Abstract
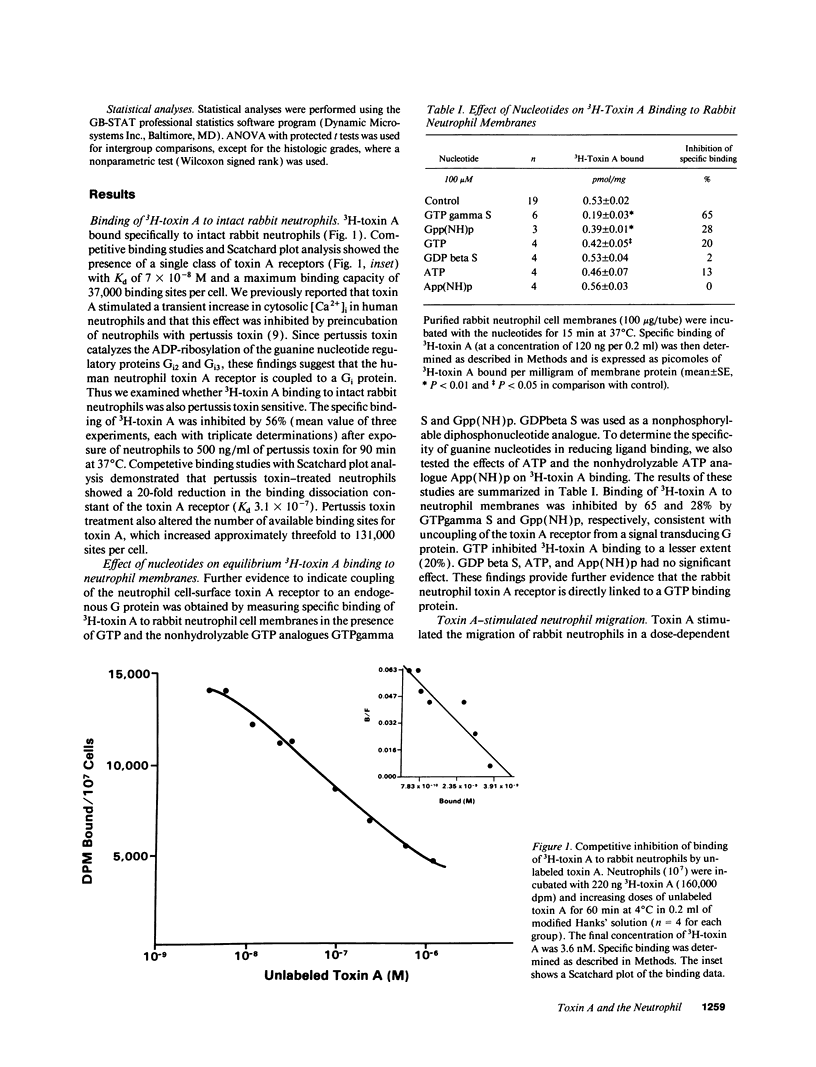
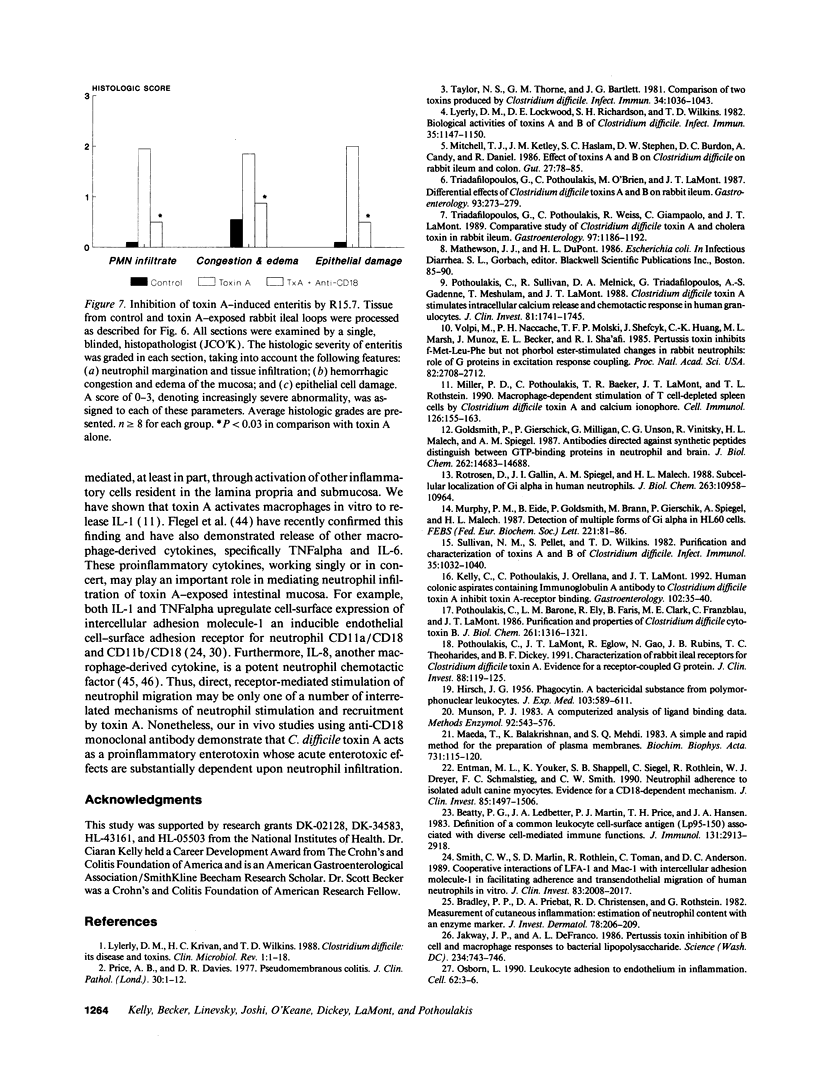
Neutrophil infiltration is a prominent feature of Clostridium difficile-associated enteritis and colitis. The aim of this study was to examine the importance of neutrophil recruitment and neutrophil-mediated tissue damage in C. difficile toxin A-induced enteritis. Competitive binding experiments using purified 3H-toxin A demonstrated the presence of a single class of medium affinity receptors on rabbit neutrophils (Kd 7 x 10(-8) M). Pertussis toxin and the nonhydrolyzable GTP analog GTPgamma S both inhibited 3H-toxin A binding (by 56 and 65%, respectively), indicating that the rabbit neutrophil toxin A receptor is G protein linked. Toxin A elicited a dose-dependent (25-200 micrograms/ml) stimulation of neutrophil migration in vitro, and this functional effect was also pertussis toxin sensitive (69% inhibition). Treatment of neutrophils with R15.7, a blocking monoclonal antibody to the leuocyte adhesion molecule CD18, inhibited toxin A-stimulated neutrophil migration by 85% in vitro. Pretreatment of rabbits with R15.7 also prevented neutrophil infiltration of toxin A-exposed ileal loops in vivo as determined by histologic examination and by ileal tissue myeloperoxidase levels. Furthermore, R15.7 effected a substantial inhibition of fluid secretion (by 65%), mannitol permeability (by 66%), and histologic damage in toxin A-exposed ileal loops. Anti-CD18 (R15.7) had no inhibitory effect on cholera toxin enterotoxicity. These data demonstrate that C. difficile toxin A is a proinflammatory toxin whose enterotoxic effects are substantially dependent upon neutrophil recruitment.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Spits H., Terhorst C., Pitt J., Todd R. F., 3rd Deficiency of a leukocyte surface glycoprotein (LFA-1) in two patients with Mo1 deficiency. Effects of cell activation on Mo1/LFA-1 surface expression in normal and deficient leukocytes. J Clin Invest. 1984 Oct;74(4):1291–1300. doi: 10.1172/JCI111539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga H. S., Walker R. K., Winberry L. K., Rittenhouse S. E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J Biol Chem. 1987 Nov 5;262(31):14871–14874. [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Crowley C. A., Curnutte J. T., Rosin R. E., André-Schwartz J., Gallin J. I., Klempner M., Snyderman R., Southwick F. S., Stossel T. P., Babior B. M. