Abstract
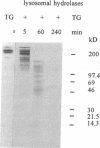
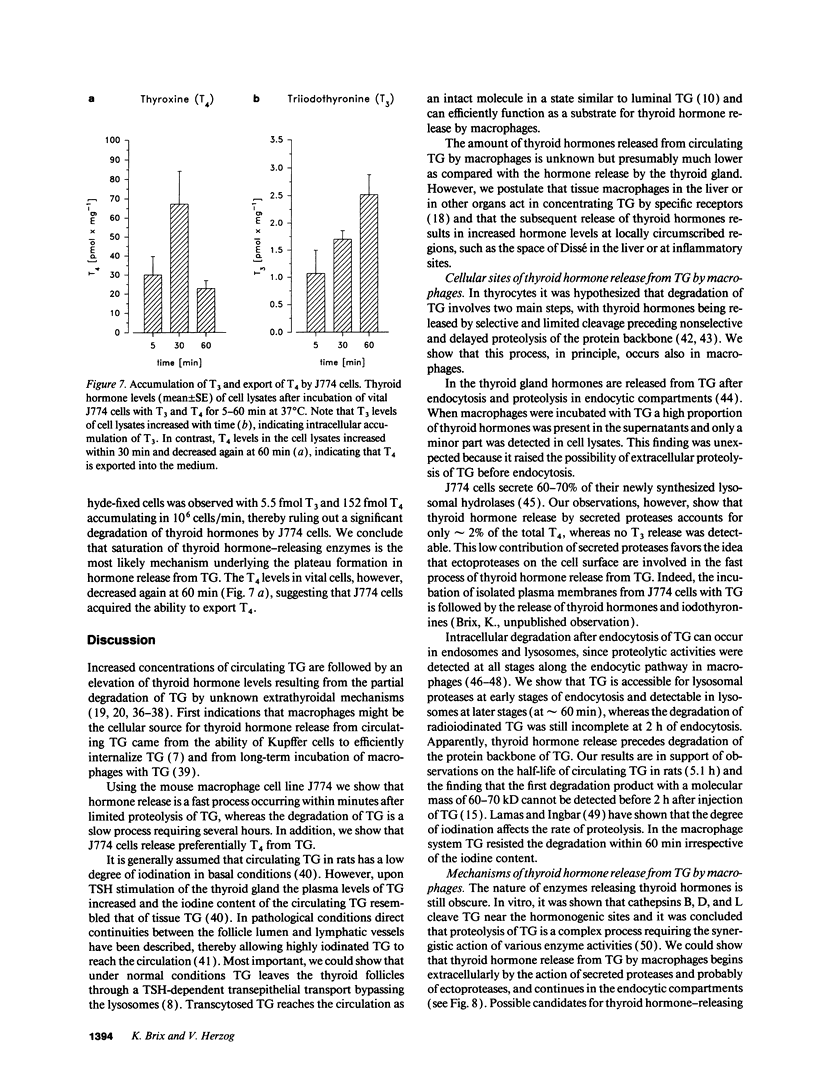
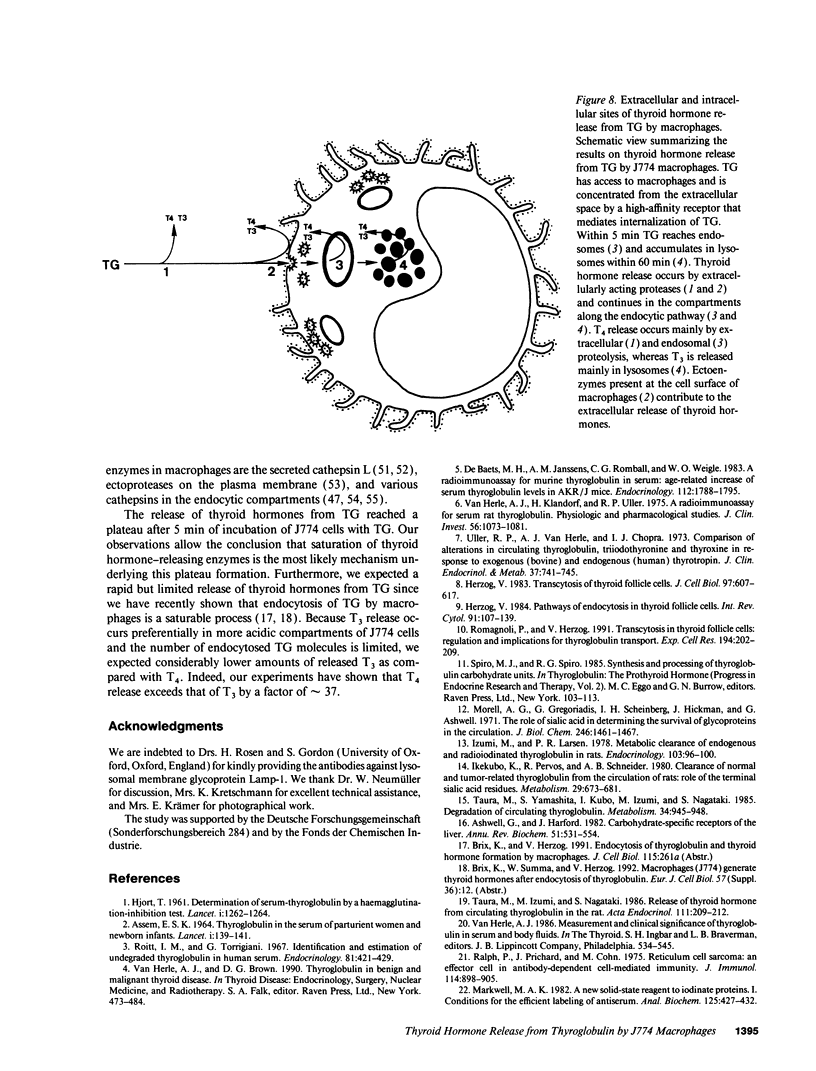
Thyroglobulin appears in the circulation of vertebrates at species-specific concentrations. We have observed that the clearance of thyroglobulin from the circulation occurs in the liver by macrophages. Here we show that the thyroid hormones T3 and T4 were released by incubation of mouse macrophages (J774) with thyroglobulin. Thyroid hormone release was a fast process, with an initial rate of approximately 20 pmol T4/mg per min and approximately 0.6 pmol T3/mg per min, indicating that macrophages preferentially release T4. The bulk of released thyroid hormones appeared after 5 min of incubation of macrophages with thyroglobulin, whereas degradation of the protein was detectable only after several hours. During internalization of thyroglobulin, endocytic vesicles and endosomes were reached at 5 min and lysosomes at 60 min. T4 release started extracellularly by secreted proteases and continued along the endocytic pathway of thyroglobulin, whereas T3 release occurred mainly intracellularly when thyroglobulin had reached the lysosomes. This shows that the release of both hormones occurred at distinct cellular sites. Our in vitro observations suggest that macrophages in situ represent an extrathyroidal source for thyroid hormones from circulating thyroglobulin.
