Abstract
Activation of inositol-requiring enzyme 1α (IRE1α) requires autophosphorylation to elicit the cellular unfolded protein response (UPR) and is functionally connected with insulin biosynthesis in pancreatic β-cells. We found that in pancreatic β-cells and primary islets the scaffold protein receptor for activated C-kinase 1 (RACK1) interacted with IRE1α in a glucose-stimulated or endoplasmic reticulum (ER) stress-responsive manner. RACK1 mediated the glucose-inducible assembly of a complex containing IRE1α, RACK1, and protein phosphatase 2A (PP2A) to promote dephosphorylation of IRE1α by PP2A, thereby inhibiting glucose-stimulated IRE1α activation and attenuating IRE1α-dependent increases in insulin production. Moreover, IRE1α activation was increased and RACK1 abundance was decreased in a mouse model of diabetes. Thus, our findings demonstrate that RACK1 functions as a key component in regulating the IRE1α signaling pathway in pancreatic β-cells.
Introduction
The unfolded protein response (UPR) plays a pivotal role in cellular homeostasis by expanding the protein folding capacity of the endoplasmic reticulum (ER) to cope with unfolded or misfolded proteins (1). IRE1α is an ER transmembrane protein kinase that functions as an endonuclease (2, 3) and senses increased demand for protein folding in the ER lumen. It is activated through autophosphorylation to initiate a key signaling arm of the mammalian UPR pathways (1). IRE1α acts through non-conventional splicing of the mRNA encoding transcription factor X-box binding protein-1 (XBP-1) and coordinates the gene expression programs of the UPR (4, 5), thus playing a role in various cellular events, including differentiation of plasma cells (6–8). IRE1α activation and attenuation is also linked to the decision of cell fate between survival and death during ER stress responses (9). In secretory pancreatic β-cells, IRE1α activation plays a role in modulating insulin biosynthesis (10). In response to acute glucose stimulation, increased phosphorylation of IRE1α is coupled to insulin production through mechanisms that do not involve XBP-1 splicing, whereas prolonged activation of IRE1α leads to suppression of insulin production after chronic exposure to high glucose (10). Under metabolic stress conditions such as in the state of genetic or diet-induced obesity, chronic IRE1α activation is also thought to be implicated in the progression of insulin resistance and diabetes mellitus (11–14). Despite increasing progress towards our understanding of the functional importance of IRE1α signaling, the molecular machinery that governs the dynamics of IRE1α activation and inactivation remains largely elusive.
RACK1, which was originally identified as an adaptor protein for activated protein kinase C (15), is a scaffold protein that contains seven Trp-Asp 40 (WD40) repeats. RACK1 binds to membrane receptors and protein kinases and coordinates the interactions between signaling components in multiple cellular processes (16). Although studies have also implicated RACK1 in mediating distinct types of cell stress responses (17, 18), whether RACK1 plays a role in the UPR-related signaling events has yet to be explored. Here, we show that in pancreatic β-cells and primary islets, RACK1 functions as an adaptor in regulating IREα activation through distinct modes of interactions in assembling a complex with IRE1α and protein phosphatase PP2A in responses to glucose stimulation or ER stress signals. Our findings provide mechanistic evidence establishing RACK1 as a key component in the cellular regulatory machinery that governs the dynamic activation of the IRE1α signaling platform in β-cells.
Results
RACK1 interacts with IRE1α in a glucose-stimulated or ER stress-induced fashion in pancreatic β-cells
To identify IRE1α-interacting partners that may regulate the IRE1α signaling pathway, we performed a yeast two-hybrid screen using the cytoplasmic portion of human IRE1α as bait. The scaffold protein RACK1 was isolated from a human liver cDNA library as a candidate IRE1α interactor. We first confirmed the IRE1α-RACK1 interaction in human embryonic kidney (HEK) 293T cells by coimmunoprecipitation analysis of overexpressed IRE1α and RACK1 proteins (fig. S1). To determine whether the IRE1α-RACK1 interaction occurs endogenously under physiological or ER stress conditions, coimmunoprecipitations were performed with an antibody against RACK1 and extracts from INS-1 β-cells that were stimulated with increasing concentrations of glucose or treated with two chemicals that induce ER stress, thapsigargin (Tg) and tunicamycin (Tm). In contrast to treatment with 2.5 mM glucose, stimulation with high glucose at 16.7 or 25 mM induced the association of IRE1α with RACK1 (Fig. 1A), which was accompanied by increased phosphorylation at the Ser724 activation site in IRE1α as detected by a phospho-specific antibody. On the other hand, the interaction of IRE1α with RACK1 also increased in response to pharmacological induction of ER stress (Fig. 1B). Moreover, glucose at concentrations within the physiological ranges enhanced the IRE1α-RACK1 interaction as well as IRE1α phosphorylation in a dose-dependent manner (Fig. 1C). However, in contrast to the observations under ER stress (Fig. 1B and fig. S2), glucose-stimulated phosphorylation of IRE1α did not increase the splicing of XBP-1 mRNA or the expression of typical UPR target genes (fig. S2) (19), but did decrease phosphorylation of eukaryotic initiation factor 2α (eIF2α) as previously reported (Fig. 1A) (20, 21). Unlike the activation of IRE1α by inducers of ER stress, glucose-induced phosphorylation did not cause a shift of the IRE1α protein as detected by immunoblot analysis (fig. S3), suggesting that IRE1α has a distinct phosphorylation status after glucose stimulation. In addition, neither fatty acid palmitate (fig. S4A), which induces ER stress markers (22), nor insulin (fig. S4B) stimulated IRE1α phosphorylation in INS-1 cells, and blocking insulin signaling or its secretion did not influence glucose-stimulated phosphorylation of IRE1α (fig. S4B). Thus, the induction of IRE1α phosphorylation is a signaling response that is specific to glucose stimulation in β-cells, ruling out the involvement of indirect autocrine action from glucose-stimulated insulin secretion. Together, these results demonstrate that stimulation of the IRE1α-RACK1 interaction accompanies glucose-induced IRE1α phosphorylation and represents a specific glucose-sensing event in pancreatic β-cells; however, this physiological stimulation by glucose, as opposed to ER stress, does not trigger the IRE1α-dependent splicing of XBP-1 mRNA or the UPR.
Fig. 1. Glucose or ER stress induces the endogenous IRE1α-RACK1 interaction in β-cells.
