Abstract
The auditory cortex is known to be a necessary neural structure for the perception of acoustic signals, particularly the spatial location and the temporal features of complex auditory stimuli. Previous studies have indicated that there is no topographic map of acoustic space in the auditory cortex and it has been proposed that spatial locations are represented by some sort of population code. Additionally, in spite of temporal processing deficits being one of the hallmark consequences of normal aging, the temporal coding of acoustic stimuli remains poorly understood. This report will address these two issues by discussing the results from several studies describing responses of single auditory cortical neurons in the non-human primate. First, we will review studies that have addressed potential spike-rate population codes of acoustic space in the caudal belt of auditory cortex. Second, we will present new data on the neuronal responses to gap stimuli in aged monkeys and compare them to published reports of gap detection thresholds. Together these studies indicate that the alert macaque monkey is an excellent model system to study both spatial and temporal processing in the auditory cortex at the single neuron level.
Keywords: macaque monkey, auditory cortex, acoustic space, temporal processing
Introduction
The mammalian auditory cortex is a critical neural structure for the perception of acoustic stimuli. Auditory perceptions include determining where the sound is located in space and what the stimulus is based on the spectral and temporal properties of the sounds. Lesion studies in a variety of mammals have shown that while the cerebral cortex is crucial for the percepts, it may not be necessary to process the information in a manner necessary to elicit an involuntary behavior. For example, auditory cortical lesions result in a severe deficit in the ability to perceive the location of a sound in contralesional space (e.g. Heffner and Heffner, 1990), but animals can still reflexively orient to the sound (e.g. Beitel and Kaas, 1993). Thus, while non-cortical areas can process acoustic space information, the cerebral cortex is necessary for the perception of the spatial location of acoustic stimuli.
The perception of an acoustic stimulus also depends on the spectral and temporal properties of the stimulus. Normal aging is known to have dramatic effects on the central mechanisms of hearing, particularly in the temporal domain (e.g. Frisina and Frisina, 1997; Gordon-Salant and Fitzgibbons, 1993, 2001). These temporal processing deficits lead to a disruption of the ability to process complex sounds, such as human speech. Consequently, aged individuals suffer speech processing deficits, especially under natural noisy listening conditions. Again, the auditory cortex is known to be critical in this perceptual function, yet very little is understood about how natural aging affects the temporal response properties of cortical neurons.
This report will review these two different aspects of auditory cortical function, spatial and temporal processing, based on studies in alert macaque monkeys. The macaque monkey and human auditory cortex are functionally organized in a homologous fashion (Hackett et al., 2001) and are divided into three principal divisions consisting of core, belt and parabelt areas. We will describe the spatial and temporal response properties of three of these areas, the primary auditory cortex of the core (A1) and the caudolateral (CL) and caudomedial (CM) fields of the belt (see Figure 1). We will explore response properties of neurons in these areas both at the single neuron level as well as across the population of neurons within each field in an attempt to account for potential neural codes for these perceptions.
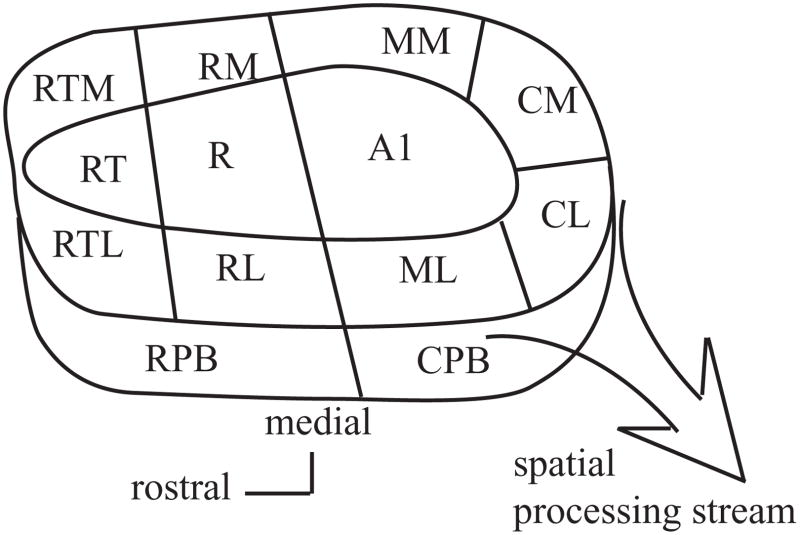
Figure 1.