Full text
PDF


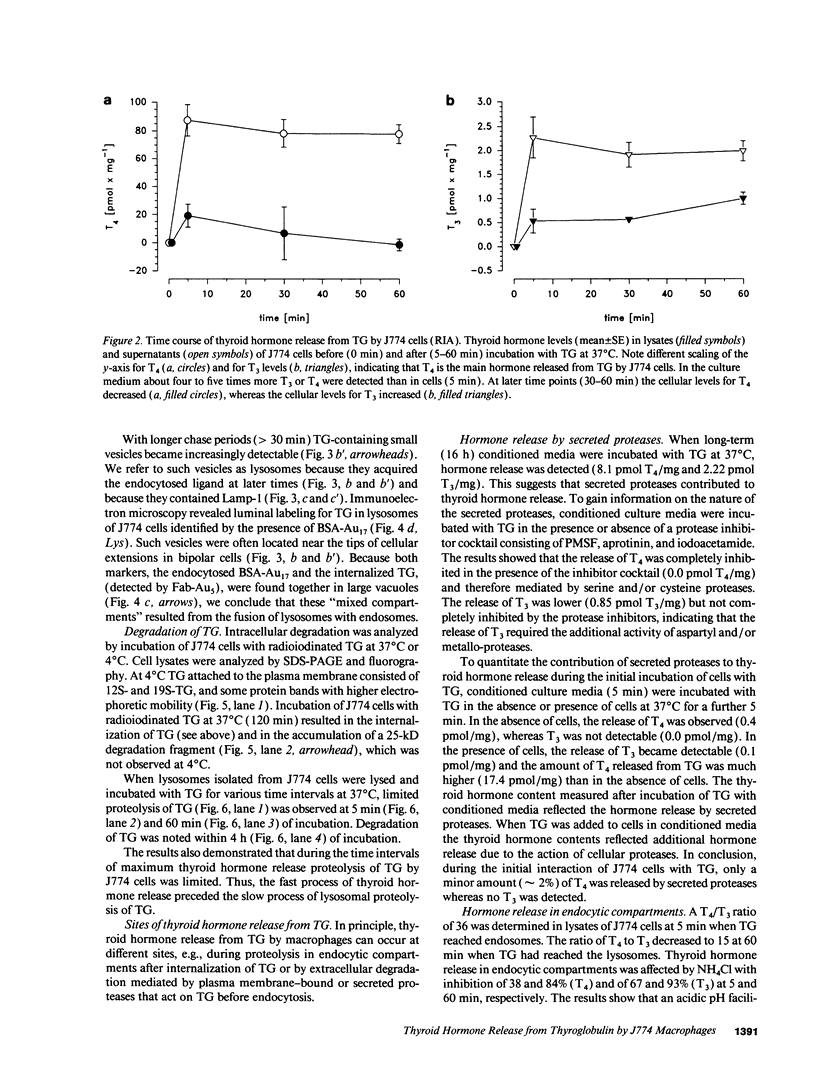

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASSEM E. S. THYROGLOBULIN IN THE SERUM OF PARTURIENT WOMEN AND NEWBORN INFANTS. Lancet. 1964 Jan 18;1(7325):139–141. doi: 10.1016/s0140-6736(64)92224-x. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- BROWN F., JACKSON H. The fate of 131I-labelled homologous and heterologous thyroglobulins in the rat, dog, monkey and rabbit. Biochem J. 1956 Feb;62(2):295–301. doi: 10.1042/bj0620295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B., Sancéau J., Wietzerbin J. Human U937 cell surface peptidase activities: characterization and degradative effect on tumor necrosis factor-alpha. Eur J Immunol. 1992 Apr;22(4):923–930. doi: 10.1002/eji.1830220407. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- De Baets M. H., Janssens A. M., Romball C. G., Weigle W. O. A radioimmunoassay for murine thyroglobulin in serum: age-related increase of serum thyroglobulin levels in AKR/J mice. Endocrinology. 1983 May;112(5):1788–1795. doi: 10.1210/endo-112-5-1788. [DOI] [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Diment S., Stahl P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J Biol Chem. 1985 Dec 5;260(28):15311–15317. [PubMed] [Google Scholar]
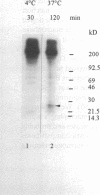
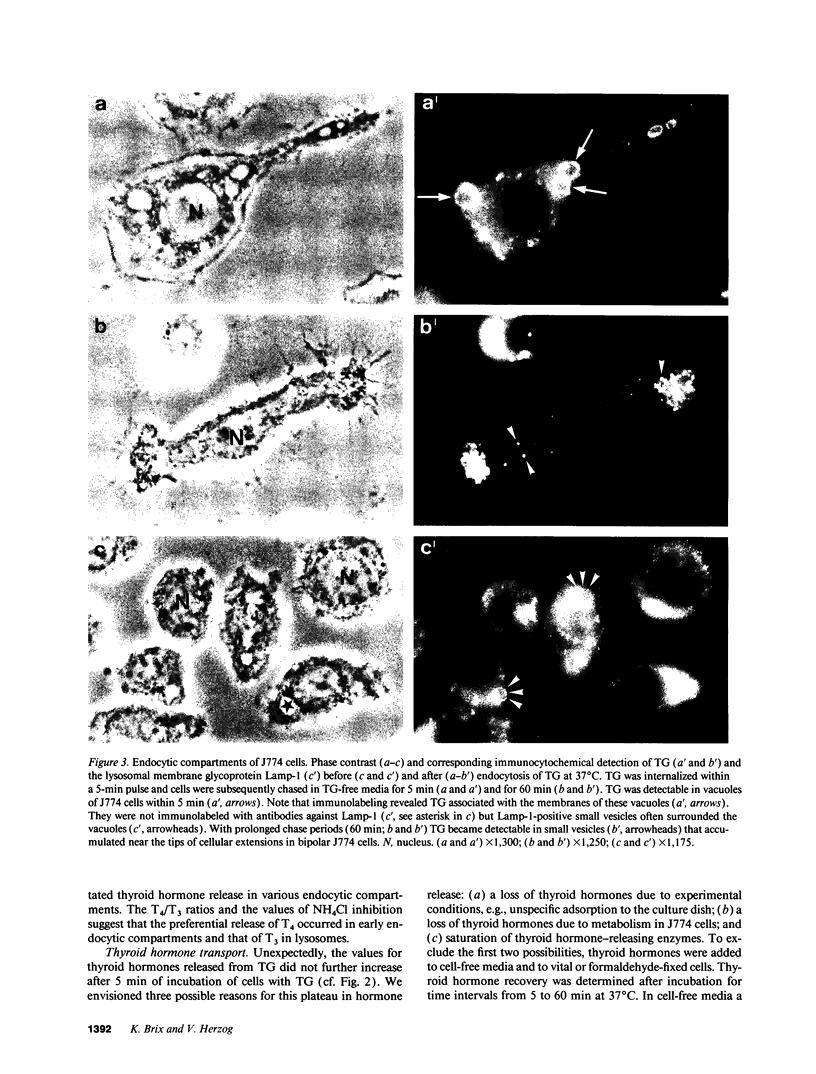
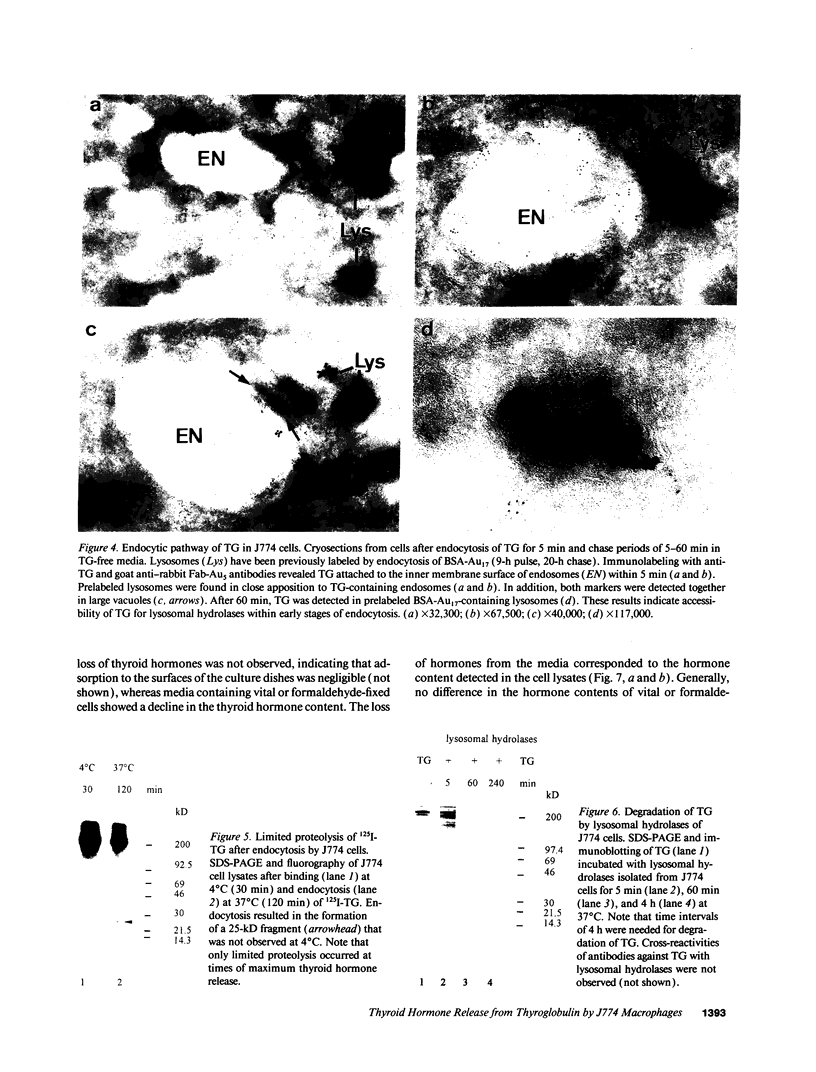
- Dunn A. D., Crutchfield H. E., Dunn J. T. Thyroglobulin processing by thyroidal proteases. Major sites of cleavage by cathepsins B, D, and L. J Biol Chem. 1991 Oct 25;266(30):20198–20204. [PubMed] [Google Scholar]
- Fujita H. Functional morphology of the thyroid. Int Rev Cytol. 1988;113:145–185. doi: 10.1016/s0074-7696(08)60848-7. [DOI] [PubMed] [Google Scholar]