(A to C) INS-1 832/3 β-cells maintained in 5 mM glucose (Glc) for 18 hours were cultured for 3 hours in medium with glucose at 2.5, 16.7, or 25 mM (A), or treated with DMSO, thapsigargin (Tg, 2 μM), or tunicamycin (Tm, 10 μg/ml) for 1 or 3 hours (B), or cultured for 3 hours in medium with glucose at the indicated concentrations ranging from 2.5 to 16.7 mM (C). Immunoprecipitation was performed with anti-RACK1. Antibodies against IRE1α, eIF2α, RACK1, and α-tubulin were used for immunoblotting. Phosphorylated IRE1α (P-IRE1α) and eIF2α (P-eIF2α) in cell lysates were detected by antibodies against phospho-Ser724 in IRE1α and phospho-Ser52 in eIF2α, respectively. Results are representative of three (A and B) or two (C) independent experiments.
To determine how glucose exerts its effect on IRE1α activity, we tested whether glucose metabolism or subsequent intracellular calcium mobilization is required for glucose-stimulated IRE1α phosphorylation and IRE1α-RACK1 association in INS-1 β-cells. Indeed, inhibition of glucose glycolysis by 2-deoxyglucose (2-DG) blunted glucose-stimulated phosphorylation of IRE1α and its interaction with RACK1 (fig. S5), as did blocking of calcium influx by EGTA (23) or calcium release from the ER by 2-APB (2-aminoethoxy-diphenyl borate) or dantrolene (which inhibits inositol trisphosphate or ryanodine receptors) (24). These data suggest that glucose-regulated IRE1α phosphorylation and formation of the IRE1α-RACK1 complex are mediated by glucose metabolism-dependent activation of the cellular calcium signaling pathways in β-cells.
To examine the structural requirements for IRE1α interaction with RACK1, we generated a series of IRE1α deletion mutants (fig. S6A) and analyzed their ability to associate with RACK1 through coimmunoprecipitation assays. Whereas IRE1α molecules with deletions of the RNase domain (ΔR) or both the kinase and RNase domains (ΔKR) interacted with RACK1, IRE1α with a deletion of the linker region (ΔL) did not interact with RACK1 (fig. S6B). These findings were confirmed by an in vitro pull-down assay using the purified GST-RACK1 fusion protein (fig. S7). This demonstrates that the linker region of IRE1α is required for RACK1 interaction. The Asp123→Pro123 (D123P) mutation in the lumenal domain of IRE1α (fig. S6A), which abrogates IRE1α dimerization and activation (25), also abolished the interaction of IRE1α with RACK1 (fig. S6B). These results indicate that IRE1α association with RACK1 is mediated through its linker region and may require the dimerization of IRE1α.
RACK1 is essential for attenuation of glucose-stimulated IRE1α phosphorylation
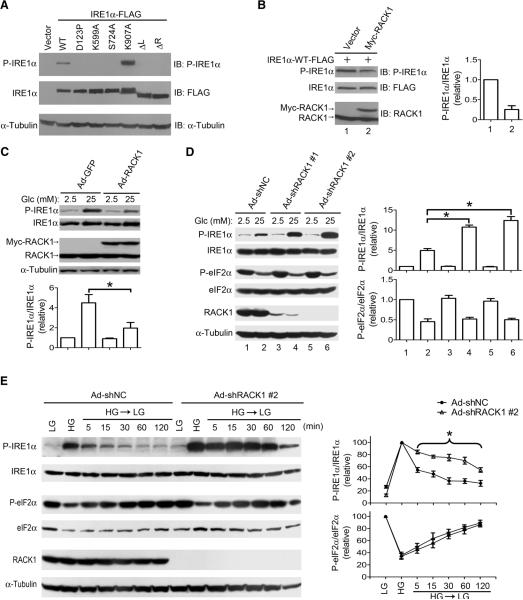
To investigate a role for RACK1 in regulating IRE1α signaling, we assessed whether RACK1 interaction altered the phosphorylation status of IRE1α. We first examined the ability of different IRE1α mutants to autophosphorylate when overexpressed in HEK 293T cells. Consistent with previously reported findings (10, 26, 27), overexpression of wild-type IRE1α resulted in its spontaneous autophosphorylation at Ser724, as detected by the phospho-site-specific antibody that did not recognize the Ser724→Ala724 (S724A) substitution mutant of IRE1α (Fig. 2A). Similarly to IRE1α mutants lacking kinase activity or the ability to dimerize, the IRE1α-ΔL mutant was not phosphorylated at Ser724, suggesting that the linker region of IRE1α contains unique structural elements essential for its autophosphorylation. Although deletion of the RNase domain (ΔR) eliminated autophosphorylation, the Lys907→Ala907 (K907A) mutation that abrogates its RNase activity (27) did not alter IRE1α phosphorylation at Ser724 (Fig. 2A). Moreover, cotransfection experiments demonstrated that exogenously expressed RACK1 significantly decreased phosphorylation of IRE1α (by 74.4 ± 9.4%, Fig. 2B). INS-1 β-cells infected with Ad-GFP showed increased IRE1α phosphorylation when stimulated with high glucose concentration (25 mM) (Fig. 2C). In contrast, INS-1 β-cells infected with Ad-RACK1 showed reduced (by 57.6 ± 5.3%) glucose-stimulated phosphorylation of IRE1α (Fig. 2C). In contrast, Ad-RACK1 infection did not affect ER stress-induced IRE1α phosphorylation or XBP-1 mRNA splicing (fig. S8A and S8B).
Fig. 2. RACK1 attenuates IRE1α phosphorylation.
(A) Autophosphorylation of overexpressed IRE1α proteins. Cell lysates from HEK 293T cells transfected with the indicated FLAG-tagged IRE1α constructs were analyzed by immunoblotting with anti-IRE1α [pSer724]. The membrane was stripped and subsequently immunoblotted with an antibody against FLAG. Shown is the representative of two independent experiments. (B) RACK1 overexpression reduces IRE1α phosphorylation. HEK 293T cells were cotransfected with the indicated plasmids and immunoblotting was performed using the indicated antibodies. IRE1α phosphorylation was determined by densitometric quantification of the immunoblots, and the relative ratios of P-IRE1α normalized to total IRE1α are presented as means ± SEM (n=3 independent experiments). (C) Overexpression of RACK1 decreases glucose-stimulated IRE1α phosphorylation in β-cells. INS-1 cells were infected at an MOI of 10 for 48 hours with recombinant adenoviruses expressing GFP or Myc-tagged RACK1. Infected cells maintained in 5 mM glucose for 18 hours were cultured in 2.5 or 25 mM glucose for 3 hours. Relative P-IRE1α/IRE1α ratios were determined from densitometric quantifications of the immunoblots and are shown as means ± SEM (n=3 independent experiments). *P<0.05 compared to Ad-GFP-infected cells cultured in 25 mM glucose by two-way ANOVA. (D) RACK1 deficiency augments glucose-stimulated IRE1α phosphorylation in β-cells. INS-1 cells were infected at an MOI of 40 for 72 hours with adenoviruses Ad-shNC, Ad-shRACK1 #1, or Ad-shRACK1 #2. Cells were subsequently maintained in 5 mM glucose for 18 hours, followed by incubation with 2.5 or 25 mM glucose for 3 hours. Relative P-IRE1α/IRE1α and P-eIF2α/eIF2α ratios are shown as means ± SEM (n=3 independent experiments). *P<0.05 compared to Ad-shNC-infected cells cultured in 25 mM glucose by two-way ANOVA. (E) Knockdown of RACK1 attenuates dephosphorylation of IRE1α after β cells are shifted from high to low glucose conditions. INS-1 cells were infected with adenoviruses Ad-shNC or Ad-shRACK1 #2. Cells were either maintained in low glucose (LG) at 2.5 mM for 3 hours or stimulated with 25 mM high glucose (HG) for 3 hours, then incubated in 2.5 mM glucose for the indicated time intervals. Changes in the relative ratios of P-IRE1α/IRE1α and P-eIF2α/eIF2α, which were normalized to their respective HG controls (which were set at 100), were tracked over the indicated time period, and are shown as means ± SEM (n=3 independent experiments). *P<0.05 compared to the Ad-shNC control by two-way ANOVA.
