Abstract
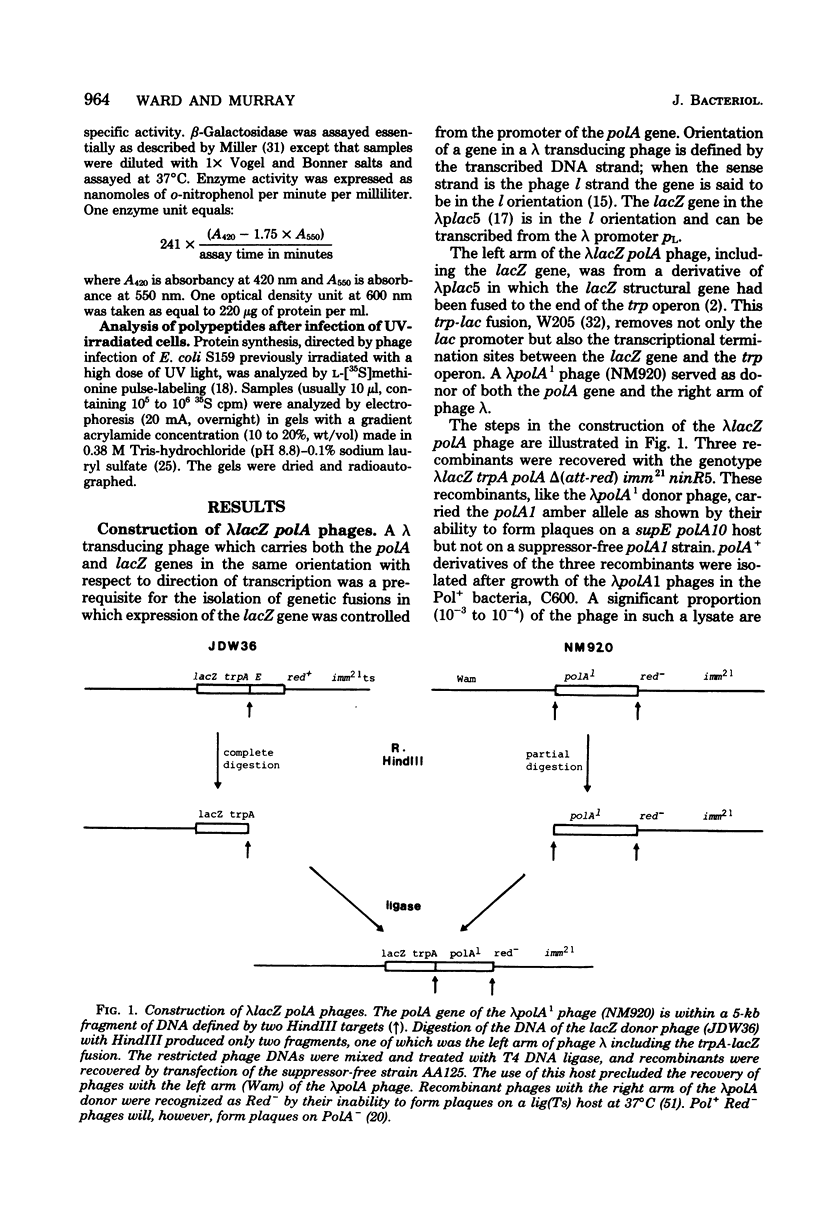
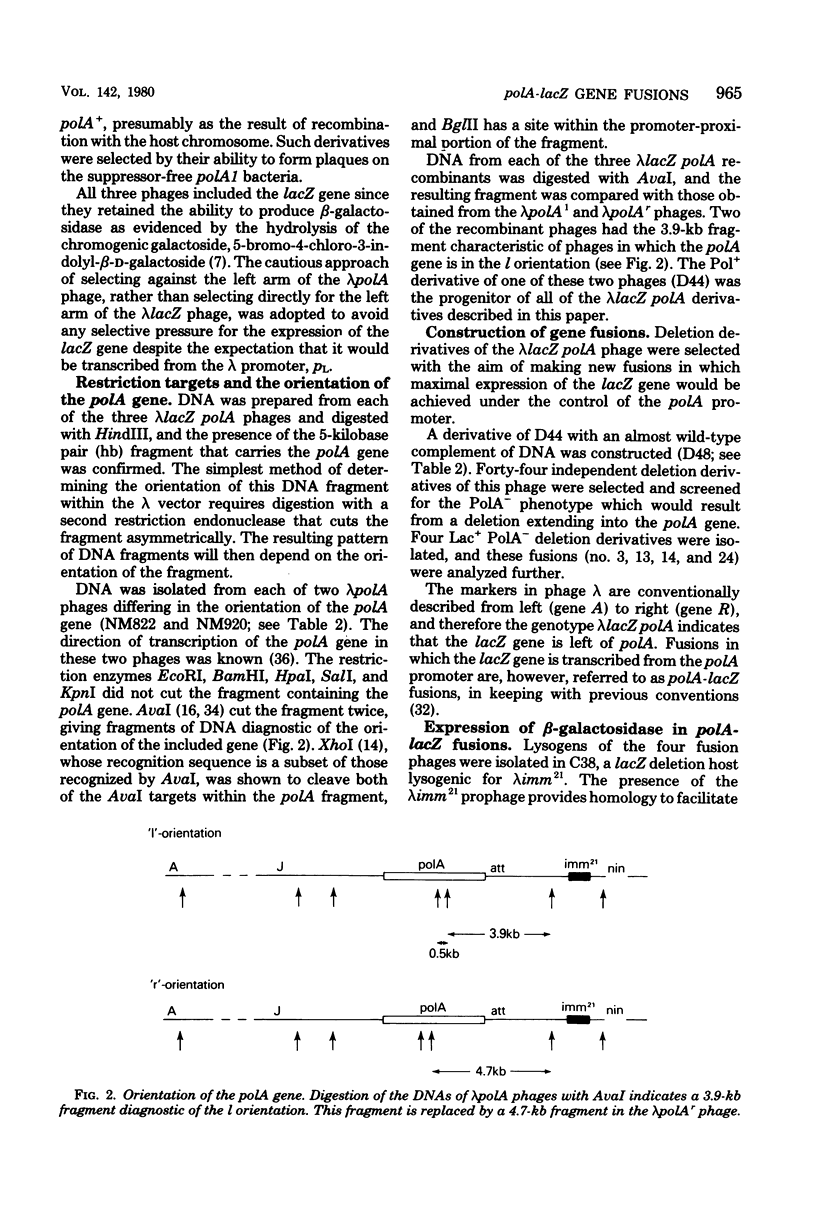
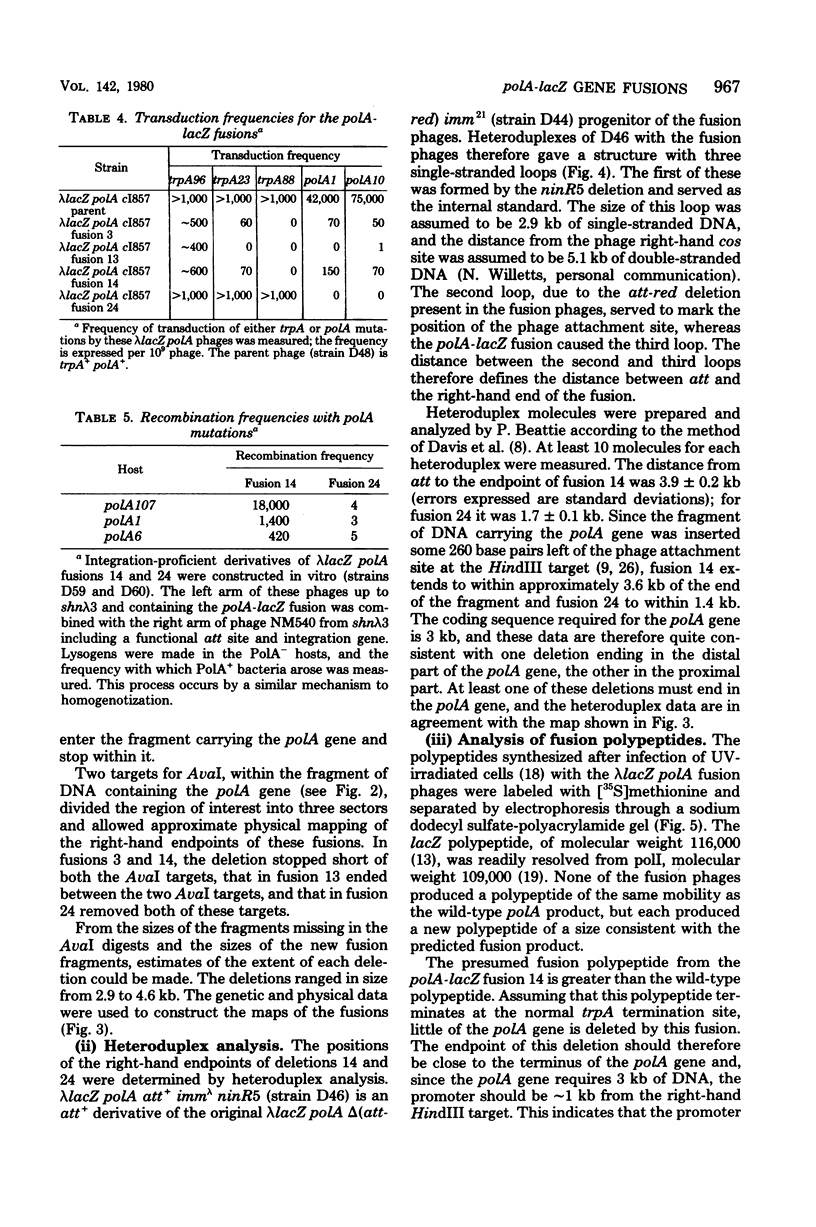
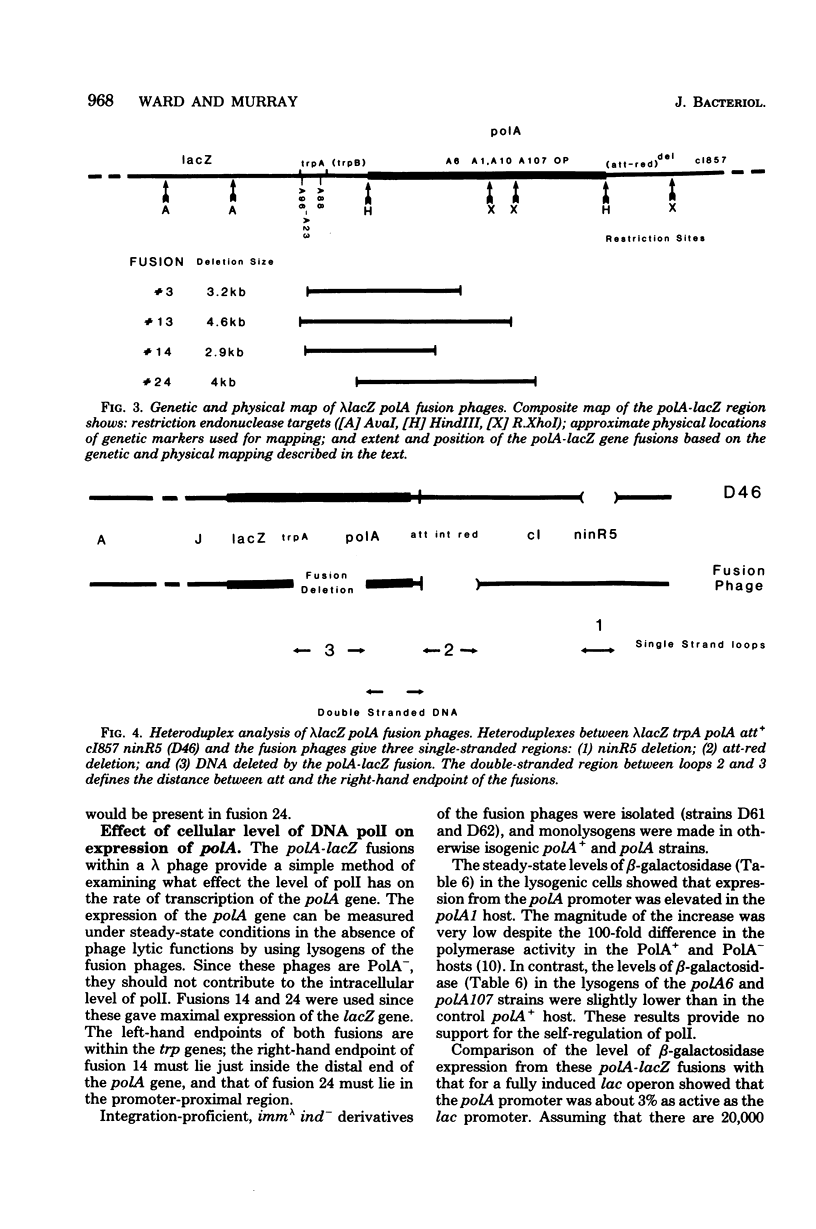
The promoter of the polA gene of Escherichia coli K-12 was fused to the lacZ gene by selecting deletions within a lambda lacZ polA transducing phage. Four fusions, deleting varying amounts of the polA gene, were characterized. The polA promoter was found to be approximately 3% as active as the fully induced lac promoter. This figure is compatible with the normal intracellular level of deoxyribonucleic acid polymerase I. No evidence was found for outogenous regulation of transcription from the polA promoter. Expression from this promoter was influenced by neither recA nor mitomycin C, but uvrD and uvrE mutations reduced expression slightly.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M., Siegel R. B., Reznikoff W. S. The construction of lambda transducing phages containing deletions defining regulatory elements of the lac and trp operons in E. coli. Mol Gen Genet. 1974 Mar 27;129(3):201–215. doi: 10.1007/BF00267913. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Davies J., Jacob F. Genetic mapping of the regulator and operator genes of the lac operon. J Mol Biol. 1968 Sep 28;36(3):413–417. doi: 10.1016/0022-2836(68)90165-4. [DOI] [PubMed] [Google Scholar]