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980 May 22;302(21):1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Youker K., Shappell S. B., Siegel C., Rothlein R., Dreyer W. J., Schmalstieg F. C., Smith C. W. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest. 1990 May;85(5):1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel W. A., Müller F., Däubener W., Fischer H. G., Hadding U., Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991 Oct;59(10):3659–3666. doi: 10.1128/iai.59.10.3659-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht G., Pothoulakis C., LaMont J. T., Madara J. L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988 Nov;82(5):1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakway J. P., DeFranco A. L. Pertussis toxin inhibition of B cell and macrophage responses to bacterial lipopolysaccharide. Science. 1986 Nov 7;234(4777):743–746. doi: 10.1126/science.3095921. [DOI] [PubMed] [Google Scholar]
- Kelly C. P., Pothoulakis C., Orellana J., LaMont J. T. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology. 1992 Jan;102(1):35–40. doi: 10.1016/0016-5085(92)91781-x. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Balakrishnan K., Mehdi S. Q. A simple and rapid method for the preparation of plasma membranes. Biochim Biophys Acta. 1983 May 26;731(1):115–120. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- Miller P. D., Pothoulakis C., Baeker T. R., LaMont J. T., Rothstein T. L. Macrophage-dependent stimulation of T cell-depleted spleen cells by Clostridium difficile toxin A and calcium ionophore. Cell Immunol. 1990 Mar;126(1):155–163. doi: 10.1016/0008-8749(90)90308-e. [DOI] [PubMed] [Google Scholar]
- Mitchell T. J., Ketley J. M., Haslam S. C., Stephen J., Burdon D. W., Candy D. C., Daniel R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986 Jan;27(1):78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J. LIGAND: a computerized analysis of ligand binding data. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Eide B., Goldsmith P., Brann M., Gierschik P., Spiegel A., Malech H. L. Detection of multiple forms of Gi alpha in HL60 cells. FEBS Lett. 1987 Aug 31;221(1):81–86. doi: 10.1016/0014-5793(87)80356-3. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Makgoba M. W. Leucocyte adhesion to cells. Molecular basis, physiological relevance, and abnormalities. Scand J Immunol. 1989 Aug;30(2):129–164. doi: 10.1111/j.1365-3083.1989.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Pothoulakis C., Barone L. M., Ely R., Faris B., Clark M. E., Franzblau C., LaMont J. T. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986 Jan 25;261(3):1316–1321. [PubMed] [Google Scholar]