Knockdown of endogenous RACK1 by Ad-shRACK1 #1 or #2, which express one of two small hairpin (sh) RNAs directed against RACK1, increased phosphorylation of IRE1α in response to high glucose, compared to cells infected with a scrambled negative control virus (Ad-shNC) (10.8 ± 0.5 or 12.4 ± 1.0-fold compared to 4.9 ± 0.5-fold; Fig. 2D). Furthermore, shifting cells expressing the RACK1 shRNAs from high to low glucose conditions did not decrease phosphorylation of IRE1α such that 69.9 ± 5.6% of the maximal IRE1α phosphorylation was sustained for up to 60 min (Fig. 2E). In contrast, shifting Ad-shNC-infected control cells from high to low glucose conditions caused a decrease in IRE1α phosphorylation (Fig. 2E). However, RACK1 knockdown did not alter glucose-induced dephosphorylation of eIF2α (Fig. 2D and 2E) (20, 21), suggesting that RACK1 knockdown selectively affects the phosphorylation of IRE1α. In contrast, IRE1α phosphorylation was decreased in RACK1 knockdown INS-1 cells after 3 hours of Tg treatment (fig. S8C). Moreover, RACK1 knockdown did not affect XBP-1 mRNA splicing in INS-1 cells stimulated with high glucose or treated with Tg (fig. S8D). Thus, RACK1 may exert distinct actions in the regulation of IRE1α signaling in response to a physiological stimulus (such as glucose) as opposed to ER stress, with RACK1 associating with IRE1α to specifically decrease glucose-stimulated IRE1α phosphorylation.
RACK1 controls IRE1α phosphorylation through regulated assembly of a ternary IRE1α-RACK1-PP2A complex
RACK1 associates with various signaling molecules (16), including protein phosphatase 2A (PP2A), which can dissociate from RACK1 in an insulin-like growth factor I (IGF-I)-regulated manner (28). Thus, RACK1 may operate in a fashion analogous to growth arrest and DNA damage-inducible 34 (GADD34), which mediates the dephosphorylation of eIF2α by recruiting PP1c, thereby inhibiting the pancreatic endoplasmic reticulum eIF2α kinase (PERK)-eIF2α arm of the UPR pathways (29). However, two protein phosphatases in yeast, Ptc2p (a serine-threonine phosphatase of type 2C) and Dcr2 (the dose-dependent cell-cycle regulator 2 phosphatase), interact directly with Ire1p (the yeast homologue of IRE1α) to mediate its dephosphorylation (30, 31). To test whether RACK1-mediated downregulation of mammalian IRE1α phosphorylation involves a protein phosphatase, we examined the effect of PP1c, PP2A, or PP2C (the mammalian homologue of yeast Ptc2p) on IRE1α phosphorylation through cotransfection experiments. Specifically, overexpression of PP2A reduced (by 75.1 ± 8.0%) the phosphorylation of IRE1α (Fig. 3A). Moreover, adenoviral overexpression of PP2A, but not that of GFP, in INS-1 cells impaired high glucose-stimulated phosphorylation of IRE1α by 51.2 ± 3.0% (Fig. 3B). To rule out possible non-specific effects caused by the overexpression of PP2A, we examined the effect of okadaic acid (OA), a phosphatase inhibitor with a higher potency against PP2A than PP1c (Ki of ~0.032 nM compared to ~147 nM) (32), on IRE1α phosphorylation. Consistent with an action on endogenous PP2A, OA treatment decreased the dephosphorylation of IRE1α in cells shifted from high glucose to low glucose, resulting in increased IRE1α phosphorylation at concentrations of >50 nM (Fig. 3C), but did not affect the phosphorylation status of eIF2α.
Fig. 3. RACK1 promotes IRE1α dephosphorylation through PP2A.
(A) Overexpression of PP2A reduces IRE1α autophosphorylation. Plasmids encoding HA-tagged PP1c, PP2A, or PP2C were co-transfected with FLAG-tagged wild-type IRE1α into HEK 293T cells. IRE1α phosphorylation was analyzed by immunoblotting at 48 hours post-transfection. The expression of PP1c, PP2A, or PP2C was detected by anti-HA. Relative P-IRE1α/IRE1α ratios are shown as means ± SEM (n=3 independent experiments). *P<0.05 compared to the vector control by one-way ANOVA. (B) Adenoviral overexpression of PP2A decreases glucose-induced IRE1α phosphorylation in β-cells. INS-1 cells infected at an MOI of 10 for 48 hours with adenoviruses expressing GFP or PP2A were maintained in 5 mM glucose for 18 hours and then cultured in 2.5 or 25 mM glucose for 3 hours. Relative P-IRE1α/IRE1α ratios are presented as means ± SEM (n=3 independent experiments). *P<0.05 compared to Ad-GFP-infected cells cultured in 25 mM glucose by two-way ANOVA. (C) Okadaic acid (OA) inhibits dephosphorylation of IRE1α, but not eIF2α, in β-cells shifted from high to low glucose conditions (HG → LG). INS-1 cells maintained with 5 mM glucose for 18 hours were cultured with low glucose (LG) at 2.5 mM or high glucose (HG) at 25 mM glucose for 3 hours, followed by incubation in fresh medium for another 2 hours (compare lane 1 to lane 2); cells pre-treated with 25 mM glucose for 3 hours were then incubated in medium with 2.5 mM glucose in the presence of increasing concentrations of OA for 2 hours as indicated (lanes 3 to 8). Relative P-IRE1α/IRE1α and P-eIF2α/eIF2α ratios are shown as means ± SEM (n=3 independent experiments). *P<0.05 compared to cells cultured with low glucose by one-way ANOVA. (D) RACK1 associates with both IRE1α and PP2A. Plasmids encoding HA-tagged PP2A or PP2C were cotransfected with FLAG-tagged WT IRE1α into HEK 293T cells. Immunoprecipitation was performed at 48 hours post-transfection with anti-RACK1, followed by immunoblotting with antibodies against FLAG, HA, or RACK1. Results are representative of two independent experiments.