- Gabel C. A., Goldberg D. E., Kornfeld S. Identification and characterization of cells deficient in the mannose 6-phosphate receptor: evidence for an alternate pathway for lysosomal enzyme targeting. Proc Natl Acad Sci U S A. 1983 Feb;80(3):775–779. doi: 10.1073/pnas.80.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebel F., Studer H. Malignant follicles of a differentiated thyroid carcinoma releasing iodinated thyroglobulin into the lymphatic vessels. Clin Endocrinol (Oxf) 1984 Apr;20(4):457–462. doi: 10.1111/j.1365-2265.1984.tb03442.x. [DOI] [PubMed] [Google Scholar]
- HJORT T. Determination of serum-thyroglobulin by a haemagglutination-inhibition test. Lancet. 1961 Jun 10;1(7189):1262–1264. doi: 10.1016/s0140-6736(61)92767-2. [DOI] [PubMed] [Google Scholar]
- Herzog V. Pathways of endocytosis in thyroid follicle cells. Int Rev Cytol. 1984;91:107–139. doi: 10.1016/s0074-7696(08)61315-7. [DOI] [PubMed] [Google Scholar]
- Herzog V. Transcytosis in thyroid follicle cells. J Cell Biol. 1983 Sep;97(3):607–617. doi: 10.1083/jcb.97.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988 Jan;9(1):28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- Hoddevik G., Seljelid R. The uptake and degradation of sheep thyroglobulin by macrophages in vitro. Exp Cell Res. 1975 Jun;93(1):152–158. doi: 10.1016/0014-4827(75)90434-6. [DOI] [PubMed] [Google Scholar]
- Ikekubo K., Pervos R., Schneider A. B. Clearance of normal and tumor-related thyroglobulin from the circulation of rats: role of the terminal sialic acid residues. Metabolism. 1980 Jul;29(7):673–681. doi: 10.1016/0026-0495(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Izumi M., Larsen P. R. Metabolic clearance of endogenous and radioiodinated thyroglobulin in rats. Endocrinology. 1978 Jul;103(1):96–100. doi: 10.1210/endo-103-1-96. [DOI] [PubMed] [Google Scholar]
