Abstract
Insects have evolved diverse methods of predator avoidance, many of which implicate complex adaptations of their wings (e.g., Phylliidae, Nymphalidae, Notodontidae). Among these, angiosperm leaf mimicry is one of the most dramatic, although the historical origins of such modifications are unclear owing to a dearth of paleontological records. Here, we report evidence of pinnate leaf mimesis in two lacewings (Neuroptera): Bellinympha filicifolia Y. Wang, Ren, Liu & Engel gen. et sp. nov. and Bellinympha dancei Y. Wang, Ren, Shih & Engel, sp. nov., from the Middle Jurassic, representing a 165-million-year-old specialization between insects and contemporaneous gymnosperms of the Cycadales or Bennettitales. Furthermore, such lacewings demonstrate a preangiosperm origin for leaf mimesis, revealing a lost evolutionary scenario of interactions between insects and gymnosperms. The current fossil record suggests that this enigmatic lineage became extinct during the Early Cretaceous, apparently closely correlated with the decline of Cycadales and Bennettitales at that time, and perhaps owing to the changing floral environment resulted from the rise of flowering plants.
Keywords: mimicry, Neuroptera, Jurassic, Inner Mongolia, gymnosperm
Crypsis, or the ability to avoid detection, is a pervasive and effective method of defense used by insects against potential predators. In particular, mimicry of other species in morphology or signals (behavioral or chemical) represents a significant theme in arthropod evolution. On the other hand, mimesis, whereby the dupe is indifferent to the model, is less common (1, 2). Mimesis is most readily known through the evolution of plant mimics that have appeared in various orders of insects, ranging from the stick and leaf insects of the Phasmatodea, to flower- and leaf-mimicking mantises (Mantodea), to the famous thorn-mimicking treehoppers of the membracoid Hemiptera, among others (1). Within this diversity of mimics, leaf specializations are the most elaborately developed among angiosperm-mimicking species of the family Phylliidae (Phasmatodea), the leaf katydids (Orthoptera), the mantises (Mantodea) of the genera Gongylus and Deroplatys, and some butterflies of the Nymphalidae and moths of the Notodontidae (Lepidoptera). All of these lineages have wings and/or body parts that vividly imitate live or dry angiosperm leaves, including the nature of leaf veins, splotches, and even markings of damage. Given the phylogenetic placement of these families and genera among their respective orders (1), such mimicry of angiosperm models likely appeared subsequent to (rather than along with) the radiation of flowering plants. Accordingly, it has been considered that leaf mimesis is a mid-Cretaceous or younger phenomenon.
Despite the diversity of leaf mimesis among living insects, evidence of this unique evolutionary adaptation in the fossil record is exceptionally rare (1). Early researchers considered the resemblance between Carboniferous roachoid wings and fern pinnules a case of early mimetic evolution (3); however, more recent studies have concluded that such alleged mimesis was due to functional convergence in laminar organs (4). More recently, an Eocene (47 million years old) fossil leaf insect, Eophyllium messelensis, was described as the earliest evidence of such unique adaptations among insects (5). Living neuropterans are themselves typically generalist predators with few specialized relationships with particular plant lineages. Although some lacewings have moderately leaf-like wings, such as Drepanepteryx phalaenoides (6) in which they loosely resemble dried angiosperm leaves in shape and coloration, the most dramatic examples of mimicry among the order are the various mimics of social wasps. Such mimesis can be so precise that color polymorphism in a single mimic species corresponds to a diversity of wasp models (7). However, neither modern neuropterans nor insects as a whole exhibit pinnate leaf mimesis. The trait is seemingly confined to the mimicking of angiosperm leaf morphologies among previously known insect mimics.