To examine whether there exists a ternary IRE1α-RACK1-PP2A complex, we performed coimmunoprecipitation experiments i n cotransfected 293T cells overexpressing IRE1α and PP2A or PP2C. Anti-RACK1 precipitated IRE1α and PP2A, but not PP2C (Fig. 3D), suggesting a specific association between IRE1α-RACK1 and PP2A. Next, to test whether RACK1 is an adaptor that bridges the interaction between IRE1α and PP2A, we used an antibody against the catalytic subunit of PP2A to conduct coimmunoprecipitation assays in INS-1 cells in the presence or absence of RACK1 (Fig. 4A). In control cells infected by Ad-shNC, PP2A was constitutively associated with RACK1. However, acute stimulation with 25 mM glucose for 3 hours was required for PP2A to coimmunoprecipitate with IRE1α. Moreover, phosphorylation of IRE1α within this tripartite complex was undetectable (Fig. 4A). In contrast, glucose stimulation of RACK1 knockdown INS-1 cells increased the phosphorylation of IRE1α, and IRE1α did not coimmunoprecipitate with PP2A from these cells (Fig. 4A). Because RACK1 deficiency did not result in increased Tg-induced phosphorylation of IRE1α, we wondered whether the assembly of the IRE1α-RACK1-PP2A complex occurs in response to ER stress. Coimmunoprecipitation analysis with anti-RACK1 revealed that, in contrast to glucose stimulation, treatment with Tg and Tm triggered the dissociation of PP2A from RACK1 in INS-1 cells (Fig. 4B). The dissociation of PP2A in response to ER stress could account for the sustained phosphorylation of IRE1α within the IRE1α-RACK1 complex (Fig. 4B). Moreover, this ER stress-induced dissociation of RACK1 from PP2A, with concomitant sustained phosphorylation of RACK1-associated IRE1α was also observed ex vivo in isolated primary mouse islets (Fig. 4B). To explore whether changes in the composition of the IRE1α-RACK1-PP2A complex contributes to sustained phosphorylation of IRE1α after chronic treatment with high glucose, we conducted the same coimmunoprecipitation assays using anti-RACK1 in INS-1 cells stimulated with 25 mM glucose for 72 hours (as compared to 3 hours). In a similar manner to ER stress (Fig. 4B), prolonged stimulation with high glucose promoted the dissociation of PP2A from RACK1, resulting in sustained phosphorylation of IRE1α associated with RACK1 (Fig. 4C). Thus, our results demonstrate that RACK1 serves as a critical adaptor in assembling distinct regulatory modules within the IRE1α signaling platform in β-cells, exerting differential effects on IRE1α phosphorylation in response to acute increases in glucose as opposed to chronic exposure to high glucose or ER stress.
Fig. 4. RACK1 regulates IRE1α phosphorylation through altered assembly of an IRE1α-RACK1-PP2A complex.

(A) RACK1 is essential for glucose-stimulated formation of a ternary IRE1α-RACK1-PP2A complex. INS-1 cells were infected at an MOI of 40 for 72 hours with adenoviruses Ad-shNC or Ad-shRACK1 #2. Following pre-culture in 5 mM glucose for 18 hours, cells were incubated in 2.5 mM or 25 mM glucose for 3 hours. Immunoprecipitation was performed using anti-PP2A, followed by immunoblotting with the desired antibodies. Results represent three independent experiments. (B) The dissocation of PP2A from RACK1, as induced by ER stress, causes the retention of RACK1-associated IRE1α phosphorylation in INS-1 β-cells and islets. INS-1 cells maintained in 5 mM glucose for 18 hours were treated with DMSO, thapsigargin (Tg, 2 μM), tunicamycin (Tm, 10 μg/ml), or high glucose (HG, 25 mM). Pancreatic islets isolated from 28 male C57BL/6 mice were pooled and maintained in 5 mM glucose for 18 hours, and were subsequently treated for 3 hours with thapsigargin (Tg, 2 μM) or high glucose (HG, 25 mM). Immunoprecipitation was performed with anti-RACK1, and immunoblotting was conducted using the indicated antibodies. Shown is the representative result of two independent experiments for INS-1 cells or pancreatic islets. (C) Chronic exposure to high glucose causes the dissociation of PP2A from RACK1 and sustained phosphorylation of RACK1-associated IRE1α in β-cells. INS-1 cells pre-cultured in 5 mM glucose were maintained in 2.5 mM glucose for 3 hours or in 25 mM glucose for 3 or 72 hours. Immunoprecipitation was performed using anti-RACK1, followed by immunoblotting with the indicated antibodies. Results represent three independent experiments.
RACK1 affects IRE1α-dependent regulation of insulin production in β-cells and pancreatic islets
To determine how RACK1 regulates IRE1α's functions, we investigated whether changes in the assembly of the IRE1α-RACK1-PP2A complex in response to acute compared to prolonged exposure of high glucose are associated with altered amounts of insulin in INS-1 cells. Chronic stimulation for 72 hours, but not acute stimulation for 3 hours, with high glucose decreased the abundance of both Insulin 1 and Insulin 2 (fig. S9B), similar to the effect of Tg treatment (fig. S9C). Because IRE1α has been implicated in degradation of certain ER-localized mRNAs including the insulin mRNAs (33–36), these data indicate the possible functional consequences of dysregulated IRE1α's activity as a result of malfunctioning of the RACK1-PP2A regulatory module under stress conditions (Fig. 4B and 4C).