- Davies R. W., Schreier P. H., Buchel D. E. Nucleotide sequence of the attachment site of coliphage lambda. Nature. 1977 Dec 22;270(5639):757–760. doi: 10.1038/270757a0. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Brammar W. J., Yanofsky C. Amino acid replacements of the glutamic acid residue at position 48 in the tryptophan synthetase A protein of Escherichia coli. J Mol Biol. 1968 Jul 28;35(2):357–367. doi: 10.1016/s0022-2836(68)80030-0. [DOI] [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta-galactosidase of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1507–1510. doi: 10.1073/pnas.74.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Myers P. A., Olson J. A., Hanberg F. A., Roberts R. J. A new specific endonuclease present in Xanthomonas holcicola, Xanthomonas papavericola and Brevibacterium luteum. J Mol Biol. 1978 Jan 5;118(1):113–122. doi: 10.1016/0022-2836(78)90247-4. [DOI] [PubMed] [Google Scholar]
- Hopkins A. S., Murray N. E., Brammar W. J. Characterization of lambdatrp-transducing bacteriophages made in vitro. J Mol Biol. 1976 Nov 15;107(4):549–569. doi: 10.1016/s0022-2836(76)80082-4. [DOI] [PubMed] [Google Scholar]
- Hughes S. G. A map of the cleavage sites for endonuclease AvaI in the chromosome of bacteriophage lambda. Biochem J. 1977 Jun 1;163(3):503–509. doi: 10.1042/bj1630503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen K., Shapiro J. A., Beckwith J. R. Transposition of the lac region to the gal region of the Escherichia coli chromosome: isolation of lambda-lac transducing bacteriophages. J Bacteriol. 