Abstract
AIM: To study the presence of Helicobacter pylori (H. pylori) virulence factors and clinical outcome in H. pylori infected patients.
METHODS: A prospective analysis of ninety nine H. pylori-positive patients who underwent endoscopy in our Endoscopy suite were included in this study. DNA was isolated from antral biopsy samples and the presence of cagA, iceA, and iceA2 genotypes were determined by polymerase chain reaction and a reverse hybridization technique. Screening for H. pylori infection was performed in all patients using the rapid urease test (CLO-Test).
RESULTS: From a total of 326 patients who underwent endoscopy for upper gastrointestinal symptoms, 99 patients were determined to be H. pylori-positive. Peptic ulceration was seen in 33 patients (33%). The main virulence strain observed in this cohort was the cagA gene isolated in 43 patients. cagA was associated with peptic ulcer pathology in 39.5% (17/43) and in 28% (16/56) of non-ulcer patients. IceA1 was present in 29 patients (29%) and iceA2 in 15 patients (15%). Ulcer pathology was seen in 39% (11/29) of patients with iceA1, while 31% (22/70) had normal findings. The corresponding values for iceA2 were 33% (5/15) and 33% (28/84), respectively.
CONCLUSION: Virulence factors were not common in our cohort. The incidence of factors cagA, iceA1 and iceA2 were very low although variations were noted in different ethnic groups.
Keywords: Ethnicity, Helicobacter pylori, Peptic ulcer disease, Virulence factors
INTRODUCTION
Helicobacter pylori (H. pylori) is a spiral, gram-negative microaerophilic bacterium that causes chronic inflammation of gastric mucosa in more than half of the population worldwide. It is a major cause of peptic ulcer (PU) disease and a recognized risk factor for gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma. The lifetime risk of an H. pylori-infected individual developing peptic ulcer disease (PUD) is estimated to be one in six.
The relationship between H. pylori genotype and its association with clinical outcome is not fully understood. The predominant H. pylori strain found in geographic locations differ with regard to genomic structures. Genetic diversity in H. pylori strains may affect the function and antigenicity of virulence factors associated with bacterial infection and, ultimately disease outcome[1].
H. pylori produces a number of virulence factors that are essential for colonization of the stomach and survival in the hostile gastric environment. In addition to urease, which plays an important role in the neutralization of gastric acid secretion and vacuolating cytotoxin, which induces vacuolar degeneration of various epithelial cell lines, there are a few other important factors. These factors are the gene products encoded by the cag pathogenicity island, which causes up-regulation of cytokines; iceA, a homologue of a gene for restriction endonuclease, induced by contact with gastric epithelium; and OipA, a pro-inflammatory protein that contributes to interleukin-8 induction. It has been proposed that cagA[1] and iceA[2] genes are markers and can identify patients with peptic ulcers.
Studies have indicated that H. pylori infection is common in Malaysia as in other developing countries. Most reports in Malaysia have focused on the prevalence and clinical patterns of gastroduodenal disease, detection of H. pylori infection, and effectiveness (or ineffectiveness) of anti-H. pylori therapies in the local population. Only a few small studies have provided information on the genotypes of the Malaysian H. pylori strains.
The aim of our study was to characterize H. pylori strains isolated from Malaysian patients and to determine if the genotypes implicated in patients with disease in the West are similar to those in the Malaysian population.
MATERIALS AND METHODS
Patients and samples
Patients found to be positive for H. pylori from those undergoing endoscopy at the Endoscopy Unit of Hospital Ampuan Afzan Kuantan were selected. This study was approved both by the Research and Ethical committees. Informed consent was obtained from each patient prior to the study. A questionnaire on demography was completed.
Gastric and duodenal pathology were identified at endoscopy. Gastritis was defined as macroscopically identifiable inflammation (antral gastritis or pangastritis) with no peptic ulcers, gastric cancer or any esophageal diseases (e.g. gastro-esophageal reflux disease and esophageal cancer). These patients were grouped as non-ulcer dyspepsia (NUD). Patients who had definite erosions or ulcers were grouped as PUD.