We then tested whether RACK1-mediated regulation of glucose-stimulated IRE1α phosphorylation alters insulin protein production in INS-1 β-cells or in pancreatic islets from animal models. In accordance with the notion that IRE1α activation is coupled to insulin biosynthesis as previously documented (10), adenoviral overexpression of IRE1α in INS-1 cells maintained at 11.1 mM glucose (a normal cell culture concentration) caused autophosphorylation of IRE1α and increased insulin content by 60.0 ± 5.2% compared to INS-1 cells overexpressing GFP (Fig. 5A). In contrast, cells overexpressing the S724A mutant, similar to cells overexpressing the K599A mutant, showed decreased phosphorylation of endogenous IRE1α and 42.8 ± 1.0% or 47.4 ± 5.3% reduction in the insulin content compared to cells overexpressing GFP (Fig. 5A), suggesting the importance of IRE1α phosphorylation at Ser724 in the regulation of insulin protein biosynthesis. Adenoviral overexpression of RACK1 also reduced insulin content; moreover, coinfection of Ad-RACK1 with Ad-IRE1α decreased insulin content and IRE1α phosphorylation (Fig. 5A). Conversely, INS-1 cells lacking RACK1 showed increased phosphorylation of IRE1α (by 1.9 ± 0.1 or 2.0 ± 0.1-fold; fig. S10A), leading to 3.5 ± 0.2 or 4.2 ± 0.1-fold increases in the insulin content (Fig. 5B). Consistent with a role for IRE1α in the regulation of insulin peptide translation (10), RACK1-mediated alterations in IRE1α phosphorylation did not change insulin mRNA abundance (fig. S11A and S11B) or glucose-stimulated insulin secretion in INS-1 cells (fig. S12). Furthermore, forced adenoviral expression ex vivo of RACK1 in isolated islets reduced IRE1α phosphorylation by 36.3 ± 4.4% (fig. S10B) and consequently decreased the islet insulin content by 33.3 ± 4.5% (Fig. 5C). These findings suggest an inverse correlation between RACK1 and IRE1α phosphorylation and that IRE1α phosphorylation is coupled with increased insulin protein abundance.
Fig. 5. RACK1 antagonizes IRE1α-dependent upregulation of insulin production in β-cells.
(A) Overexpression of RACK1 in β-cells attenuates the IRE1α phosphorylation-dependent increase in the intracellular insulin content. INS-1 cells maintained in 11.1 mM glucose were infected with control adenovirus Ad-GFP (at an MOI of 20), or co-infected at a total MOI of 20 with Ad-GFP (at an MOI of 10) plus viruses expressing the indicated forms of IRE1α, Ad-RACK1, or Ad-IRE1α-WT with Ad-RACK1. Cell lysates were analyzed by immunoblotting at 48 hours post-infection using the indicated antibodies. Intracellular insulin content was measured by radioactive immunoassay (RIA) and values are shown as means ± SEM (n=3 independent experiments). *P<0.05 compared to Ad-GFP-infected control cells, and #P<0.05 compared to cells infected with Ad-IRE1α by one-way ANOVA. (B) RACK1 knockdown increases IRE1α phosphorylation and insulin content in β-cells. INS-1 cells maintained in 11.1 mM glucose were infected at an MOI of 40 with Ad-shNC, Ad-shRACK1 #1, or Ad-shRACK1 #2. Intracellular insulin content was determined and is shown as means ± SEM (n=3 independent experiments). *P<0.05 compared to the Ad-shNC control by one-way ANOVA. (C) Adenoviral RACK1 overexpression reduces IRE1α phosphorylation and insulin content in mouse pancreatic islets. Primary islets isolated from 6–8 male C57BL/6 mice were pooled and infected at an MOI of 100 (with the assumption of 2500 cells/islet) by Ad-GFP or Ad-RACK1 for 48 hours. After pre-culture in 5 mM glucose for 18 hours, islets were incubated in 25 mM glucose for 3 hours prior to immunoblotting analysis. Insulin content was measured and are shown as means ± SEM (n=3 independent experiments). *P<0.05 by unpaired two-tailed t-test. (D) Restored expression of RACK1 reduces IRE1α phosphorylation and decreases insulin content in the islets of db/db mice. Islets isolated and pooled from 6–7 C57BL/6 db/db mice at 14–16 weeks of age were infected at an MOI of 100 with Ad-GFP or Ad-RACK1 for 48 hours or left uninfected. Uninfected islets from wild-type littermates were used as the control. Immunoblotting and RIA insulin measurement were performed, and insulin contents are shown as means ± SEM (n=3 independent experiments). *P<0.05 by unpaired two-tailed t-test.
To investigate whether RACK1-mediated control of IRE1α activation is altered in pancreatic islets undergoing metabolic stress, we employed the leptin receptor-deficient C57BL/6 db/db mice (37), which develop morbid obesity and mild diabetes as characterized by transitory hyperglycemia (fig. S13A), hyperinsulinemia, and hypertrophy of the islets (38). In comparison to wild-type littermates, phosphorylation of IRE1α but not that of eIF2α was elevated by 6.6 ± 0.8-fold; fig. S10C) in the islets of db/db mice (Fig. 5D; fig. S14A); furthermore, Xbp-1 splicing and the abundance of XBP-1 target genes such as Bip was increased (fig. S14B). In addition, the abundance of Rack1 mRNA (fig. S13B) and RACK1 protein (Fig. 5D) were decreased by 4.4 ± 0.6-fold and 5.5 ± 0.6-fold in db/db islets compared to wild-type islets, whereas no changes were detected in the abundance of PP2A protein (fig. S13C). In parallel, when normalized to total islet protein amounts, db/db islets showed increased (by 48.5 ± 0.6%) insulin content relative to wild-type islets (Fig. 5D). Restoration of RACK1 expression in db/db islets through adenoviral infection reduced IRE1α phosphorylation (by 44.2 ± 4.1%; fig. S10C) and insulin content (by 36.0 ± 1.5%;; Fig. 5D) without altering the abundance of insulin mRNA (fig. S13D). Thus, these results reveal altered regulation of IRE1α as a result of decreased RACK1 abundance in the islets of db/db mice, which may represent an adaptive response to obesity-induced metabolic stress to enhance IRE1α signaling and increase insulin production.
Discussion
Proper activation of the IRE1α signaling pathway elicits the cytoprotective actions of the UPR and is essential to maintaining cellular homeostasis and survival in response to ER stress (9). Our findings in the present study reveal a role for RACK1 in modulating the dynamic activation of the IRE1α signaling platform in pancreatic β-cells under different extracellular stimuli. As depicted in our proposed model (Fig. 6), RACK1 may switch its regulatory mode through altered capacity for associating with PP2A. RACK1 serves as an adaptor to bridge the interaction between IRE1α and PP2A, thus specifically targeting IRE1α for dephosphorylation in response to physiological stimulation of glucose; through dissociation from PP2A, however, RACK1 does not alter the phosphorylation status of IRE1α in response to chronic exposure to high glucose or ER stress signals. This contrasts with how Ire1p activity is regulated in yeast, where both Ptc2p and Dcr2 phosphatases can directly interact with phosphorylated Ire1p (30, 31). Moreover, given that the yeast Ire1p possesses a longer linker region that is essential for its activation through oligomerization (39), it is tempting to speculate that RACK1, through induced interaction with mammalian IRE1α's shorter linker region, may be an adaptor that mediates the oligomerization (or clustering) or stabilization of the activated form of IRE1α. The use of RACK1 in mammals may provide an extra regulatory layer for more precise and flexible control of IRE1α signaling, facilitating the coordination and integration of multiple UPR signaling cascades in response to a broader spectrum of physiological stimuli or stress conditions.