1971 Oct;108(1):5–9. doi: 10.1128/jb.108.1.5-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Kelley W. S., Chalmers K., Murray N. E. Isolation and characterization of a lambdapolA transducing phage. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5632–5636. doi: 10.1073/pnas.74.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. S., Grindley N. D. Mapping of the polA locus of Escherichia coli K12: orientation in the amino- and carboxy-termini of the cistron. Mol Gen Genet. 1976 Sep 23;147(3):307–314. doi: 10.1007/BF00582882. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. J Biol Chem. 1958 Jul;233(1):163–170. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landy A., Ross W. Viral integration and excision: structure of the lambda att sites. Science. 1977 Sep 16;197(4309):1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman I. R., Uyemura D. G. DNA polymerase I: essential replication enzyme. Science. 1976 Sep 10;193(4257):963–969. doi: 10.1126/science.781842. [DOI] [PubMed] [Google Scholar]
- Li S. L., Yanofsky C. Amino acid sequences of fifty residues from the amino termini of the tryptophan synthetase chains of several enterobacteria. J Biol Chem. 1972 Feb 25;247(4):1031–1037. [PubMed] [Google Scholar]
- Mitchell D. H., Reznikoff W. S., Beckwith J. R. Genetic fusions defining trp and lac operon regulatory elements. J Mol Biol. 1975 Apr 15;93(3):331–350. doi: 10.1016/0022-2836(75)90281-8. [DOI] [PubMed] [Google Scholar]
- Murray K., Hughes S. G., Brown J. S., Bruce S. A. Isolation and characterization of two sequence-specific endonucleases from Anabaena variabilis. Biochem J. 1976 Nov;159(2):317–322. doi: 10.1042/bj1590317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Murray N. E., De Ritis P. M., Foster L. A. DNA targets for the Escherichia coli K restriction system analysed genetically in recombinants between phages phi80 and lambda. Mol Gen Genet. 1973 Feb 2;120(3):261–281. doi: 10.1007/BF00267157. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Kelley W. S. Characterization of lambdapolA transducing phages; effective expression of the E. coli polA gene. Mol Gen Genet. 1979 Aug;175(1):77–87. doi: 10.1007/BF00267858. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Michels C. A., Cooper T. G., Silverstone A. E., Magasanik B. Inhibition of lacZ gene translation initiation in trp-lac fusion strains. J Bacteriol. 1974 Mar;117(3):1231–1239. doi: 10.1128/jb.117.3.1231-1239.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C. Ultraviolet-sensitive mutator strain of Escherichia coli K-12. J Bacteriol. 1973 Jan;113(1):145–160. doi: 10.1128/jb.113.1.145-160.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Ward D. F., Murray N. E. Convergent transcription in bacteriophage lambda: interference with gene expression. J Mol Biol. 1979 Sep 15;133(2):249–266. doi: 10.1016/0022-2836(79)90533-3. [DOI] [PubMed] [Google Scholar]