Two sets of gastric biopsy specimens were obtained from the antrum in all patients and one set was tested for H. pylori using the Rapid Urease test, CLO-test (Ballard Medical Products, USA) and the other specimen was selected for DNA extraction. We felt that CLO-test was the most suitable investigation to screen for H. pylori as it is quick, simple and inexpensive with sensitivity and specificity comparable to culture, histology and polymerase chain reaction (PCR)[3].
Genotyping
The biopsy tissue was stored at 4°C until DNA extraction. DNA was isolated from the biopsy tissue by the QIAamp DNA Mini kit (QIAGEN, Hilden, Germany) using the tissue protocol outlined in the manufacturer’s instructions.
The DNA yield in the eluate was obtained by measuring its absorbance at 260 nm. The reading should fall between 0.1 and 1.0. The DNA purity was obtained by calculating the A260 nm:A280 nm ratio. Ideally the ratio should be ≥ 1.7-1.9. The ratios of our DNA extracts were 1.7-1.9.
GeneAmp® PCR system 9700 (PE Applied Biosystem) was used for molecular analysis. The PCR protocol using Taq PCR Master Mix (Qiagen, Germany) was followed.
The amplification cycles for cagA, iceA1, and iceA2 consisted of an initial denaturation of target DNA at 94°C for 1 min and then denaturation at 94°C for 1 min, primer annealing at 48°C or 58°C (iceA1 and iceA2) for 1 min and extension at 72°C for 1 min (35 cycles for cagA and 40 cycles for iceA1 and A2). The final extension was another cycle lasting 15 min. The primers used to amplify the targeted genes are summarized in Table 1. A negative control (without template DNA) was included in each experiment.
Table 1.
Polymerase chain reaction primers for amplification of cagA, iceA1 and iceA2 genes
| Amplified gene | Primer destination | Sequence of primer | Size of PCR product (bp) | Ref. |
| cagA | D008 F | ATAATGCTAAATTAGACAACTTGAGCGA | 297 | |
| R008 R | TTAGAATAATCAACAAACATCACGCCAT | [4,5] | ||
| iceA1 | iceA1 F | GTGTTTTTAACCAAAGTATC | 247 | |
| iceA1 R | CTATAGCCASTYTCTTTGCA | [6] | ||
| iceA2 | iceA2 F | GTTGGGTATATCACAATTTAT | 229 or 334 | [1] |
| iceA2 R | TTRCCCTATTTTCTAGTAGGT |
PCR: Polymerase chain reaction; F: Forward primer; R: Reverse primer.
Agarose Gel Electrophoresis was used to separate and purify the extracted DNA. DNA bands were visualized under BIO-RAD UV transilluminator 2000 (Bio-Rad, UK).
RESULTS
From a total of 326 endoscopies carried out for upper gastrointestinal symptoms during the study period, 99 (30%, 99/326) were found to be CLO-test positive. All specimens were analyzed using the PCR assay.
Of the ninety nine patients, 33 patients were diagnosed with PUD (12 gastric ulcers and 21 duodenal ulcers), while 66 were categorized as NUD.
As shown in Table 2, the cagA gene was isolated in only 43 patients (43%). An association between cagA and peptic ulcer pathology was noted in 39.5% (17/43) and with NUD in 28% (16/56). This was not statistically significant.
Table 2.
Genotype and virulence factors in relation to clinical conditions n (%)
| Clinical outcome |
Starin genotypes |
Total | ||
| cagA | iceA1 | iceA2 | ||
| PUD | 17 (51.5) | 11 (33.3) | 5 (15.1) | 33 (33.3) |
| NUD | 26 (39.3) | 18 (27.2) | 10 (15.1) | 66 (66.6) |
| Total | 43 (43.3) | 29 (29.2) | 15 (15.1) | 99 (100) |
n: No. of Helicobacter pylori positive strains with the given characteristics; PUD: Peptic ulcer disease; NUD: Non ulcer dyspepsia.
iceA1 was present in 29 patients (29%) and iceA2 in 15 patients (15%). Ulcer pathology was seen in 39% (11/29) of patients with iceA1, while 31% (22/70) had normal findings. The corresponding values for iceA2 were 33% (5/15) and 33% (28/84), respectively. Again no statistical significance was noted.