- Jessup W., Dean R. T. Spontaneous lysosomal enzyme secretion by a murine macrophage-like cell line. Biochem J. 1980 Sep 15;190(3):847–850. doi: 10.1042/bj1900847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamas L., Ingbar S. H. The effect of varying iodine content on the susceptibility of thyroglobulin to hydrolysis by thyroid acid protease. Endocrinology. 1978 Jan;102(1):188–197. doi: 10.1210/endo-102-1-188. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Reconstitution of endosomal proteolysis in a cell-free system. Transfer of immune complexes internalized via Fc receptors to an endosomal proteolytic compartment. J Biol Chem. 1989 Apr 5;264(10):5392–5399. [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Muno D., Sutoh N., Watanabe T., Uchiyama Y., Kominami E. Effect of metabolic alterations on the density and the contents of cathepsins B, H and L of lysosomes in rat macrophages. Eur J Biochem. 1990 Jul 20;191(1):91–98. doi: 10.1111/j.1432-1033.1990.tb19097.x. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Rabinowitz S., Horstmann H., Gordon S., Griffiths G. Immunocytochemical characterization of the endocytic and phagolysosomal compartments in peritoneal macrophages. J Cell Biol. 1992 Jan;116(1):95–112. doi: 10.1083/jcb.116.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Rodman J. S., Levy M. A., Diment S., Stahl P. D. Immunolocalization of endosomal cathepsin D in rabbit alveolar macrophages. J Leukoc Biol. 1990 Aug;48(2):116–122. doi: 10.1002/jlb.48.2.116. [DOI] [PubMed] [Google Scholar]
- Roitt I. M., Torrigiani G. Identification and estimation of undegraded thyroglobulin in human serum. Endocrinology. 1967 Sep;81(3):421–429. doi: 10.1210/endo-81-3-421. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Herzog V. Transcytosis in thyroid follicle cells: regulation and implications for thyroglobulin transport. Exp Cell Res. 1991 Jun;194(2):202–209. doi: 10.1016/0014-4827(91)90355-x. [DOI] [PubMed] [Google Scholar]
- Rousset B., Mornex R. The thyroid hormone secretory pathway--current dogmas and alternative hypotheses. Mol Cell Endocrinol. 1991 Jun;78(1-2):C89–C93. doi: 10.1016/0303-7207(91)90176-s. [DOI] [PubMed] [Google Scholar]