Fig. 6. Schematic model for RACK1 acting as a crucial regulatory adaptor within the IRE1α signaling platform in pancreatic β-cells.
RACK1 is constitutively associated with PP2A under normal physiological conditions. It interacts with IRE1α in response to acute glucose stimulation, thereby directing PP2A to exert a feedback “brake” control on IRE1α phosphorylation as well as IRE1α-dependent upregulation of insulin biosynthesis. Conversely, ER stress or prolonged exposure to high glucose causes RACK1 to dissociate from PP2A, resulting in disruption of this tripartite regulatory module. In this scenario, the retained phosphorylation of RACK1-associated IRE1α may exert altered functional outputs during the cellular UPR.
It is remains unknown whether IRE1α-associated RACK1 proteins bear distinct functional features under acute glucose stimulation compared to prolonged exposure to high glucose or during conditions of ER stress; moreover, the exact molecular nature of modifications within the RACK1-PP2A regulatory module has yet to be dissected. At present, it is also unclear whether the induction of RACK1 dissociation from PP2A under stress conditions involves similar phosphorylation events as seen with IGF-I-induced RACK1-PP2A dissociation, an event that mediates the integration of IGF-I receptor and adhesion signaling in other cell types (40). Moreover, it has been demonstrated that IRE1α may possess distinct endonuclease target specificities upon activation through autophosphorylation as compared to that through chemical modulation of its kinase activity (35). Thus, our results raise the possibility that IRE1α-associated RACK1, after dissociation from PP2A, may influence the activation state of IRE1α and thus its endonuclease selectivity, thereby leading to the observed reductions in insulin mRNA abundance during ER stress or after chronic exposure to high glucose.
Our findings in the islets of leptin receptor-deficient db/db mice provide additional evidence for a role of RACK1-mediated mechanism in the physiological IRE1α regulation under certain metabolic stress conditions. However, the precise pathophysiological effect of chronic RACK1-dependent dysregulation of IRE1α during β-cell dysfunction remains to be further investigated. In islets from db/db mice, RACK1 suppression and the subsequent increase in IRE1α phosphorylation does not lead to the decreased insulin mRNA abundance seen in INS-1 β-cells exposed to high glucose for prolonged periods. This difference between in vitro and in vivo models may reflect an adaptive compensatory mechanism for IRE1α-dependent upregulation of insulin biosynthesis in β-cells during islet expansion. However, it is currently unknown whether decreased RACK1 abundance in the db/db islets arises directly from defective leptin signaling or from other obesity-associated metabolic stress signals. It is also not clear whether chronic IRE1α activation as a result of prolonged failure of this RACK1-operated “brake” may bring about deleterious effects on β-cell functions, contributing to the pathological development of β-cell failure. Given the essential role of IRE1α activation in governing the switch from the adaptive UPR to ER stress-associated apoptosis, it is important to fully understand how RACK1 affects the actions of other IRE1α interactors, such as TRAF2 (tumor necrosis factor receptor-associated factor 2) (41), JIK (c-Jun N-terminal inhibitory kinase) (42), and the pro-apoptotic proteins Bak and Bax (43), and the anti-apoptotic protein BI-1 (Bax inhibitor-1) (44), as well as the interplay between other RACK1-mediated actions and IRE1α-dependent cellular events during ER stress, such as JNK activation (18, 41). In this scenario, defective signaling through the RACK1-PP2A regulatory loop in the control of the IRE1α signaling pathway may represent a potential pathophysiological mechanism underlying various ER stress-related human diseases (45), including the loss of β-cells in diabetes by apoptosis (13).
Materials and Methods
Yeast two-hybrid screening
A DNA fragment encoding the cytoplasmic portion of human IRE1α (amino acid residues 469–977), derived by RT-PCR from the total cellular RNA of HT-29 cells, was subcloned into pGBKT7 (CLONTECH Laboratories) to yield a fusion protein with the Gal4 DNA binding domain. This Gal4-IRE1α fusion protein was used as the bait for screening a human liver cDNA library (CLONTECH Laboratories) in pACT2 according to the manufacturer's instructions. Yeast transformation was performed in the strain AH109 using the polyethylene glycol/lithium acetate (PEG/LiAc) method, and positive clones obtained by growing on selective media. DNA inserts from candidate clones were analyzed by sequencing.
Cell culture and transfection
HEK 293T cells were maintained in DMEM containing 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO, Invitrogen). Transfection of HEK 293T cells was performed with the polyethylenimine (PEI) method (46) (Polysciences). INS-1 832/3 cells, which were kindly provided by Dr. Christopher B. Newgard (Duke University), were maintained in RPMI-1640 containing 11.1 mM D-glucose, 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin, 10 mM Hepes, 2 mM L-glutamine, 1 mM sodium-pyruvate, and 0.05 mM 2-β-mercaptoethanol. INS-1 cells between passage numbers 24–30 were used.
Protein expression constructs
For expression of the wild-type and K599A and K907A mutants of human IRE1α, cDNA fragments were amplified by PCR from the previously described pED-IRE1α-WT, pED-IRE1α-K599A and pED-IRE1α-K907A constructs (27), respectively, and subcloned into pCMV-Tag 4A (Stratagene) with the FLAG tag in-frame at the C-terminus. Expression plasmids for IRE1α (D123P) and IRE1α (S724A) were derived from pCMV-Tag 4A-IRE1α-WT using Muta-direct™ site-directed mutagenesis kit (SBS Genetech, China) to introduce the desired mutations at Asp123 and Ser724, respectively. Human RACK1 cDNA, which was generated by RT-PCR from the total cellular RNA of HEK 293T cells, was cloned into pCMV-Myc (CLONTECH Laboratories) with the Myc tag in-frame at the N-terminus. Expression vectors for rat PP1c, PP2A, and PP2C were constructed by cloning into pCMV-HA (CLONTECH Laboratories) their respective RT-PCR products amplified from total cellular RNA of INS-1 β-cells, with the HA tag in-frame at the N-terminus. The expression plasmid for GST-RACK1 fusion protein was created by subcloning into pGEX-4T1 (Amersham Biosciences) the DNA fragment encoding human RACK1 derived by PCR from pCMV-Myc-RACK1, with GST in-frame at the N-terminus. All constructs used were subsequently verified by DNA sequencing.
