Abstract
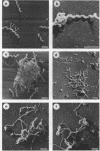
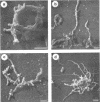
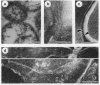
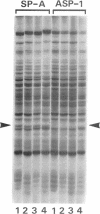
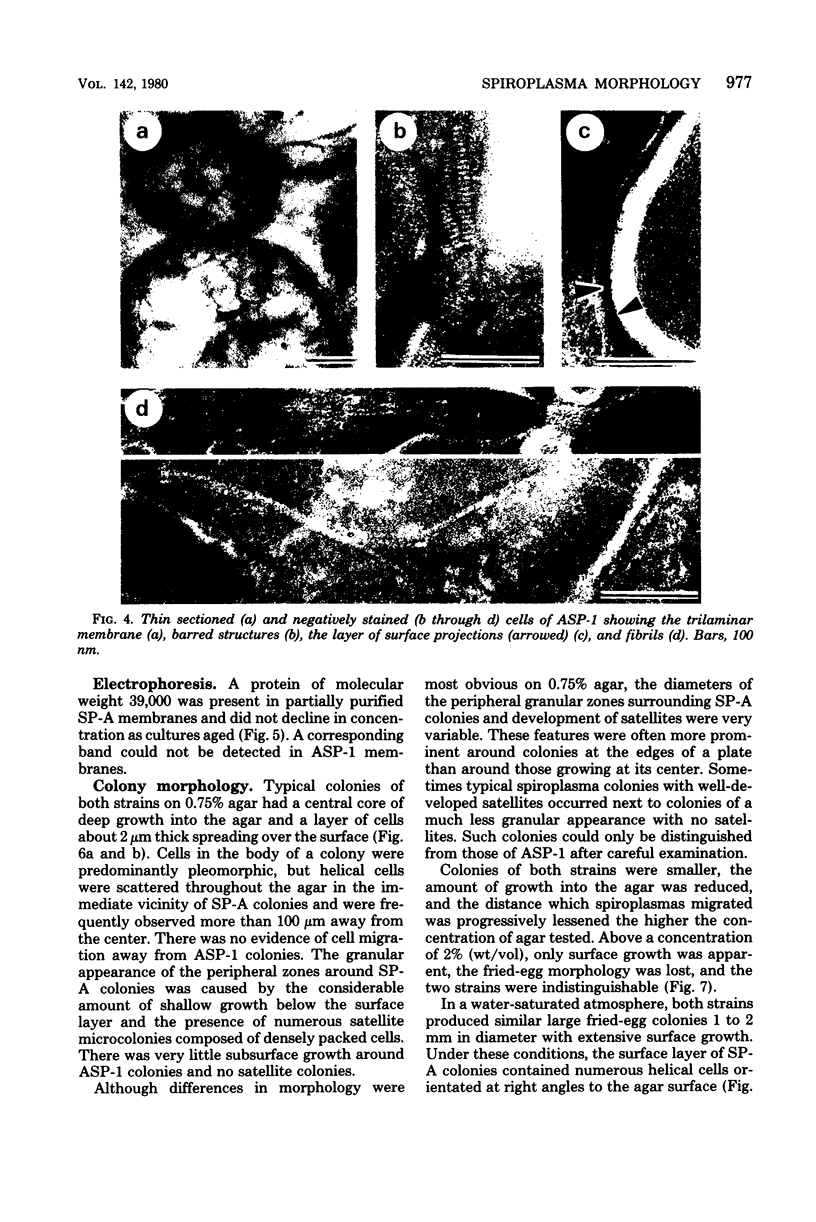
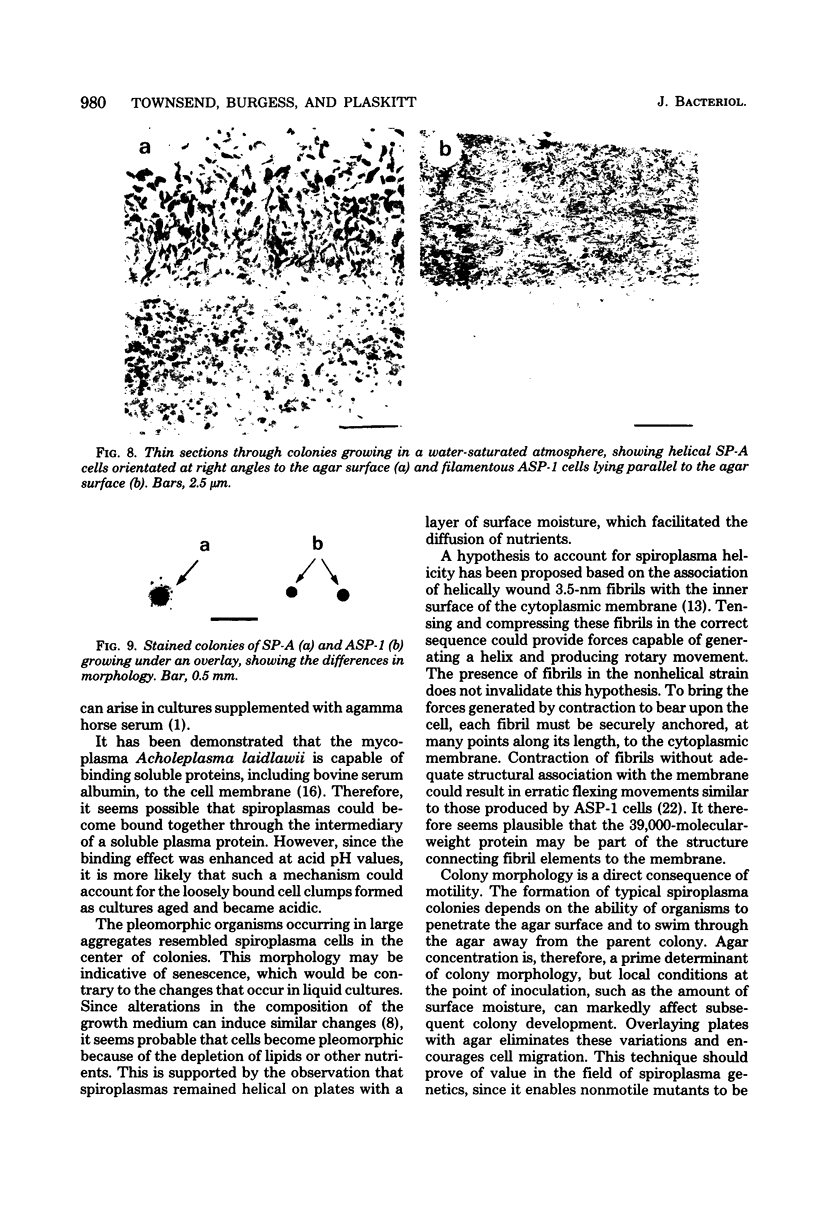
Cells of the nonhelical strain of Spiroplasma citri underwent changes of morphology comparable to those which occurred in the normal helical strain. Cells of the nonhelical strain had the same ultrastructural features as helical cells and released long flexible fibrils similar to those seen in other spiroplasmas. Nonhelical organisms showed an increased tendency to aggregate, forming cell clusters of an unusual annular form. The cytoplasmic membrane of the nonhelical strain lacked a single protein present in all helical strains. Loss of helicity associated with the senescence of spiroplasma cells was not accompanied by the disappearance of this protein. Differences in colony morphology were shown to be a consequence of motility, and a technique was developed which facilitated the identification of nonmotile organisms.