- Rousset B., Selmi S., Bornet H., Bourgeat P., Rabilloud R., Munari-Silem Y. Thyroid hormone residues are released from thyroglobulin with only limited alteration of the thyroglobulin structure. J Biol Chem. 1989 Jul 25;264(21):12620–12626. [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatumi K., Suzuki Y., Sinohara H. Clearance of circulating desialylated thyroglobulins in the rat. Biochim Biophys Acta. 1979 Apr 3;583(4):504–511. doi: 10.1016/0304-4165(79)90067-9. [DOI] [PubMed] [Google Scholar]
- Taura M., Izumi M., Nagataki S. Release of thyroid hormone from circulating thyroglobulin in the rat. Acta Endocrinol (Copenh) 1986 Feb;111(2):209–212. doi: 10.1530/acta.0.1110209. [DOI] [PubMed] [Google Scholar]
- Taura M., Yamashita S., Kubo I., Izumi M., Nagataki S. Degradation of circulating thyroglobulin. Metabolism. 1985 Oct;34(10):945–948. doi: 10.1016/0026-0495(85)90143-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Farquhar M. N. Specific measurement of DNA in nuclei and nucleic acids using diaminobenzoic acid. Anal Biochem. 1978 Aug 15;89(1):35–44. doi: 10.1016/0003-2697(78)90724-8. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller R. P., Van Herle A. J., Chopra I. J. Comparison of alterations in circulating thyroglobulin, triiodothyronine and thyroxine in response to exogenous (bovine) and endogenous (human) thyrotropin. J Clin Endocrinol Metab. 1973 Nov;37(5):741–745. doi: 10.1210/jcem-37-5-741. [DOI] [PubMed] [Google Scholar]
- Van Herle A. J., Klandorf H., Uller R. P. A radioimmunoassay for serum rat thyroglobulin. Physiologic and pharmacological studies. J Clin Invest. 1975 Nov;56(5):1073–1081. doi: 10.1172/JCI108181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herle A. J., Uller R. P., Matthews N. I., Brown J. Radioimmunoassay for measurement of thyroglobulin in human serum. J Clin Invest. 1973 Jun;52(6):1320–1327. doi: 10.1172/JCI107303. [DOI] [PMC free article] [PubMed] [Google Scholar]