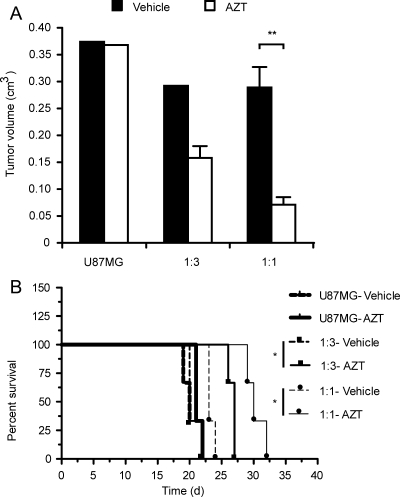
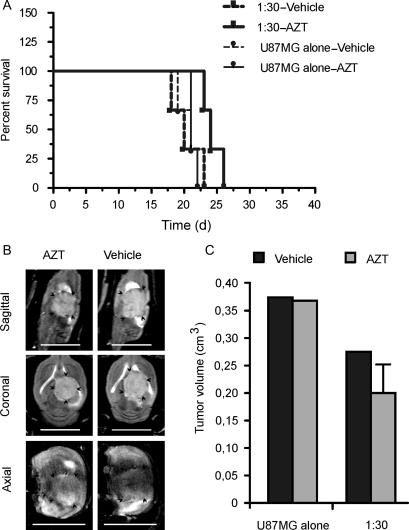
For the in vivo bystander effect, we mixed NGC-407-toTK1 cells with nontransduced U87MG cells at 1:1, 1:3, and 1:30 ratios and implanted them intracranially into nude rats. A fourth group of animals received only parental U87MG cells. The rats were treated with AZT or an equal volume of vehicle intraperitoneally for up to 21 days starting 1 day after implantation. Tumor growth was followed by MRI on days 12–13 and 22–23 postimplantation. Images were acquired at 3 anatomical projections (sagittal, coronal, and axial) to simulate 3D imaging. Comparison of tumor size between the first and second MRI follow-ups in the vehicle-treated animals showed that after initial establishment, the tumor grew very fast. Upon exposure to AZT for 3 weeks, the tumor size was substantially reduced in all toTK1-treated animals (Fig. 3). Tumor volume was measured using the MRI images. The observed tumor volumes were 25% and 50% of the respective vehicle-treated tumors at 1:1 and 1:3 ratios of NGC-407-toTK1 and U87MG cells. There was no difference in tumor size where only U87MG cells were injected, which indicates that AZT does not have an effect in the absence of toTK1 (Fig. 4A). The AZT-treated rats that received cells at 1:1 and 1:3 ratios survived on average 6–7 days longer than the vehicle-treated rats (Fig. 4B). The improvement of survival was statistically significant (P < .05). At the 1:30 ratio, exposure to AZT resulted in an apparent reduced tumor size and increased survival by 3 days, although this was statistically insignificant (Fig. 5). However, the 1:30 survival curves along with the curves presented in Fig. 4B show a significant trend (P = .0008, α = 0.05, n = 8; log-rank test for trend). Some vehicle-treated animals of the U87MG-alone, 1:3, and 1:30 groups and some AZT-treated animals of the U87MG-alone group reached the experimental end point before the scheduled MRI experiments. This indicates the very fast-growing nature of the GBM xenografts in the absence of toTK1 treatment and emphasizes the efficacy of the toTK1/AZT suicide gene therapy system.
Fig. 3.
MRI showing the size of GBM xenografts treated with toTK1-expressing NGC-407 cells and AZT. U87MG cells were used to create the intracranial xenografts in nude rats. toTK1-expressing NGC-407 cells were coinjected with U87MG cells at the indicated ratios. Another group received U87MG cells alone. Sagittal, coronal, and axial T2-weighted images at the largest tumor area in an animal from each of the U87MG alone, 1:3, and 1:1 (NGC-407:U87MG) groups are shown. Animals were treated with AZT or vehicle for 21 days starting 24 hours after implantation and the MRI measurements were performed on day 22 or 23 postimplantation. Arrow heads indicate the tumor border. Scale bar is 1 cm.
Fig. 4.