Schematic diagram of the primate auditory cortex. The auditory cortex is made up of multiple cortical fields organized into a core-belt-parabelt fashion. The caudal fields are believed to form a spatial processing stream. Adapted from Kaas and Hackett, 2000; Hackett et al., 2001. Core; A1: primary auditory cortex, R: rostral field, RT: rostrotemporal field. Belt; CM: caudomedial field, CL: caudolateral field, ML: middle lateral field, RL: rostrolateral field, RTL: rostrotemporal lateral field, RTM: rostrolateral lateral field, RM: rostromedial field, MM: middle medial field. Parabelt; RPB: rostral parabelt, CPB: caudal parabelt.
The Cortical Representation of Acoustic Space
Studies over the course of the past few decades have sought to understand how the auditory cortex could potentially encode acoustic space. In contrast to the primary visual and somatosensory systems, in which there are topographic representations of the sensory epithelium (retinotopy and somatotopy), early studies in anesthetized cats clearly showed that there is no topographic representation of acoustic space in the primary auditory cortex (e.g. Middlebrooks and Pettigrew, 1980; Imig et al., 1990; Rajan et al., 1990a,b). This is because, unlike vision and touch, the neural representation of the spatial location of a sound is not based on the activity at a particular location on the sensory epithelium. Rather, acoustic space must be computed by the nervous system. This computation is based on interaural timing and intensity cues, which are used to localize stimuli in the horizontal plane (azimuth). These cues are generated by the distance between the two ears and the shadowing of the sound by the head and body. Spectral cues also contribute to horizontal localization, but are more important in localizing sounds in elevation. Such cues are based on the interactions of the sounds with the body, head and especially the pinna (see Blauert, 1997).
Many previous studies have approached the problem of how acoustic space is represented in the cortex by asking how well individual auditory cortical neurons are tuned to spatial location. In general, the findings are that individual neurons have very large spatial receptive fields, where many have some level of response to the same sound regardless of the spatial location. Often times each cell is tested at one or more stimulus intensities that are based on the threshold of that particular neuron, so it becomes difficult to address how the population of neurons responds at a particular stimulus intensity. This also makes it difficult to correlate the spatial tuning of the neurons with sound localization acuity. These technical details have made it extremely challenging to appreciate potential neural codes of acoustic space.
One way around these technical issues is to record the responses of single neurons in alert animals to a particular stimulus and then compare them to the sound localization performance for that stimulus. This has been tackled in our laboratory in a pair of studies in macaque monkeys, the first targeted localization in both azimuth and elevation in frontal space as a function of the stimulus spectrum, and the second targeted localization in horizontal space as a function of stimulus intensity. Although several cortical fields were tested, for the purposes of this paper we will concentrate on the responses of neurons in one core (A1) and two belt (CM and CL) fields. These caudal areas have been implicated in the putative pathway that is involved in processing spatial information (see Rauschecker et al., 1995, 1997; Rauschecker and Tian, 2004; Tian et al., 2001; Tian and Rauschecker 2004; Kaas and Hackett, 2000; Romanski et al., 1999).
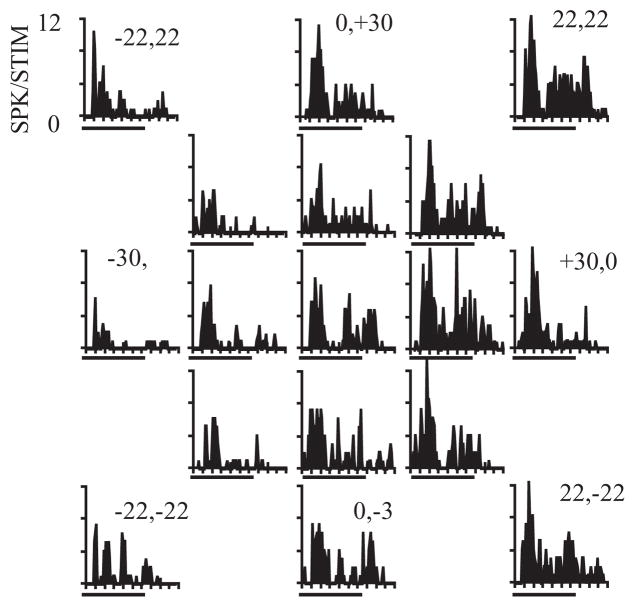
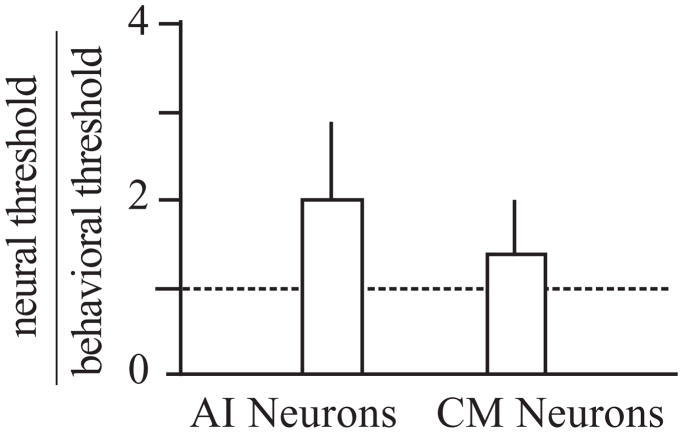