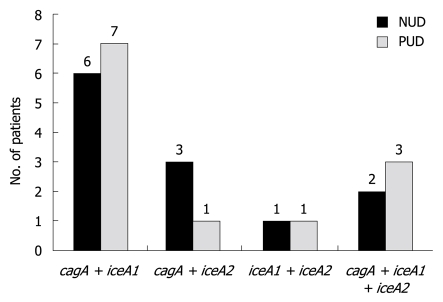
A combination of cagA and iceA1 was observed in 13 isolates and a combination of cagA and iceA2 was noted in 4 patients. Only two patients had a combination of iceA1 and iceA2. A total of 5 isolates were positive for all three virulence factors as shown in Figure 1. There was no significant difference noted between the combinations and clinical outcome.
Figure 1.
Patients with a combination of virulence factors and the relation to non ulcer dyspepsia and peptic ulcer disease in the study sample. NUD: Non ulcer dyspepsia; PUD: Peptic ulcer disease.
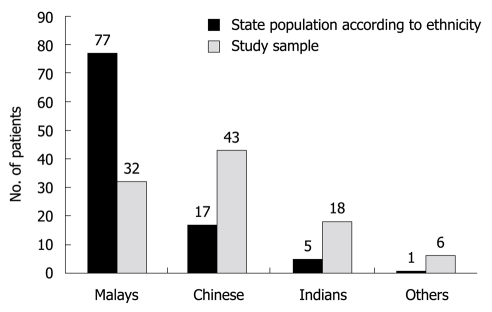
Malaysia has a unique population consisting of three main ethnic groups namely Malays, Chinese and Indians. The other minority groups are categorized as others. As shown in Figure 2, the distribution of our cohort according to their ethnicity and also the distribution of these same groups in the state population highlight the diverse nature of the prevalence of H. pylori among the different ethnic groups.
Figure 2.
Distribution of ethnicity in our cohort. This shows the population of the state of Pahang according to ethnicity and the detection of Helicobacter pylori infection among the different groups in our cohort.
Table 3 shows the variable distribution of the virulence factors among the different ethnic groups. The overall rate is low when compared to other regional studies. This was very evident especially in the Malay patients.
Table 3.
Distribution of virulence factors cagA, iceA1 and iceA2 among different ethnic groups in the study sample n (%)
| Ethnic group |
Strain genotypes |
Total | ||
| cagA | iceA1 | iceA2 | ||
| Malays | 12 (37) | 5 (15) | 2 (6) | 32 |
| Chinese | 21 (48) | 17 (39) | 9 (20) | 43 |
| Indians | 7 (38) | 4 (22) | 2 (11) | 18 |
| Others | 3 (50) | 3 (50) | 2 (33) | 6 |
n: Total No. of patients of the respective ethnicity.
DISCUSSION
The presence of H. pylori in the gastric mucosa cannot be considered a disease in itself but as a potential risk factor for the development of upper gastrointestinal tract diseases. It is estimated that about 10% of these individuals will subsequently develop PUD and a smaller percentage of about 1%-2% will develop gastric malignancy[7]. The International Agency for Research on Cancer and the World Health Organization in 1994 concluded that H. pylori has a causal link with gastric carcinogenesis and classified it as a Group 1 or definite carcinogen in humans.
Two of the three major ethnic groups in Malaysia, the Chinese and Indians are migrant populations and have been in Malaysia for nearly three generations. The prevalence of H. pylori varies among the different ethnic groups. Several studies have demonstrated a high prevalence ranging from 68%-75% in the Indian community, 45%-66.6% in the Chinese and a lower prevalence of 8%-43.3% among Malays[8,9]. According to the last census in 2000, Malays and other indigenous groups (Bumiputras) constitute 58%; Chinese, 24%; persons of Indian descent, 8%; and other groups, 10%[10]. The state of Pahang where these patients were recruited has an ethnic distribution of 77% Malays, 17% Chinese and 5% Indian. The ethnic distribution of H. pylori patients recruited from Pahang to our study is shown in Figure 2. Studies from the West show that the prevalence of H. pylori is often considerably higher among first and second-generation immigrants[11,12]. Similar reasons may be attributed to the higher incidence in Chinese and Indian populations in Malaysia. The lower rate of H. pylori infection in Malay patients in our cohort is similar to other studies in Malaysia. There is no clear explanation for this but may reflect improvements in the standards of household hygiene with a clear shift in this group to the middle income category. Studies in the United States of America show socioeconomic status and household hygiene during childhood as being very significant factors for the variation in prevalence of H. pylori infection in different races.