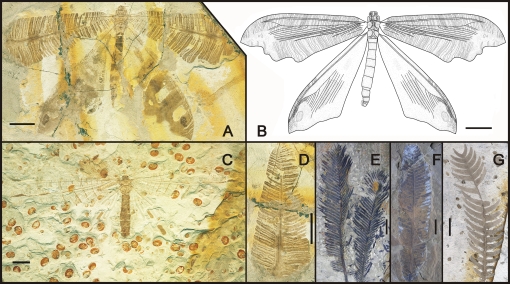
Two extraordinary fossil lacewings, Bellinympha filicifolia Y. Wang, Ren, Liu & Engel gen. et sp. nov. and Bellinympha dancei Y. Wang, Ren, Shih & Engel sp. nov. (Neuroptera), from the Jiulongshan Formation in northeastern China, preserve wings that are dramatically modified to resemble pinnate leaves (Fig. 1). These are the earliest evidence of leaf mimesis and predate Eophyllium by nearly 120 million years. Bellinympha demonstrates that lacewings of the late Middle Jurassic (165 million years ago) already had evolved highly specialized mimicry of pinnate leaves as a strategy for avoiding predators such as contemporaneous mammals, pterosaurs, dinosaurs, birds, spiders, and other predacious insects, suggesting a much richer biotic world in the Mesozoic Era.
Fig. 1.
Species of Bellinympha and potential model plants from the Middle Jurassic of China. (A) Specimen CNU-NEU-NN2010240-1 of B. filicifolia sp. nov., exhibiting outstretched wings, apices of forewings not preserved [for photo of counterpart (CNU-NEU-NN2010240-2) refer to Fig. S1]. (B) reconstruction of B. filicifolia. (C) Specimen CNU-NEU-NN2010241-1 of B. dancei sp. nov., exhibiting similar wing position to B. filicifolia although the coloration preservation is poorer [for photo of counterpart (CNU-NEU-NN2010241-2) refer to Fig. S2]. (D) Forewing of specimen CNU-NEU-NN2010240-1, depicting distinct pinnate leaf markings. (E–G) Potential models of pinnate-leaved plants. (E) Holozamites (Cycadales) (30). (F) Unnamed leaf of a cycadophyte. (G) Nilssonia (Cycadales). (Scale bars, 10 mm in A–D, 20 mm in E–G.)
Systematic Paleontology
Insecta Linnaeus, 1758; Neuroptera Linnaeus, 1758; Saucrosmylinae Ren & Yin, 2003; Bellinympha Y. Wang, Ren, Liu, Shih & Engel gen. nov.; Bellinympha filicifolia Y. Wang, Ren, Liu & Engel gen. et sp. nov. (type species of genus, here designated); Bellinympha dancei Y. Wang, Ren, Shih & Engel sp. nov.
Etymology
The generic name is a combination of belli (Latin, meaning beautiful) and nympha (Latin, meaning girl) and is of feminine gender. The specific epithet filicifolia (Latin) is derived from the pinnate leaf-like forewing. The specific name dancei is a patronym honoring Mr. Tom Dance, an excellent business leader and mentor providing guidance, motivation, and inspiration to C.S.
Diagnosis
Antenna filiform, shorter than forewing. Forewing extremely broadened, with undulating postero-apical margin, apex somewhat falcate. Membrane with distinct pinna-like markings (Fig. 1 A and C and Figs. S1 and S2). Nygma present in center of wing. Costal veinlets forked distally, with numerous interconnecting veinlets (Figs. S3 and S4). Rs region with two to five rows of cells; Rs arching anteriorly in apical quarter of wing; first fork of Rs near origin of Rs; in forewing distal branches of Rs apically curving toward posterior margin. MP2 with many pectinate branches. CuA forked at midwing, forming complicated pectinate branches.
B. filicifolia Y. Wang, Ren, Liu & Engel gen. et sp. nov
Holotype.
CNU-NEU-NN2010240-1 and -2, part and counterpart. A well-preserved almost complete body with most of four wings but missing wing apexes and part of antennae (Fig. 1A and Fig. S1). Deposited in the Key Laboratory of Insect Evolution and Environmental Changes, Capital Normal University, Beijing, China.