In the first study, the activity of single neurons was recorded in A1 and CM in animals that were actively localizing sounds across 30 degrees of frontal space (Recanzone et al., 2000b). The recording sites reported in that study almost certainly included neurons in CL, but these were not differentiated from those that were in area CM. Typical responses to a broadband stimulus are shown in Figure 2, where there is clear spatial tuning in both azimuth and elevation in contralateral space based on the firing rate of the cell. For approximately 80% of neurons, the firing rate was statistically significantly correlated with the spatial location of either a tone or noise stimulus (spatially sensitive neurons), however, there was little variation in the response as a function of the elevation of tonal stimuli, consistent with the difficulty in localizing tones in this plane (see Blauert, 1997). These changes in the firing rates of cortical neurons as a function of spatial location were consistent with the behavioral thresholds measured in the same monkeys. The monkeys were trained to release a lever when they detected a change in the location of a sequentially presented stimulus from directly ahead to a different location from 5 to 30 degrees in eccentricity along the vertical or horizontal plane. Stimuli included broadband noise, one-octave band-passed noise and tones. One key advantage of this study is that the same stimuli were used in the behavioral studies as well as the physiological studies, regardless of the threshold and best frequency of the tested neuron. At the level of the individual neuron, there was no correlation between behavioral thresholds and neural activity. In order to determine if the population of cortical neurons contained sufficient information to account for the behavioral thresholds, the response of each spatially sensitive neuron was first normalized to the peak firing rate for that neuron. Neurons recorded under the different stimulus conditions were then pooled together and the variance in this pooled response was used to calculate how much spatial separation was necessary for an ideal observer to determine whether the stimuli came from straight ahead or from a different location in either azimuth or elevation for the broadband noise and each of the band-passed and tonal stimuli. This is precisely what the monkeys were trained to do, and the results of this analysis comparing the behaviorally measured thresholds to those predicted by the pooled neural response are shown in Figure 3. Neurons from CM, in contrast to those in A1, were much more accurate at discriminating spatially disparate stimuli. This was true not only of locations in azimuth but also locations in elevation, and across the different frequencies and spectral bandwidths, giving rise to the relatively small error bars. These data support the notion that population coding of neurons in the caudal regions of the auditory cortex can accurately encode spatial location.
Figure 2.
Example responses of a single neuron to broadband noise stimuli from different locations in frontal space. Each PSTH is for a stimulus located directly ahead (center), or along one of two concentric rings at 15 or 30 degrees eccentricity. Numbers along the outer circle of PSTHs denote the location in degrees of azimuth and elevation. Stimuli were 200 ms of ‘unfrozen’ noise. The firing rate of this cell was greater toward the contralateral (right) side of acoustic space. Adapted from Recanzone et al., 2000b.
Figure 3.
Population coding of acoustic space. Each bar of the histogram shows the ratio of the neural/behavioral spatial discrimination threshold based on the firing rates of neurons that were significantly tuned. Threshold ratios were based on the distribution of firing rates as a function of spatial location in azimuth and elevation for the broadband noise, three one-octave band-passed noise and six tones tested both electrophysiologically and behaviorally. Neurons in the caudal belt, labeled CM but likely included many CL neurons as well, were more accurate than A1 neurons, and their estimates were not significantly different from a ratio of 1.0 (no difference). Adapted from Recanzone et al., 2000b.