It has been postulated that the functional diversity of cagA may have an important relationship with disease outcome. According to Yamaoka et al[13], more than 90% of H. pylori strains are cagA positive in East Asian countries, irrespective of clinical presentation. This was not the case in our study. cagA was positive in only 43% of our samples but was associated with peptic ulcer pathology in only 39.5% (17/43) of patients compared to 28% (16/56) of non-ulcer patients. This was not statistically significant. Eradication failure was also significantly higher in cagA strain-positive patients (cure rate 73%) as compared to cagA-negative (cure rate 84%) in a meta-analysis by Suzuki et al [14].
The other virulence factor studied was the iceA gene. iceA gene (“induced by contact with epithelium”) which has two allelic variants (iceA1 and iceA2). Studies suggest an association between the iceA variant and PUD. According to Yamaoka et al[6] iceA1 was the predominant subtype in an east Asian population, while the iceA2 subtype was predominant in Columbia and the USA. Conflicting data has emerged from other parts of the world. In an analysis of iceA alleles from H. pylori strains among Finnish and African patients, the presence of iceA was significantly less in the former (35%) than the latter group (93%)[15]. In another study in Germany involving 141 H. pylori patients, the iceA gene was detected in 98% of H. pylori isolates (138 of 141)[16]. Similar results were reported by groups from Turkey, where 74.7% were positive for iceA1 and 25.3% for iceA2, and in Shanghai where iceA1 and iceA2 were found in 74.5% and 15.6%, respectively[17,18]. Our study showed a completely different picture. The corresponding values in our cohort for iceA1 and iceA2 were 29% and 15%, respectively. iceA1 was predominant as in other east Asian populations but was very low compared to the other studies. Data from Thailand, which is close to Malaysia, reported higher levels in a study involving 112 H. pylori isolates. The positive rates for cagA, iceA1 and iceA2 were 98.2%, 45.5% and 33.1%, respectively[19]. The reason for the low values in our study may be due to the reduced incidence of H. pylori infection among the major ethnic group, the Malays. The Malay community had positive rates of only 15% and 6% for iceA1 and iceA2, respectively. This trend was also noted in the other communities in our study (Table 3).
Is the presence of peptic ulcer related to the virulence factors? We were able ascertain peptic ulcer pathology in only 39% (17/43) of cagA, 37% (11/29) of iceA1 and 33% (5/15) of iceA2 isolates. Momenah et al[20] from Saudi Arabia reported much higher values. Their study revealed that 100% of ulcer cases were infected with iceA1, and iceA2 was present in 94.6% of their gastritis and in 90.9% of normal patients. Caner et al[21] also reported similar conflicting findings in a study involving a total of 46 patients. Isolates from 20 patients with chronic gastritis (66.6%) were iceA2-positive, while iceA1 was predominant in those with duodenal ulcers (68.8%).
As shown in Figure 2, combinations of cagA with iceA1 or iceA2 were not significantly different among the NUD and the PUD groups, which is in concordance with results from other Asian countries[11].
In conclusion we feel that the prevalence of H. pylori infection in Malaysia is lower than that in most countries in Southeast Asia. This may be partly due to the consistently lower incidence reported in the Malay community. The Chinese and Indian communities both have high a incidence of H. pylori infection but not as high as those noted in mainland China or India. This trend is similar to studies from the West that show the prevalence of H. pylori being persistently higher among first and second-generation immigrants[11,22]. The Chinese and Indian cohorts in our study were at least third-generation immigrants.
Of the virulence factors studied, cagA was noted to be present in 43% of patients which was much lower than most other countries in the region. A recent study from Malaysia[23] showed the occurrence of cagA diversity in the same population, where most of the isolates from Chinese patients carried East Asian cagA type and most of the isolates from Indians and Malays carried the Western cagA type. This may explain the lower rates seen here. The prevalence of iceA1 and iceA2 were very low and there were no significant differences noted between these virulence factors and any pathology either individually or in combination.