NGC-407 NPCs expressing toTK1 reduce tumor volume and improve survival in animals with human GBM xenografts. NGC-407 cells expressing toTK1 were coimplanted with U87MG glioma cells at 1:3 (n = 6) or 1:1 (n = 6) ratios. Each group of animals was then subgrouped and treated with AZT or vehicle for up to 21 days starting 24 hours after implantation. U87MG cells alone were injected in another group (n = 6), which was also treated similarly. (A) Tumor volumes after the stem cell delivery of toTK1 into intracranial GBM xenografts. Bar graphs show the volume of xenografts measured from multislice MRI images in the sagittal, coronal, and axial planes on day 22 or 23 postimplantation. Comparisons were made between AZT and vehicle treatment. **P < .01 (2-tailed, α = 0.05, n = 3; Student's t-test for unpaired values). Values are means ± SEM in the presence of error bars. (B) Kaplan–Meier survival plots showing improved survival on exposure to AZT. Comparison was made between these 2 treatment groups. *P < .05 (α = 0.05, n = 3) in both comparisons (log-rank [Mantel–Cox] test). The survival curves along with the curves for 1:30 ratio presented in Fig. 5 show a significant trend (P < .001, α = 0.05, n = 8; log-rank test for trend).
Fig. 5.
Effects of toTK1-expressing NGC-407 cells coimplanted with U87MG cells at a 1: 30 ratio. (A) Kaplan–Meier survival plots of nude rats that received only U87MG cells (n = 6) or the NGC-407 and U87MG cells (1:30; n = 6) and were exposed to AZT or vehicle for 14 days. P = .0629 for 1:30 group (α = 0.05, n = 3) and P = .4855 for U87MG-alone group (α = 0.05, n = 3), when treatment subgroups were compared (log-rank [Mantel–Cox] test). (B) Sagittal, coronal, and axial MRI images after coimplantation of NGC-407 and U87MG cells at a 1:30 ratio. One AZT-treated and 1 vehicle-treated animal are shown. MRI experiments were performed on day 22 or 23 postimplantation. Scale bar is 1 cm. (C) Bar graphs showing the volume of xenograft tumors after implantation of NGC-407 and U87MG cells at a 1:30 ratio or U87MG cells alone and upon exposure to AZT or vehicle. Volumes are measured from multislice images of MRI experiments.
Discussion
The success of suicide gene therapy relies on the comprehensive catalytic activity of the enzyme encoded by the suicide gene, targeted gene delivery, a suitable prodrug with adequate access into the tumor, and sufficient transgene expression as well as an efficient bystander effect.7,25,34,35 In this paper, we isolated and characterized a novel suicide gene, toTK1, which has high specificity for the prodrug AZT. toTK1/AZT demonstrated a more efficient cell killing of GBM cells in vitro compared with the widely tested HSV-tk/GCV system. In a GBM xenograft model in nude rats, toTK1 delivered by NPCs, reduced the tumor volume substantially and improved survival following exposure to AZT.
With respect to enzyme kinetics, the Vmax and Km parameters of toTK1 for Thd and AZT were very similar (Table 2), suggesting that this kinase may be considered as a Thd/AZT kinase. This property is also important from a therapeutic point of view, since it will prevent an in vivo substrate out-competition of AZT by Thd. The kinetic parameters obtained with our toTK1 system (Table 2) suggest that this would be an ideal suicide gene with respect to specificity for phosphorylation of AZT compared with Thd. Although direct translation of kinetic parameters measured in vitro to an in vivo condition is difficult, the observed kcat/Km values in Table 2 suggest that toTK1 catalyzes the phosphorylation of AZT more efficiently than that of Thd.
An additional important feature of toTK1 is its ability to phosphorylate AZT-MP to AZT-DP. To our knowledge, this is the first dNK reported to phosphorylate AZT-MP. Human thymidylate kinase has restricted substrate specificity and cannot substantially phosphorylate AZT-MP into the AZT-DP form.22 This differential property of the human TK1 and toTK1 is an important safety feature of using AZT, since the conversion of the prodrug to the therapeutically active form will be considerably more effective in cells expressing toTK1 and leave the toTK1-minus cells less affected.