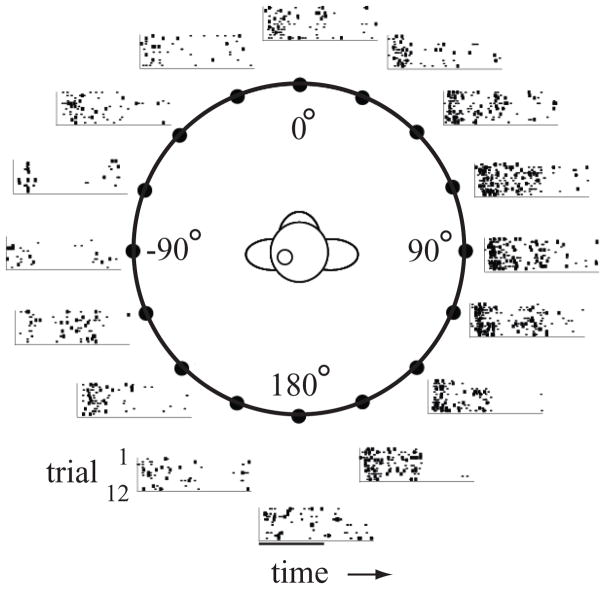
The second study was similar in concept but was designed to ask if population coding could also account for spatial localization as a function of stimulus intensity (Woods et al., 2006). Previous studies in humans (Altshuler and Comalli, 1975; Comalli and Altshuler, 1976; Su and Recanzone, 2001; Sabin et al., 2005) as well as macaque monkeys (Recanzone and Beckerman, 2004) had shown that sound localization performance is degraded near the detection threshold but is maintained across a broad intensity range. However, the firing rates of single neurons can be quite varied over this same range of stimulus intensity, and it remains unclear if a mismatch between firing rates and behavioral performance would remain across the population of active neurons. To address this issue, the responses of single neurons were recorded across the core region A1 as well as both CM and CL to stimuli near and well above localization thresholds. Typical neuronal responses for a single intensity are shown in Figure 4, where there is a clear increase in the firing rate in contralateral space compared to ipsilateral space. What is not clear from this figure, however, is that there was a great deal of individual variability across neurons in all three cortical fields. For example, neurons could have their sharpest spatial tuning for any of the four different stimulus intensities, but usually the highest intensity stimulus elicited the greatest response. The neuronal population showed both a variety of best directions and best intensities, but there was no evidence for any kind of topographic or modular organization. What was clear, however, is that neurons in the caudal belt areas did have sharper spatial tuning than those from A1 at all stimulus intensities tested.
Figure 4.
Example of a neural response across 360 degrees of space. Each raster shows the response to a 200 msec broadband noise presented from one of 16 locations. Recordings were made in the left auditory cortex. Responses were greater for locations in the right hemifield (contralateral) compared to the left hemifield. Data taken from Woods et al., 2006.
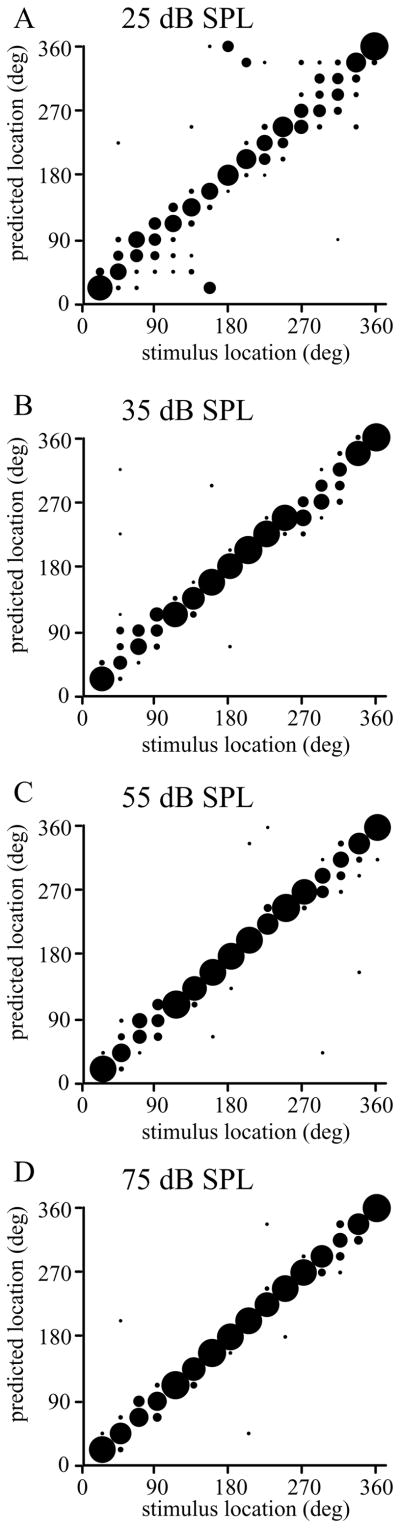
In order to compare these neuronal responses to the ability to localize sounds, localization estimates based on neuronal firing rates were compared to behavioral data obtained from human subjects. This inter-species comparison was necessary as it is technically extremely difficult to train head-restrained monkeys to localize sounds outside of their visual range. However, comparisons between human and monkey subjects when localizing acoustically simple sounds such as these, especially when performed using the same stimuli from the same speakers and equivalent behavioral responses, show that the two species have the same thresholds (e.g. see Recanzone et al., 1998, 2000b; Su and Recanzone 2001; Recanzone and Beckerman, 2004). In this task, the same broadband noise stimuli at the same intensities were presented from the same experimental apparatus and the subjects were asked to identify from which speaker the sound came from. A typical example is shown in Figure 5, where the proportion of responses are shown as the size of the circle, with errors for a particular location shown in the vertical direction. Sound localization performance was quite good at the highest intensities (no difference between 75 and 55 dB SPL; p > 0.05; see Miller and Recanzone, 2009), but was progressively worse at 35 (p < 0.01) and 25 dB SPL (p < 0.01 vs. 35 dB SPL).