Locality and Age.
Collected from the Jiulongshan Formation, the late Middle Jurassic (Bathonian-Callovian boundary interval) of Daohugou Village, Shantou Township, Ningcheng County, Inner Mongolia, China. Daohugou strata are well known for yielding diverse lacewings and belong to a lacustrine deposit, representing a humid and warm-temperate climate (8, 9).
Diagnosis.
Forewing CuP long, extending beyond midlength of CuA; hind wing costal cross-veins forked distally; wings broader and shorter, with more tightly spaced markings, pinna-like markings prominent.
Description and Comparisons.
Body, fairly well preserved, is 39.8 mm long. Compound eyes protrudent, antennae filiform and incompletely preserved. Thorax distinctly differentiated; prothorax narrow; sclerite in meso- and metathorax well developed. Forelegs only with femora preserved, not crassate, with small, fine setae. Abdomen with 10 segments and with an appendix close to caudal segment. The appendix resembles the gonapophysis lateralis of Osmylidae, which represents a primitive character of Neuroptera (10).
Forewing 54.4 mm long and 19.7 mm wide, with numerous pinna-like markings (Fig. 1 A and B). Trichosors and nygmata present. Outer margin with conspicuous undulating projections, similar to those in Kempynus (a genus of Osmylidae: Kempyninae). Costal cross-veins forked distally, interlinked by numerous veinlets (Fig. S3). Rs expanded in middle and bent anteriorly sharply toward R1. Space between Rs and R1 broad, forming two to five rows of cells. Rs with 19 branches, each with complicated distal forks and angled toward posterior apical margin. Cross-veins in radial sector numerous, arranged irregularly. MA coalescent with Rs. MP forked close to wing base, MP2 with eight to nine distal pectinate branches. Cu forked at wing base. CuA with numerous oblique pectinate branches medially, forming a large triangular region; CuP deeply dark; long, extending beyond midlength of CuA. Anal region incompletely preserved. Two rows of cells between A1 and A2.
Hind wing, poorly preserved, is 53.8 mm long and 19.2 mm wide. Membrane with numerous oblique stripes close to apex, resembling the pinna-like forewings. Trichosors well spread across outer margin and part of anterior margin. Venation is similar to that of the forewing: costal cross-veins forked distally, interconnected by veinlets; Rs bent anteriorly toward R1 distally, forming a large space between R1 and Rs; cross-veins in radial sector numerous, arranged irregularly.
B. dancei Y. Wang, Ren, Shih & Engel sp. nov
Holotype.
CNU-NEU-NN2010241-1 and -2, part and counterpart. All wings are unfolded but poorly preserved, body and antennae are intact (Fig. 1C and Fig. S2). Deposited in the Key Laboratory of Insect Evolution and Environmental Changes, Capital Normal University, Beijing, China.
Locality and Age.
Same as those of B. filicifolia.
Diagnosis.
Forewing CuP short, not extending beyond midlength of CuA; hind wing costal cross-veins simple; wings more narrow and longer, markings more sparse, pinna-like markings weaker.
Descriptions and Comparisons.
Body, well preserved, is 36.6 mm long. Compound eyes protrudent, antennae filiform and completely preserved. Thorax distinctly differentiated; prothorax short; sclerite in meso- and metathorax well developed. Forelegs only with tibiae and tarsi preserved, with small, fine setae; pretarsal claws distinct, simple. Abdomen with 10 segments, and caudal segment small and triangular dorsally.