toTK1/AZT tested in vitro showed that the toTK1/AZT combination eradicated GBM cells efficiently and was found superior to the HSV-tk/GCV system. Coculture experiments with toTK1-transduced and nontransduced cells also demonstrated that GBM cell killing by a gap junction–mediated bystander effect is also substantial (Fig. 1). In order to investigate the bystander effect in vivo, we used the NGC-407 stem cell line as a delivery vehicle of toTK1. This cell line has been expanded and propagated according to good manufacturing practice for future evaluation in clinical protocols for gene therapy. Although NGC-407 cells were immortalized for in vitro expansion, this will not create any adverse effects in vivo, as the stem cells carrying the suicide gene will die along with the tumor cells. This is a built-in safety in this cell-mediated suicide gene therapy. NGC-407 cells expressing toTK1 were implanted into nude rat brain along with human GBM cells. The growth of the GBM xenografts was attenuated substantially on exposure to AZT, as indicated by MRI and tissue sections. A significant survival benefit was also observed for the treated animals. It is important to note here that the reduced tumor size and improved survival was observed in immunocompromized animals, which were used to prevent rejection of human cell grafts. Immune response is known to improve the efficacy of suicide gene therapy by enhancing the bystander effect.36 Therefore, although the neural stem cells used here are less immunogenic compared with viral vectors,25 the observed effect may be even greater in immunocompetent animals or in a clinical setting. By using the toTK1/AZT suicide gene-prodrug system presented here, several of the previously encountered problems with this paradigm may be prevented. The optimal level of toTK1 expression in the vector cells is one issue that is yet to be defined, as is the tropism and migratory capacity of the stem cells carrying suicide gene to the site of tumor cells. If this can be achieved in an in vivo model, the novel toTK1/AZT suicide gene system may become available for clinical testing.
Funding
The work was supported by the Swedish Cancer Foundation (T.J.E., J.P., and P.M.A.), Karolinska Institutet (T.J.E.), Danish Cancer Society, Swedish Research Council, and the Meyer Foundation (J.P.), and Danish Research Council and Novo Nordisk Foundation (B.M.-P.).
Acknowledgments
The development of the NGC-407 cells was done in collaboration between NsGene A/S, Denmark, and the laboratory of Prof. Olle Lindvall, Lund University.
Conflict of interest statement. None declared.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 3.Oldfield EH, Ram Z, Culver KW, Blaese RM, DeVroom HL, Anderson WF. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993;4:39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- 4.Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 5.Palu G, Cavaggioni A, Calvi P, et al. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: a pilot study in humans. Gene Ther. 1999;6:330–337. doi: 10.1038/sj.gt.3300805. [DOI] [PubMed] [Google Scholar]
- 6.Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 7.Black ME, Newcomb TG, Wilson HM, Loeb LA. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proc Natl Acad Sci USA. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niculescu-Duvaz I, Springer CJ. Introduction to the background, principles, and state of the art in suicide gene therapy. Mol Biotechnol. 2005;30:71–88. doi: 10.1385/MB:30:1:071. [DOI] [PubMed] [Google Scholar]
- 9.Wiewrodt R, Amin K, Kiefer M, et al. Adenovirus-mediated gene transfer of enhanced herpes simplex virus thymidine kinase mutants improves prodrug-mediated tumor cell killing. Cancer Gene Ther. 2003;10:353–364. doi: 10.1038/sj.cgt.7700589. [DOI] [PubMed] [Google Scholar]
- 10.Desaknai S, Lumniczky K, Esik O, Hamada H, Safrany G. Local tumour irradiation enhances the anti-tumour effect of a double-suicide gene therapy system in a murine glioma model. J Gene Med. 2003;5:377–385. doi: 10.1002/jgm.357. [DOI] [PubMed] [Google Scholar]
- 11.Grignet-Debrus C, Cool V, Baudson N, et al. Comparative in vitro and in vivo cytotoxic activity of (E)-5-(2-bromovinyl)-2'-deoxyuridine (BVDU) and its arabinosyl derivative, (E)-5-(2-bromovinyl)-1-beta-D-arabinofuranosyluracil (BVaraU), against tumor cells expressing either the varicella zoster or the herpes simplex virus thymidine kinase. Cancer Gene Ther. 2000;7:215–223. doi: 10.1038/sj.cgt.7700108. [DOI] [PubMed] [Google Scholar]