Full text
PDF
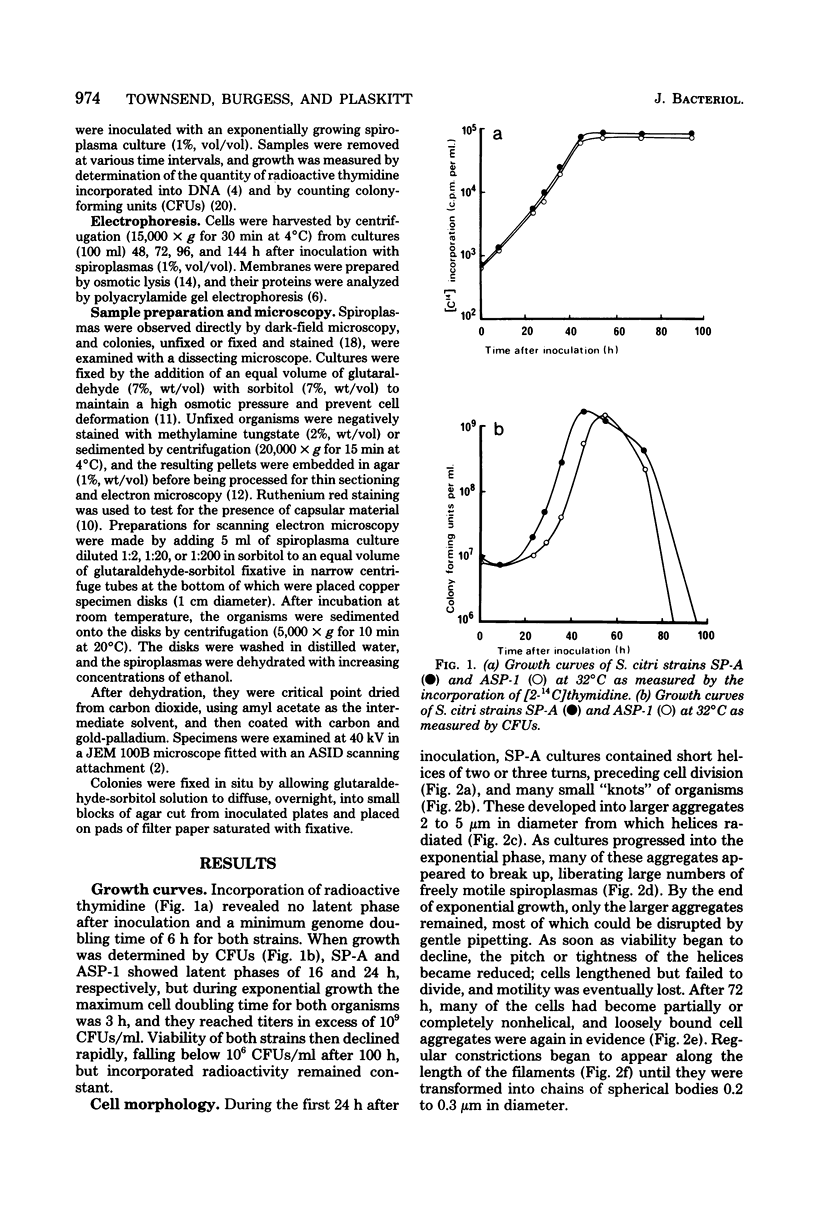
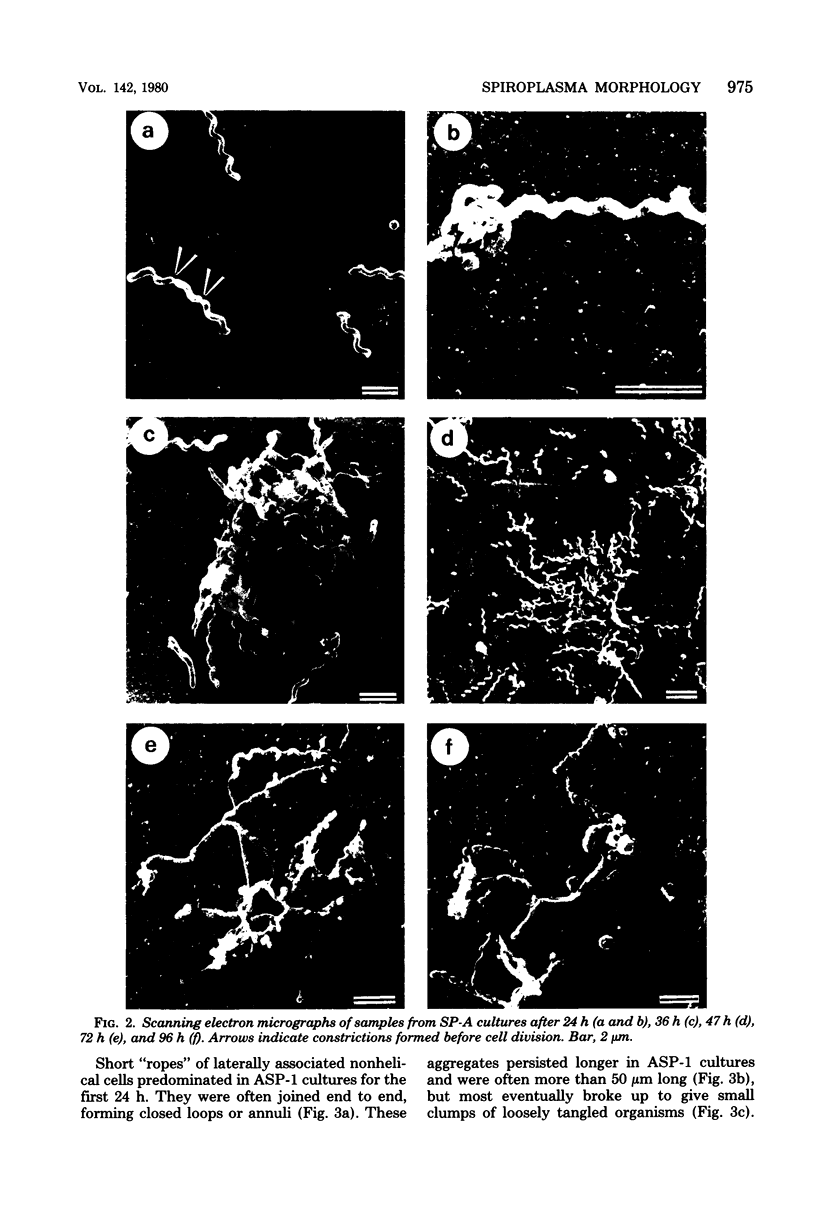
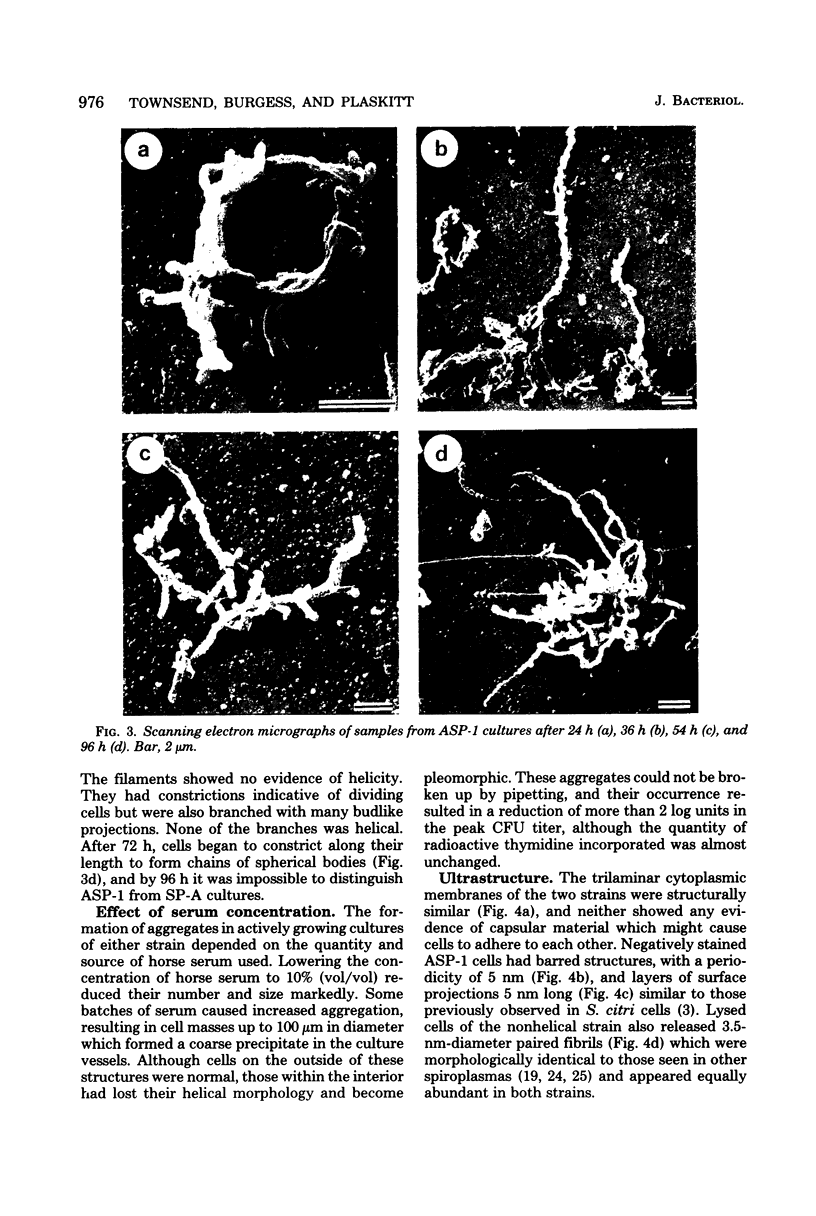



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boatman E. S., Kenny G. E. Morphology and ultrastructure of Mycoplasma pneumoniae spherules. J Bacteriol. 1971 Jun;106(3):1005–1015. doi: 10.1128/jb.106.3.1005-1015.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Tully J. G., Popkin T. J., Bové J. M. Morphology, ultrastructure, and bacteriophage infection of the helical mycoplasma-like organism (Spiroplasma citri gen. nov., sp. nov.) cultured from "stubborn" disease of citrus. J Bacteriol. 1973 Jul;115(1):367–384. doi: 10.1128/jb.115.1.367-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J., Meddins B. M. Polyacrylamide gel electrophoresis of mycoplasma proteins in sodium dodecyl sulphate. J Gen Microbiol. 1973 May;76(1):239–242. doi: 10.1099/00221287-76-1-239. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Sissenstein R., McManus T. T., Woodward J. E., Lee I. M., Mudd J. B. Lipid composition and lipid metabolism of Spiroplasma citri. J Bacteriol. 1976 Mar;125(3):946–954. doi: 10.1128/jb.125.3.946-954.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Gourley R. N. An electron-microscopic examination of certain bovine mycoplasmas stained with ruthenium red and the demonstration of a capsule on Mycoplasma dispar. J Gen Microbiol. 1974 Aug;83(2):393–398. doi: 10.1099/00221287-83-2-393. [DOI] [PubMed] [Google Scholar]
- Lemcke R. M. Osmolar concentration and fixation of mycoplasmas. J Bacteriol. 1972 Jun;110(3):1154–1162. doi: 10.1128/jb.110.3.1154-1162.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S., OLIVER O. Morphogenesis of Mycoplasma and bacterial L-form colonies. J Gen Microbiol. 1961 Feb;24:225–237. doi: 10.1099/00221287-24-2-225. [DOI] [PubMed] [Google Scholar]
- Razin S., Hasin M., Ne'eman Z., Rottem S. Isolation, chemical composition, and ultrastructural features of the cell membrane of the mycoplasma-like organism Spiroplasma citri. J Bacteriol. 1973 Dec;116(3):1421–1435. doi: 10.1128/jb.116.3.1421-1435.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hasin M., Razin S. Binding of proteins to mycoplasma membranes. Biochim Biophys Acta. 1973 Apr 16;298(4):876–886. doi: 10.1016/0005-2736(73)90392-1. [DOI] [PubMed] [Google Scholar]
- Townsend R. Arginine metabolism by spiroplasma citri. J Gen Microbiol. 1976 Jun;94(2):417–420. doi: 10.1099/00221287-94-2-417. [DOI] [PubMed] [Google Scholar]
- Whitcomb R. F., Williamson D. L. Helical wall-free prokaryotes in insects: multiplication and pathogenicity. Ann N Y Acad Sci. 1975;266:260–275. doi: 10.1111/j.1749-6632.1975.tb35109.x. [DOI] [PubMed] [Google Scholar]
- Williamson D. L. Unusual fibrils from the spirochete-like sex ratio organism. J Bacteriol. 1974 Feb;117(2):904–906. doi: 10.1128/jb.117.2.904-906.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]