- Pothoulakis C., LaMont J. T., Eglow R., Gao N., Rubins J. B., Theoharides T. C., Dickey B. F. Characterization of rabbit ileal receptors for Clostridium difficile toxin A. Evidence for a receptor-coupled G protein. J Clin Invest. 1991 Jul;88(1):119–125. doi: 10.1172/JCI115267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C., Sullivan R., Melnick D. A., Triadafilopoulos G., Gadenne A. S., Meshulam T., LaMont J. T. Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granulocytes. J Clin Invest. 1988 Jun;81(6):1741–1745. doi: 10.1172/JCI113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. B., Davies D. R. Pseudomembranous colitis. J Clin Pathol. 1977 Jan;30(1):1–12. doi: 10.1136/jcp.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Walker R., Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987 Oct 1;139(7):2419–2423. [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I., Spiegel A. M., Malech H. L. Subcellular localization of Gi alpha in human neutrophils. J Biol Chem. 1988 Aug 5;263(22):10958–10964. [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. J., Prpic V., Johns J. A., Powers F. S., Graber S. E., Forbes J. T., Exton J. H. Bacterial toxins affect early events of T lymphocyte activation. J Clin Invest. 1989 Jan;83(1):234–242. doi: 10.1172/JCI113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., O'Brien M. J., LaMont J. T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987 Aug;93(2):273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., Weiss R., Giampaolo C., Lamont J. T. Comparative study of Clostridium difficile toxin A and cholera toxin in rabbit ileum. Gastroenterology. 1989 Nov;97(5):1186–1192. doi: 10.1016/0016-5085(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Winn R. K., Rice C. L., Chi E. Y., Arfors K. E., Harlan J. M. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988 Mar;81(3):939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Arfors K. E., McKnight G. W. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991 Apr;100(4):878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Oppenheim J. J., Leonard E. J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987 Aug 1;139(3):788–793. [PubMed] [Google Scholar]