Forewing elongate, 69.8 mm long, with numerous pinna-like markings. The pinna-like forewing resembles those of B. filicifolia; however, the markings of B. dancei are sparse and less distinct compared with B. filicifolia. Trichosors and nygmata undetected. MA space covering a distinct, dark, rachis-like marking. Because of the incomplete preservation, the undulating margin is not well discerned in B. dancei. Costal cross-veins forked distally and interlinked by numerous veinlets (Fig. S4). Sc and R1 fused distally but cross-vein sc-r1 not apparent. Rs resembles B. filicifolia: Rs with numerous branches, and the first branch diverging from Rs stem with deep fork. MA fused with Rs; MP forked close to wing base. CuA long, with numerous complicated branches from middle to apical termination; CuP shorter than CuA, with four branches distally; cross-veins cua-cup complicated, forming two rows of cells medially. A1 long, with six pectinate branches; A2 short, nearly half the length of A1; cross-veins a1-a2 form two rows of cells.
Hind wing poorly preserved, with undulating margin; apex pointed. Membrane with three continuous markings. Venation resembles the forewing except for the simple costal cross-veins.
The venation of B. dancei is quite similar to that of B. filicifolia; however, CuP in B. dancei is distinctly shorter than the elongate condition observed in B. filicifolia. In addition, the wings of B. dancei are narrower, longer, and more slender, with sparser markings, than those of B. filicifolia. The undulating margin is not preserved and unknown for the forewing of B. dancei, whereas the hind wing has a distinctly undulating postero-apical margin.
Discussion
The two species of Bellinympha reported here are exceptionally rare, representing only two specimens out of more than 250,000 insect fossils in the Capital Normal University (Beijing) collection. The genus is noteworthy for the large body size, elongate forewings, long filiform antennae, protrudent compound eyes, undulating margins to the forewings, disruptive pattern of coloration resembling leaf pinnae, distinctly thickened MP branches resembling a leaf rachis, complicated venation, and an appendage close to the caudal segment (Fig. 1B) that resembles the gonapophysis lateralis of Osmylidae, a relatively primitive feature among Neuroptera (10). Although moderate- to small-sized lacewings are dominant among extant Neuroptera, such large species are frequently found in the Middle Jurassic, notably species of the Aetheogrammatidae, Kalligrammatidae, and Grammolingiidae (11–13). The larger body sizes render such species more vulnerable to predators because they are more conspicuous, and so it is not surprising that genera in these families often exhibit forewing wing modifications. Like Bellinympha and its relatives, Kalligrammatidae, Aetheogrammatidae, and Grammolingiidae have complicated venational patterns and specialized markings on the forewing, frequently resembling “eye spots” and presumably used to startle potential predators as in modern moths (14).
Unique among these Mesozoic giant lacewings, the forewing shape and patterns of Bellinympha are obviously irrelevant to angiosperm leaves both in construction and temporal occurrence. The numerous pinna-like markings on the wing membranes are remarkably similar to the pinnate leaves of Mesozoic Cycadales and Bennettitales: the dark posterior median (MP) region of the wing resembles the rachis of the leaf, whereas the oblique stripes resemble the pinnae (compare Fig. 1 A, C, and D–G). Among the two species, B. filicifolia has a more obvious pattern of pinnate leaves due to a darker and better-defined coloration (Fig. 1 A, B, and D), whereas B. dancei has a more pronounced central rachis-like region (MP region), with a similar zigzag shape near the apical area, but the oblique pinna-like stripes are less developed (Fig. 1C). Pinna-like markings on the forewings imitate contemporaneous pinnate leaves of Cycadales and Bennettitales that are frequently found in the Daohugou strata. If the distal end of the wing is aligned with the proximal end of these fossil leaves, there is a close match between insect wings and leaves (Fig. 1 D–G). This marks as an important defensive strategy that evolved before the more extensively known leaf mimesis after the rise of angiosperms. Naturally, had Bellinympha been perched at the apex of young leaves, which are frequently the feeding target in living cycads, it might be difficult for a predator to distinguish the insect from the surrounding foliage (Fig. S5).