- 12.Miura F, Moriuchi S, Maeda M, et al. Sustained release of low-dose ganciclovir from a silicone formulation prolonged the survival of rats with gliosarcomas under herpes simplex virus thymidine kinase suicide gene therapy. Gene Ther. 2002;9:1653–1658. doi: 10.1038/sj.gt.3301860. [DOI] [PubMed] [Google Scholar]
- 13.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 14.Sandrini MP, Piskur J. Deoxyribonucleoside kinases: two enzyme families catalyze the same reaction. Trends Biochem Sci. 2005;30:225–228. doi: 10.1016/j.tibs.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Knecht W, Rozpedowska E, Le Breton C, et al. Drosophila deoxyribonucleoside kinase mutants with enhanced ability to phosphorylate purine analogs. Gene Ther. 2007;14:1278–1286. doi: 10.1038/sj.gt.3302982. [DOI] [PubMed] [Google Scholar]
- 16.Mercer KE, Ahn CE, Coke A, Compadre CM, Drake RR. Mutation of herpesvirus thymidine kinase to generate ganciclovir-specific kinases for use in cancer gene therapies. Protein Eng. 2002;15:903–911. doi: 10.1093/protein/15.11.903. [DOI] [PubMed] [Google Scholar]
- 17.Springer CJ, Niculescu-Duvaz I. Prodrug-activating systems in suicide gene therapy. J Clin Invest. 2000;105:1161–1167. doi: 10.1172/JCI10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brewster ME, Raghavan K, Pop E, Bodor N. Enhanced delivery of ganciclovir to the brain through the use of redox targeting. Antimicrob Agents Chemother. 1994;38:817–823. doi: 10.1128/aac.38.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klecker RW, Jr, Collins JM, Yarchoan R, et al. Plasma and cerebrospinal fluid pharmacokinetics of 3'-azido-3'-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987;41:407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- 20.Blum MR, Liao SH, Good SS, de Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988;85:189–194. [PubMed] [Google Scholar]
- 21.Rolinski B, Bogner JR, Sadri I, Wintergerst U, Goebel FD. Absorption and elimination kinetics of zidovudine in the cerebrospinal fluid in HIV-1-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:192–197. doi: 10.1097/00042560-199707010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Lavie A, Schlichting I, Vetter IR, Konrad M, Reinstein J, Goody RS. The bottleneck in AZT activation. Nat Med. 1997;3:922–924. doi: 10.1038/nm0897-922. [DOI] [PubMed] [Google Scholar]
- 23.Furman PA, Fyfe JA, St Clair MH, et al. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rainov NG, Kramm CM. Recombinant retrovirus vectors for treatment of malignant brain tumors. Int Rev Neurobiol. 2003;55:185–203. doi: 10.1016/s0074-7742(03)01008-0. [DOI] [PubMed] [Google Scholar]
- 25.Lawler SE, Peruzzi PP, Chiocca EA. Genetic strategies for brain tumor therapy. Cancer Gene Ther. 2006;13:225–233. doi: 10.1038/sj.cgt.7700886. [DOI] [PubMed] [Google Scholar]
- 26.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown AB, Yang W, Schmidt NO, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 28.Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 29.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 30.Khan Z, Akhtar M, Asklund T, Juliusson B, Almqvist PM, Ekstrom TJ. HDAC inhibition amplifies gap junction communication in neural progenitors: potential for cell-mediated enzyme prodrug therapy. Exp Cell Res. 2007;313:2958–2967. doi: 10.1016/j.yexcr.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Roybon L, Hjalt T, Christophersen NS, Li JY, Brundin P. Effects on differentiation of embryonic ventral midbrain progenitors by Lmx1a, Msx1, Ngn2, and Pitx3. J Neurosci. 2008;28:3644–3656. doi: 10.1523/JNEUROSCI.0311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fick J, Barker FG, 2nd, Dazin P, Westphale EM, Beyer EC, Israel MA. The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proc Natl Acad Sci USA. 1995;92:11071–11075. doi: 10.1073/pnas.92.24.11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammerpohl O, Thormeyer D, Khan Z, et al. HDACi phenylbutyrate increases bystander killing of HSV-tk transfected glioma cells. Biochem Biophys Res Commun. 2004;324:8–14. doi: 10.1016/j.bbrc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Basilion JP, Ichikawa T, Chiocca EA. Gene therapy of brain tumors: problems presented by physiological barriers. Neurosurg Focus. 2000;8:1–7. [Google Scholar]
- 35.Portsmouth D, Hlavaty J, Renner M. Suicide genes for cancer therapy. Mol Aspects Med. 2007;28:4–41. doi: 10.1016/j.mam.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Freeman SM, Ramesh R, Marrogi AJ. Immune system in suicide-gene therapy. Lancet. 1997;349:2–3. doi: 10.1016/S0140-6736(97)22001-5. [DOI] [PubMed] [Google Scholar]