COMMENTS
Background
Helicobacter pylori (H. pylori) produces a number of virulence factors that are essential for colonization of the stomach and survival in the hostile gastric environment. Studies have shown considerable inconsistencies with regard to the presence of virulence factors and their associations, depending on the population or geographic origin. The authors’ aim was to determine the presence of these strains and their relationship with clinical outcome in a multi-ethnic cohort.
Research frontiers
The population of Malaysia is unique in that it comprises three major ethnic groups: Malays, Chinese and Indians. Studies have shown a low prevalence of H. pylori infection among the Malay community while Indians have the highest, however, the Chinese community have the highest rate of peptic ulcer pathology. Does the distribution of diverse strains among the groups have any relation to this observation?
Innovations and breakthroughs
The rate of H. pylori infection in Malaysia is lower than most countries in Southeast Asia. This may be partly due to the consistently lower incidence reported in the Malay community. The Chinese and Indian communities being third-generation immigrants have a high incidence but not as high as those noted in India or China. This is a trend noted in studies from the West which shows a persistently higher prevalence of H. pylori infection among first- and second-generation immigrants.
Applications
By creating awareness of the inconsistent distribution of H. pylori strains in this multi-ethnic group, this study may represent a change in strategy that is needed to address the management of dyspeptic symptoms in this part of the world. A large multicenter study to confirm these observations at national level is required in Malaysia.
Peer review
In this manuscript, Amjad et al reported their studies in the presence of H. pylori virulence factors (cagA and iceA) and clinical outcome in Malaysian patients. The authors found that the H. pylori isolates from their patients carried an overall low rate of cagA and iceA genes as compared to other geographical regions including Southeast Asia. cagA was present in less than half the patients and the presence of iceA1 and A2 were not significantly related to any pathology.
Footnotes
Supported by The Research Management Centre, International Islamic University Malaysia
Peer reviewer: Dr. Wang-Xue Chen, Institute for Biological Sciences, National Research Council Canada, 100 Sussex Drive, Room 3100, Ottawa, Ontario K1A 0R6, Canada
S- Editor Wang YR L- Editor Webster JR E- Editor Lin YP
References
- 1.van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 2.Xu Q, Blaser MJ. Promoters of the CATG-specific methyltransferase gene hpyIM differ between iceA1 and iceA2 Helicobacter pylori strains. J Bacteriol. 2001;183:3875–3884. doi: 10.1128/JB.183.13.3875-3884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract Res Clin Gastroenterol. 2007;21:299–313. doi: 10.1016/j.bpg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Domingo D, Alarcón T, Prieto N, Sánchez I, López-Brea M. cagA and vacA status of Spanish Helicobacter pylori clinical isolates. J Clin Microbiol. 1999;37:2113–2114. doi: 10.1128/jcm.37.6.2113-2114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sillakivi T, Aro H, Ustav M, Peetsalu M, Peetsalu A, Mikelsaar M. Diversity of Helicobacter pylori genotypes among Estonian and Russian patients with perforated peptic ulcer, living in Southern Estonia. FEMS Microbiol Lett. 2001;195:29–33. doi: 10.1111/j.1574-6968.2001.tb10493.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cats A, Meuwissen SG, Forman D, Craanen ME, Kuipers EJ. Helicobacter pylori: a true carcinogen? Eur J Gastroenterol Hepatol. 1998;10:447–450. doi: 10.1097/00042737-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Tan HJ, Rizal AM, Rosmadi MY, Goh KL. Distribution of Helicobacter pylori cagA, cagE and vacA in different ethnic groups in Kuala Lumpur, Malaysia. J Gastroenterol Hepatol. 2005;20:589–594. doi: 10.1111/j.1440-1746.2005.03783.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramelah M, Aminuddin A, Alfizah H, Isa MR, Jasmi AY, Tan HJ, Rahman AJ, Rizal AM, Mazlam MZ. cagA gene variants in Malaysian Helicobacter pylori strains isolated from patients of different ethnic groups. FEMS Immunol Med Microbiol. 2005;44:239–242. doi: 10.1016/j.femsim.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10. Available from: http://www.nationsencyclopedia.com/Asia-and-Oceania/Malaysia-ETHNIC-GROUPS.html#ixzz0csvN9hKw.