Generation of recombinant adenoviruses and viral infection
Recombinant adenoviruses for the overexpression of GFP, IRE1α (wild-type and mutants), RACK1, and PP2A were generated using the AdEasy™ System (Stratagene) according to the manufacturer's instructions. Briefly, DNA fragments encoding the target proteins were first subcloned into pShuttle-CMV, which were then used to produce recombinant adenoviral plasmids through homologous recombination with pAdEasy-1 in E. coli BJ5183 cells. Transfection of HEK 293A cells (Invitrogen) was conducted using the linearized recombinant plasmids to produce the desired recombinant viruses.
The RACK1-targeting recombinant adenovirus Ad-shRACK1 and control adenovirus Ad-shNC were generated using the BLOCK-iT™ Adenoviral RNAi Expression System (Invitrogen) in HEK 293A cells according to the manufacturer's instructions. Briefly, DNA fragments encoding shRNAs against rat RACK1 or containing a general scrambled sequence (as the negative control) were introduced into pENTR™/U6 vector under the control of the human U6 promoter. The two viruses designed for RACK1 knockdown contained the following RACK1 target sequences: Ad-shRACK1 #1, 5'-GGATGAGAGTCATTCAGAATG-3' and Ad-shRACK1 #2, 5'-GCTAAAGACCAACCACATTGG-3'. The control virus, Ad-shNC, had a core scrambled sequence of 5'-GTTCTCCGAACGTGTCACGTTT-3'.
The multiplicity of infection (MOI) of the generated adenoviruses was measured according to the manufacturer's instructions.
INS-1 cells were infected by adenoviruses of the indicated MOI for 48 hours in the overexpression experiments or for 72 hours in the RACK1 knockdown experiments. Adenoviral infections of INS-1 cells under these conditions, as assessed by Ad-GFP or Ad-shNC by cresyl violet or MTT assays, did not show significant effects on the cellular viability (fig. S15). Isolated pancreatic islets were infected at an MOI of 100 for 48 hours, with an assumption of ~2500 cells per islet. Adenoviral infection efficiencies of islets were estimated to be between 40–50% as determined from analysis of islets infected by Ad-GFP under similar experimental conditions.
Chemical reagents, antibodies, and Western immunoblotting
Thapsigargin (Tg), tunicamycin (Tm), 2-deoxy-D-glucose (2-DG), ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA), 2-aminoethoxy-diphenyl borate (2-APB), somatostatin-14, and palmitate were purchased from Sigma; dantrolene (Dant) was from Tocoris; hydroxy-2-naphthalenylmethylphosphonic acid tris acetoxymethyl ester [HNMPA(AM)3] was from Alexis Biochemicals.
FLAG M2 and α-tubulin monoclonal antibodies were purchased from Sigma; Myc antibody was kindly provided by Dr. Jinqiu Zhou (IBCB, SIBS, CAS); HA, IRE1α, eIF2α, AKT [pSer473], and AKT antibodies were from Cell Signaling; GAPDH antibody was from Kangcheng, Shanghai, China; IRE1α [pSer724] antibody was from Novus Biologicals; monoclonal antibodies against RACK1 and the catalytic α subunit of PP2A were from BD Transduction Laboratories; human eIF2α [pSer52] antibody was from Biosource; BiP monoclonal antibody was from Stressgen, and GST antibody was generated by our laboratory. For Western immunoblotting, proteins from cells or islet extracts were separated by SDS-PAGE and transferred to PVDF filter membrane (Amersham Biosciences). After incubation with the desired antibodies, the blots were developed using Thermo Scientific's SuperSignal® West Pico Chemiluminescent Substrate or Millipore's Immobilon™ Western Chemiluminescent HRP Substrate.
Coimmunoprecipitation
For coimmunoprecipitation analysis, cells or primary pancreatic islets were lysed with the lysis buffer [20 mM Tris-HCl, pH7.5, 100 mM KCl, 0.1% Nonidet P-40, 1 mM EDTA and 10% Glycerol containing 1 mM PMSF, 1% protease inhibitors cocktail (Sigma) and 1% phosphatase inhibitors cocktail I/II (Sigma)] for 0.5 hour at 4°C (with sonication for islets). After incubation with the desired primary antibody for 18 hours at 4°C via gentle rocking, immune complexes were captured by mixing with a final concentration of 2.5% protein G Sepharose beads (Amersham Biosciences) for 2 hours at 4°C on a rotator. When using the anti-RACK1 IgM antibody in the coimmunoprecipitation assays, goat anti-mouse IgM (Upstate) was included as the bridging antibody. Beads were subsequently washed three times with the washing buffer [20 mM Tris-HCl pH7.5, 150 mM KCl, 0.5% Nonidet P-40, 1 mM EDTA, and 10% glycerol supplemented with 1 mM PMSF, 1% protease inhibitors cocktail (Sigma), and 1% phosphotase inhibitors cocktail I/II (Sigma)], followed by SDS-PAGE and immunoblotting analysis after elution by boiling in 2× SDS loading buffer.
GST pull-down assays
For pull-down assays, bacterially expressed GST or GST-RACK1 fusion protein was purified with glutathione-Sepharose 4B beads (GE Healthcare Bio-Sciences) according to the manufacturer's instructions. Five μg of purified GST or GST-RACK1 protein was incubated for 3 hours at 4°C with lysates of HEK 293T cells (~5 × 106) transfected with IRE1α expression vectors. Subsequently, a total of 20 μl of glutathione-Sepharose-4B beads were added to the mixture and incubated for 1 hour at 4°C on a rotator. After five washes with lysis buffer, bound proteins were eluted in 2× SDS loading buffer and analyzed by SDS-PAGE and immunoblotting.
Measurement of insulin content and secretion
Intracellular insulin content in INS-1 cells or islets was measured as described previously with some modifications (47). INS-1 cells, which were lysed by incubation in 1 ml of a mixture of ethanol-water-concentrated HCl (750:235:15) overnight at 4°C, were scraped into the acid-ethanol buffer and centrifuged briefly at 4°C. Likewise, islets were lysed in 200 μl of the acid-ethanol by mild sonication on ice followed by incubation at 4°C overnight. After measurement of total cellular protein, the supernatant from INS-1 cells or islets was diluted by 1:100 or 1:250 in PBS containing 0.1% BSA before insulin measurement using an radioimmunoassay Kit (Linco Research).
Glucose-stimulated insulin secretion from INS-1 cells was determined as described previously (48). Briefly, adenovirus-infected cells were washed with 0.8 ml of HBSS (HEPES-balanced salt solution), followed by a pre-incubation for 2 hours in 0.8 ml of HBSS. Secreted insulin was then measured after incubation for 2 hours in 0.8 ml of HBSS containing 2.5 mM or 25 mM glucose. Cell lysates were prepared for subsequent analysis of total cellular proteins.