Figure 5.
Localization of broadband noise in human subjects. Each plot shows the response of a single subject to each of 16 locations using the same stimuli as used in the monkey physiology experiments. The actual target location is on the x-axis, and the estimates by the subject are on the y-axis, thus, errors are shown vertically. The size of the circle is proportional to the percentage of responses at each location on the y-axis. This subject showed the most errors at the lowest intensity stimulus (25 dB, panel A), particularly toward the rear hemifield. The performance improved with increasing stimulus intensity (panels B – D). Adapted from Miller and Recanzone, 2009.
In order to determine whether the firing rates of populations of cortical neurons could account for this sound localization performance, a logarithmic maximum likelihood estimator model was applied to these neural data (Miller and Recanzone, 2009). This model had previously been shown to be a good predictor of direction of motion discrimination based on the responses of area MT neurons (Jazayeri and Movshon, 2006). Each neuron was assigned to one of 16 different sub-populations depending on the stimulus location that elicited the peak firing rate. The responses of single neurons across these sub-populations were randomly sampled to determine how well a winner-take-all model could predict the actual stimulus location. This model has the advantages in that it takes into account the variance in the single trial responses, and that the estimate is based on a single value, i.e. there is no arbitrarily set threshold. Additionally, these estimates could be assigned an error value identical to the psychophysical data and therefore the two datasets could be directly compared.
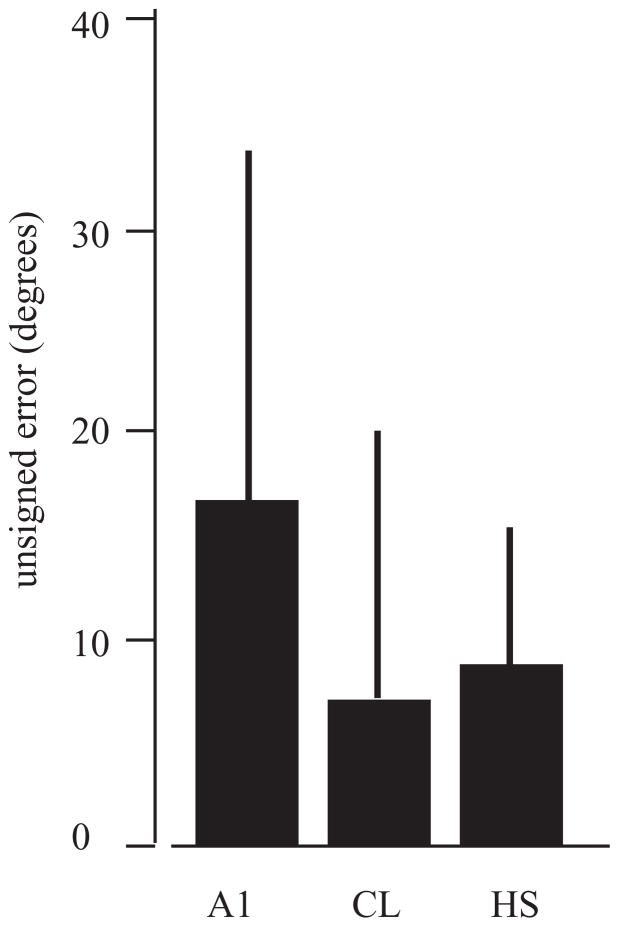
When this was model was applied, the estimates for ipsilateral locations were very poor and for contralateral locations quite accurate, consistent with the hemisphere-specific effects of unilateral lesions (Heffner and Heffner, 1990; Jenkins and Merzenich, 1984). However, the model was less accurate than expected for midline locations, which could be due to either under-sampling of neurons or compensation of sound location encoding by both hemispheres (see Miller and Recanzone, 2009). The second finding was that the model accuracy differed between cortical areas. Modeled data from CL neurons were not different from the human psychophysical data in contralateral space, whereas errors from A1 neurons were greater than the human estimates (Figure 6). This indicates that the firing rates from the population of cortical neurons recorded in CL contain enough information to account for sound localization performance.