When compared with coincident leaf fossils, Bellinympha’s imitation was not as advanced as those of living insects whereby mimesis can show an almost perfect match with various angiosperm leaves. Nonetheless, perfect representation of the mimic is not necessary to acquire an advantage because the morphology need only be close enough to elicit the appropriate response from a potential dupe. Accordingly, only a subset of visual signals may be necessary to confer sufficient crypsis as to avoid detection.
According to the material recovered to date, most wings of Bellinympha and its relatives are preserved in out-stretched positions rather than folded roof-like over the body as in many neuropterans. The same posture is also observed in other large and extinct lacewings contemporaneous with Bellinympha, such as the aforementioned families Aetheogrammatidae, Kalligrammatidae, and Grammolingiidae. It is likely that the out-stretched forewing among extremely large lacewings stems from a behavioral adaptation of “adaptive stillness” (5). Bellinympha with their pinnate leaf-like wings consisting of disruptive maculations likely rested and fed on the terminal pinnae of the leaves, remaining motionless or swaying gently to resemble leaves moving in the breeze, similar to modern Phasmatodea.
Bellinympha may not have been a swift flyer, similar to many other large neuropterans. Accordingly, there may have been several potential predators for such species among the Daohugou fauna, including, as noted, early birds, mammals, dinosaurs, pterosaurs, spiders, and other predacious insects. Although records are not numerous, early studies have documented examples of birds capturing neuropterans (15). Primitive birds, pterosaurs, and prosauropod dinosaurs discovered in the Daohugou biota possibly had also insectivorous habits, particularly arboreal dinosaurs or lizards certainly may have preyed on Bellinympha and its relatives (16–19). Feathered dinosaurs such as Epidexipteryx hui from Daohugou were likely also insectivorous (16). Most notably, this fauna contained an early gliding mammal, Volaticotherium antiquus, which possessed specialized dentition for feeding on insects (20). The same strata harbor other early insectivorous mammals (21, 22), demonstrating a robust fauna of potential vertebrate predators, particularly for any large, slow-flying neuropterans such as Bellinympha, aetheogrammatids, kalligrammatids, and the like. In addition, spiders were common predators in the Middle Jurassic, and there are numerous records from Daohugou (23). Extant neuropterans are also frequently attacked by predacious insects, such as dragonflies (Odonata), predatory flies (Diptera), and hangingflies (Mecoptera) (15). The Daohugou insect-biota was diverse, with various potential generalist insect predators, including the aforementioned dragonflies (24, 25) and hangingflies (26). Considering the multitude of contemporaneous predators, it is clear that predatory pressures were significant, and mechanisms to ease or escape predation, such as mimesis, would have been greatly favored. Certainly various behavioral and chemical mechanisms of avoiding detection must have existed, but these have left few or no fossil traces or may be determined only by specialized morphologies (e.g., nocturnality by ocellar development, integumental pigmentation). Similarly, instances of camouflage, whereby a species blends into its abiotic surroundings, may have existed in species for which coloration is not preserved. Certainly among easily discernible forms of mimicry, nothing as complex or dramatic as leaf mimesis is known before Bellinympha.
The eventual disappearance of Bellinympha and its relatives is likely the same story as has been played out by innumerable highly specialized lineages of plant-associated insects; that is, that dependent specialization that is susceptible to rapid extinction should affect the model species. In the case of Bellinympha, its intimate association within a world comprising abundant gymnospermous plants with pinnate leaves left it dependent on such a flora. When the floral composition of the environment shifted in the Early Cretaceous toward a more angiosperm-dominated ecosystem and Bellinympha’s cycadalean and bennettitalean models waned in diversity and abundance, so too did the mimic. The disappearance of pinnate leaf mimesis coincident with declines in Cycadales and Bennettitales (27) supports the conclusion of an evolutionary tie between such plants and these Bellinympha neuropterans. As fewer and fewer pinnate-leaf plants were present in the flora, Bellinympha and its relatives would have become increasingly obvious to visual predators, leading to sharp declines in their numbers. Without rapid reversal to a more standard neuropteran wing shape and pattern or new adaptation to the increasingly abundant leaf morphologies of flowering plants, such insects were likely doomed. Such uniquely specialized morphology would have become more of a hindrance in such a changed environment and, if coupled with losses of fitness due to changing climates or its own food resources, may have led to a rapid decline. Regardless, Bellinympha and its relatives demonstrate that tightly associated leaf mimicry had evolved early in the Mesozoic and well before the ecological radiation of flowering plants, and that such adaptations at one time included mimicking pinnate leaf morphologies among gymnosperms.