RT-PCR analysis
Total RNA was isolated from INS-1 cells using TRIzol (Invitrogen) or from isolated islets using RNeasy Plus Mini Kit (Qiagen). After reverse transcription by M-MLV Reverse Transcriptase (Invitrogen), regular PCR was performed with TaKaRa Taq kits (Takara) and quantitative real-time PCR with an ABI 7500 Fast Real-Time PCR System using Power SYBR® Green PCR Master Mix (Applied Biosystems). Gapdh was used as an internal control for normalization, and the oligonucleotide primers for each target genes examined were listed in Supplementary Materials.
Animal care and pancreatic islet isolation
C57BL/6 db/+ mice (Model Animal Research Center of Nanjing University, China) were used for breeding to yield db/db animals as previously described in detail (49). Animals were weaned at 22 days of age and housed at a temperature of 22 ± 3°C under a 12 hour dark/light cycle with ad libitum access to a standard chow (Shanghai Laboratory Animals Co.) and water in an accredited animal facility at Shanghai Institute for Biological Sciences, CAS. Fasted glucose concentrations were measured from blood collected from tail vein using a glucometer (FreeStyle). All experimental protocols were approved by the Institutional Animal Care and Use Committees at the Institute for Nutritional Sciences, SIBS, CAS.
Pancreatic islets were isolated from male db/db mice or their wild type littermates at 14–16 weeks of age by the Liberase (Roche) digestion method, using pancreas perfused with digestion buffer as previously described (50).
Statistical analysis
Statistical analysis was performed with unpaired two-tailed t-test or one-way or two-way ANOVA followed by Bonferroni's post test using Graphpad Prism 5.0. P<0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
This work was supported by grants from National Natural Science Foundation (No. 90713027, 30830033 and 30988002), the Ministry of Science and Technology (973 Program 2006CB503900 and 2007CB947100), Chinese Academy of Sciences (The Knowledge Innovation Programs No.KSCX1-YW-02 and KSCX2-YW-R-115, the CS program-SIBS2008006 and the CAS/SAFEA International Partnership Program) and Science and Technology Commission of Shanghai Municipality (No. 08dj1400601) to YL and WJL; and the Ministry of Science and Technology (No. 2006BAI23B00) to XG. Portions of this work were supported by NIH grants DK042394, HL052173, and HL057346 to RJK. RJK is an investigator of the Howard Hughes Medical Institute. We thank J. Zhou for providing the Myc antibody, and L. Rui and C. E. Samuel for critical reading of the manuscript.
Footnotes
This manuscript has been accepted for publication in Science Signaling. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 3.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28− related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 5.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 6.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 7.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 8.Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 12.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 15.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci U S A. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 17.Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10:1324–1332. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C [corrected] Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez E, Powell ML, Greenman IC, Herbert TP. Glucose-stimulated protein synthesis in pancreatic beta-cells parallels an increase in the availability of the translational ternary complex (eIF2-GTP.Met-tRNAi) and the dephosphorylation of eIF2 alpha. J Biol Chem. 2004;279:53937–53946. doi: 10.1074/jbc.M408682200. [DOI] [PubMed] [Google Scholar]
- 21.Vander Mierde D, Scheuner D, Quintens R, Patel R, Song B, Tsukamoto K, Beullens M, Kaufman RJ, Bollen M, Schuit FC. Glucose activates a protein phosphatase-1-mediated signaling pathway to enhance overall translation in pancreatic beta-cells. Endocrinology. 2007;148:609–617. doi: 10.1210/en.2006-1012. [DOI] [PubMed] [Google Scholar]
- 22.Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–859. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- 23.Khoo S, Cobb MH. Activation of mitogen-activating protein kinase by glucose is not required for insulin secretion. Proc Natl Acad Sci U S A. 1997;94:5599–5604. doi: 10.1073/pnas.94.11.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, McGlynn K, Vanderbilt CA, Cobb MH. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278:32517–32525. doi: 10.1074/jbc.M301174200. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci U S A. 2006;103:14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirasophon W, Lee K, Callaghan B, Welihinda A, Kaufman RJ. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 2000;14:2725–2736. doi: 10.1101/gad.839400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiely PA, O'Gorman D, Luong K, Ron D, O'Connor R. Insulin-like growth factor I controls a mutually exclusive association of RACK1 with protein phosphatase 2A and beta1 integrin to promote cell migration. Mol Cell Biol. 2006;26:4041–4051. doi: 10.1128/MCB.01868-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welihinda AA, Tirasophon W, Green SR, Kaufman RJ. Protein serine/threonine phosphatase Ptc2p negatively regulates the unfolded-protein response by dephosphorylating Ire1p kinase. Mol Cell Biol. 1998;18:1967–1977. doi: 10.1128/mcb.18.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Polymenis M. Dcr2 targets Ire1 and downregulates the unfolded protein response in Saccharomyces cerevisiae. EMBO Rep. 2006;7:1124–1127. doi: 10.1038/sj.embor.7400813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 34.Lipson KL, Ghosh R, Urano F. The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PLoS One. 2008;3:e1648. doi: 10.1371/journal.pone.0001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 38.Hummel KP, Coleman DL, Lane PW. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem Genet. 1972;7:1–13. doi: 10.1007/BF00487005. [DOI] [PubMed] [Google Scholar]
- 39.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiely PA, Baillie GS, Lynch MJ, Houslay MD, O'Connor R. Tyrosine 302 in RACK1 is essential for insulin-like growth factor-I-mediated competitive binding of PP2A and beta1 integrin and for tumor cell proliferation and migration. J Biol Chem. 2008;283:22952–22961. doi: 10.1074/jbc.M800802200. [DOI] [PubMed] [Google Scholar]
- 41.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 42.Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 43.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 44.Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, Hetz C. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999;6:1380–1388. doi: 10.1038/sj.gt.3300976. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Iynedjian PB. Modulation of glucose responsiveness of insulinoma beta-cells by graded overexpression of glucokinase. Proc Natl Acad Sci U S A. 1997;94:4372–4377. doi: 10.1073/pnas.94.9.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 49.Jiang L, You J, Yu X, Gonzalez L, Yu Y, Wang Q, Yang G, Li W, Li C, Liu Y. Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc Natl Acad Sci U S A. 2008;105:18619–18624. doi: 10.1073/pnas.0804589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gan Z, Zhao L, Yang L, Huang P, Zhao F, Li W, Liu Y. RNA editing by ADAR2 is metabolically regulated in pancreatic islets and beta-cells. J Biol Chem. 2006;281:33386–33394. doi: 10.1074/jbc.M604484200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.