- 11.Zheng PY, Hua J, Yeoh KG, Ho B. Association of peptic ulcer with increased expression of Lewis antigens but not cagA, iceA, and vacA in Helicobacter pylori isolates in an Asian population. Gut. 2000;47:18–22. doi: 10.1136/gut.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai CJ, Perry S, Sanchez L, Parsonnet J. Helicobacter pylori infection in different generations of Hispanics in the San Francisco Bay Area. Am J Epidemiol. 2005;162:351–357. doi: 10.1093/aje/kwi207. [DOI] [PubMed] [Google Scholar]
- 13.Yamaoka Y, Orito E, Mizokami M, Gutierrez O, Saitou N, Kodama T, Osato MS, Kim JG, Ramirez FC, Mahachai V, et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180–184. doi: 10.1016/s0014-5793(02)02617-0. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Matsuo K, Sawaki A, Ito H, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, et al. Systematic review and meta-analysis: importance of CagA status for successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;24:273–280. doi: 10.1111/j.1365-2036.2006.02994.x. [DOI] [PubMed] [Google Scholar]
- 15.Karhukorpi J, Yan Y, Kolho KL, Rautelin H, Lahti M, Sirviö A, Riipinen K, Lindahl H, Verkasalo M, Fagerholm R, et al. cagA, vacA and iceA virulence genes of Helicobacter pylori isolates of children in Finland. Eur J Clin Microbiol Infect Dis. 2000;19:790–793. doi: 10.1007/s100960000366. [DOI] [PubMed] [Google Scholar]
- 16.Miehlke S, Yu J, Schuppler M, Frings C, Kirsch C, Negraszus N, Morgner A, Stolte M, Ehninger G, Bayerdörffer E. Helicobacter pylori vacA, iceA, and cagA status and pattern of gastritis in patients with malignant and benign gastroduodenal disease. Am J Gastroenterol. 2001;96:1008–1013. doi: 10.1111/j.1572-0241.2001.03685.x. [DOI] [PubMed] [Google Scholar]
- 17.Erzin Y, Koksal V, Altun S, Dobrucali A, Aslan M, Erdamar S, Dirican A, Kocazeybek B. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA, babA2 genotypes and correlation with clinical outcome in Turkish patients with dyspepsia. Helicobacter. 2006;11:574–580. doi: 10.1111/j.1523-5378.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 18.Han YH, Liu WZ, Zhu HY, Xiao SD. Clinical relevance of iceA and babA2 genotypes of Helicobacter pylori in a Shanghai population. Chin J Dig Dis. 2004;5:181–185. doi: 10.1111/j.1443-9573.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 19.Chomvarin C, Namwat W, Chaicumpar K, Mairiang P, Sangchan A, Sripa B, Tor-Udom S, Vilaichone RK. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis. 2008;12:30–36. doi: 10.1016/j.ijid.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Momenah AM, Tayeb MT. Helicobacter pylori cagA and iceA genotypes status and risk of peptic ulcer in Saudi patients. Saudi Med J. 2007;28:382–385. [PubMed] [Google Scholar]
- 21.Caner V, Yilmaz M, Yonetci N, Zencir S, Karagenc N, Kaleli I, Bagci H. H pylori iceA alleles are disease-specific virulence factors. World J Gastroenterol. 2007;13:2581–2585. doi: 10.3748/wjg.v13.i18.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Perez GI, Olivares AZ, Foo FY, Foo S, Neusy AJ, Ng C, Holzman RS, Marmor M, Blaser MJ. Seroprevalence of Helicobacter pylori in New York City populations originating in East Asia. J Urban Health. 2005;82:510–516. doi: 10.1093/jurban/jti093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed R, Hanafiah A, Rose IM, Manaf MR, Abdullah SA, Sagap I, van Belkum A, Yaacob JA. Helicobacter pylori cagA gene variants in Malaysians of different ethnicity. Eur J Clin Microbiol Infect Dis. 2009;28:865–869. doi: 10.1007/s10096-009-0712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]