- Weil J., Signer E. R. Recombination in bacteriophage lambda. II. Site-specific recombination promoted by the integration system. J Mol Biol. 1968 Jul 14;34(2):273–279. doi: 10.1016/0022-2836(68)90252-0. [DOI] [PubMed] [Google Scholar]
- Wilson G. G., Tanyashin V. I., Murray N. E. Molecular cloning of fragments of bacteriophage T4 DNA. Mol Gen Genet. 1977 Nov 14;156(2):203–214. doi: 10.1007/BF00283493. [DOI] [PubMed] [Google Scholar]
- Windass J. D., Brammar W. J. Aberrant immunity behaviour of hybrid lambda imm21 phages containing the DNA of ColE1-type plasmids. Mol Gen Genet. 1979;172(3):329–337. doi: 10.1007/BF00271733. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Drapeau G. R., Guest J. R., Carlton B. C. THE COMPLETE AMINO ACID SEQUENCE OF THE TRYPTOPHAN SYNTHETASE A PROTEIN (alpha SUBUNIT) AND ITS COLINEAR RELATIONSHIP WITH THE GENETIC MAP OF THE A GENE. Proc Natl Acad Sci U S A. 1967 Feb;57(2):296–298. doi: 10.1073/pnas.57.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V. Tryptophan synthetase chain positions affected by mutations near the ends of the genetic map of trpA of Escherichia coli. J Biol Chem. 1972 Jul 25;247(14):4494–4498. [PubMed] [Google Scholar]