The discovery of Bellinympha is of great significance to understanding the Mesozoic evolution and diversification of insects, particularly in the context of early coevolution between plants and pollinators before angiosperms (28). These species reveal a unique pattern that seems to have disappeared in modern insects and adds to the growing body of evidence documenting that the evolution of insects was more complex before the radiation of angiosperms (29). These enigmatic scenarios of interactions between insects and gymnosperms were lost during the course of evolutionary history and show a more rich association of insects and their surrounding environment in past geological epochs than previously has been surmised.
Supplementary Material
Acknowledgments
This research was supported by National Natural Science Foundation of China Grants 40872022, 30811120038, 40772006, and J0630967; Nature Science Foundation of Beijing Grant 5082002; and Scientific Research Key Program, PHR Project of Beijing Municipal Commission of Education and State Key Laboratory of Paleobiology and Stratigraphy, 103109 (Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences). Support for M.S.E.’s participation was provided by K.K.M. Engel (Scantron, Lawrence, KS) and US National Science Foundation Grant DEB-0542909.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006460107/-/DCSupplemental.
References
- 1.Grimaldi DA, Engel MS. Evolution of the Insects. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 2.Gullan PJ, Cranston PS. The Insects—An Outline of Entomology. Oxford: Blackwell Publishing; 2005. pp. 356–359. [Google Scholar]
- 3.Scudder SH. Revision of the American fossil cockroaches with descriptions of new forms. Bull US Geol Surv. 1895;124:1–176. [Google Scholar]
- 4.Jarzembowski EA. Fossil cockroaches or pinnule insects? Proc Geol Assoc. 1994;105:305–311. [Google Scholar]
- 5.Wedmann S, Bradler S, Rust J. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proc Natl Acad Sci USA. 2007;104:565–569. doi: 10.1073/pnas.0606937104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspöck U, Aspöck H. Lingering diversity of a bygone blossom. On the evolution, phylogeny, and biodiversity of the Neuropterida (Insecta: Endopterygota) Denisia. 2007;20:451–516. [Google Scholar]
- 7.Opler PA. Polymorphic mimicry of polistine wasps by a neotropical neuropteran. Biotropica. 1981;13:165–176. [Google Scholar]
- 8.Ren D, et al. Stratigraphic division of the Jurassic in the Daohugou area, Ningcheng, Inner Mongolia. Geol Bull China. 2002;21:584–591. [Google Scholar]
- 9.Liu Y, et al. Daohugou biota−bearing lithostratigraphic succession on the southeastern margin of the Ningcheng basin, Inner Mongolia, and its geochronology. Geol Bull China. 2004;23:1180–1185. [Google Scholar]
- 10.Aspöck U, Aspöck H. Phylogenetic relevance of the genital sclerites of Neuropterida (Insecta: Holometabola) Syst Entomol. 2008;33:97–127. [Google Scholar]
- 11.Ren D, Yin JC. New ‘osmylid-like’ fossil Neuroptera from the Middle Jurassic of Inner Mongolia, China. J NY Entomol Soc. 2003;111:1–11. [Google Scholar]
- 12.Ren D, Yin JC. A new genus and new species of lacewings in the Jurassic of China (Neuroptera: Myrmeleontoidea) Acta Zootax Sin. 2002;27:269–273. [Google Scholar]
- 13.Ren D, Engel MS. A split-footed lacewing and two epiosmylines from the Jurassic of China (Neuroptera) Ann Zool. 2007;57:211–219. [Google Scholar]
- 14.Stevens M, Hardman CJ, Stubbins CL. Conspicuousness, not eye mimicry, makes “eyespots” effective antipredator signals. Behav Ecol. 2008;19:525–531. [Google Scholar]
- 15.Killington FJ, editor. A Monograph of the British Neuroptera. Vol. 1. London: Royal Society; 1936. pp. 179–182. [Google Scholar]
- 16.Zhang FC, Zhou ZH, Xu X, Wang XL, Sullivan C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature. 2008;455:1105–1108. doi: 10.1038/nature07447. [DOI] [PubMed] [Google Scholar]
- 17.Lü JC, Unwin DM, Jin XS, Liu YQ, Ji Q. Evidence form modular evolution in a long-tailed pterosaur with a pterodactyloid skull. Proc Biol Sci. 2009;277:383–389. doi: 10.1098/rspb.2009.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XL, Zhou ZH, Zhang FC, Xu X. A nearly completely articulated rhamphorhynchoid pterosaur with exceptionally well-preserved wing membranes and “hairs” from Inner Mongolia, northeast China. Chin Sci Bull. 2002;47:226–230. [Google Scholar]
- 19.Galton PM. Diet of prosauropod dinosaurs from the Late Triassic and Early Jurassic. Lethaia. 1985;18:105–123. [Google Scholar]
- 20.Meng J, Hu YM, Wang YQ, Wang XL, Li CK. A Mesozoic gliding mammal from northeastern China. Nature. 2006;444:889–893. doi: 10.1038/nature05234. [DOI] [PubMed] [Google Scholar]
- 21.Ji Q, Luo ZX, Yuan CX, Tabrum AR. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science. 2006;311:1123–1126. doi: 10.1126/science.1123026. [DOI] [PubMed] [Google Scholar]
- 22.Evans SE. At the feet of the dinosaurs: The early history and radiations of lizards. Biol Rev Camb Philos Soc. 2003;78:513–551. doi: 10.1017/s1464793103006134. [DOI] [PubMed] [Google Scholar]
- 23.Selden PA, Huang DY, Ren D. Palpimanoid spiders from the Jurassic of China. J Arachnol. 2008;36:306–321. [Google Scholar]
- 24.Zhang BL, et al. New isophlebioid dragonflies (Odonata: Isophlebioptera: Campterophlebiidae) from the Middle Jurassic of China. Zootaxa. 2006;1339:52–68. [Google Scholar]
- 25.Nel A, Huang DY, Zhang BL. A new genus of isophlebioid damsel-dragonflies (Odonata: Isophlebioptera: Campterophlebiidae) from the Middle Jurassic of China. Zootaxa. 2007;1642:13–22. [Google Scholar]
- 26.Petrulevičius JF, Huang DY, Ren D. A new hangingfly (Insecta: Mecoptera: Bittacidae) from the Middle Jurassic of Inner Mongolia, China. Afr Invertebr. 2007;48:145–152. [Google Scholar]
- 27.Crane PR. In: The Origin of the Angiosperms and Their Biological Consequences. Friis EM, Chaloner WG, Crane PR, editors. Cambridge, UK: Cambridge Univ Press; 1987. pp. 107–144. [Google Scholar]
- 28.Ren D, et al. A probable pollination mode before angiosperms: Eurasian, long-proboscid scorpionflies. Science. 2009;326:840–847. doi: 10.1126/science.1178338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ollerton J, Coulthard E. Evolution of animal pollination. Science. 2009;326:808–809. doi: 10.1126/science.1181154. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Li N, Wang Y, Zheng S. The discovery of whole-plant fossil cycad from the Upper Triassic in western Liaoning and its significance. Chin Sci Bull. 2009;54:3116–3119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.