Figure 6.
Mean unsigned errors for model estimates based on neuronal data taken from A1 and CL and compared to the mean unsigned errors measured in human subjects (HS). Model estimates based on the firing rates of CL neurons was not different from that of the human subjects, although estimates based on A1 neurons were worse. Adapted from Miller and Recanzone, 2009.
The representation of acoustic space based on a population code, while suspected for some time, had yet to be revealed in a plausible form across locations, intensities, and/or bandwidths (Eisenman, 1974; Recanzone et al., 2000b; Furukawa et al., 2000; Mrsic-Flogel et al., 2005; Stecker et al., 2005; Werner-Reiss and Groh, 2009). In the population firing rate model proposed herein, one could envision that each station along the cortical hierarchy acts as an estimator. Here, CL extracts information from A1 and other inputs and uses it to generate a code dominated by firing rate for spatial tuning and thereby generates the necessary information to account for sound location perception. This information is then passed along to the next level in the cortical hierarchy for further processing.
Effects of Natural Aging on Temporal Processing
Natural aging results in two common forms of age-related hearing deficits. The first is an audiometric hearing loss, where detection thresholds for different frequencies are increased (see Wiley et al., 1998; Mazelová et al., 2003). Changes in hearing detection thresholds are primarily believed to be due to peripheral factors (see Jennings and Jones, 2001; Ohlemiller, 2004; Nelson and Hinojosa, 2006). The second form, generally referred to as temporal processing deficits, consists of deficits in a variety of tasks seen in individuals with normal audiograms. These deficits have been revealed in studies of gap detection (e.g. Schneider et al., 1994; Schneider and Hamstra, 1999; Snell and Frisina, 2000), speech recognition and discrimination (Gordon-Salant and Fitzgibbons, 1993, 2001; Strouse et al., 1998; Snell and Frisina, 2000; Snell et al., 2002; Vesfeld and Dreschler, 2002) and by testing other temporal processing abilities (e.g. Gordon-Salant and Fitzgibbons, 2001). While a number of studies have investigated potential anatomical or physiological correlates of these deficits in the ascending auditory system (e.g. cochlear nucleus: Schwartz et al., 2002; nucleus of the lateral lemniscus: Willott et al., 1998; inferior colliculus: Caspary et al., 1995, 1999; Walton et al. 1997, Walton et al. 1998, Willott et al., 1998; Kazee and West, 1999), studies exploring the effects on the cerebral cortex, particularly in the non-human primate, are extremely scarce. We therefore have studied the response properties of single neurons in A1 and CL in aged macaque monkeys in order to provide the first investigations into gap discrimination processing at the level of the single cortical neuron.
Macaque monkeys age at approximately three times the rate of humans after sexual maturity, and have been shown to have age-related hearing deficits that are similar in both incidence and severity to humans (Bennett et al., 1983; Hawkins et al., 1985; Fowler et al., 2002). We have recorded the single neuron responses of two such animals, aged 24.1 to 25.8 years (monkey A; approximately 72–77 human years) and 24.4 to 26.2 years (monkey B; approximately 73 – 79 human years), while they passively sat in an acoustic chamber while a series of acoustic stimuli were presented (see Woods et al. 2006 for detailed methods). At the end of these experiments the animals were trained at a simple auditory detection task, in which they depressed a lever and an acoustic stimulus was presented within the following 2 – 7 seconds. If they correctly heard the sound and released the lever they received a fluid reward and the stimulus intensity was decreased by 1 dB for the next trial. If they did not release the lever within 800 msec the trial was aborted and the stimulus was increased by 1 dB for the next trial. The detection threshold was defined as 8–16 reversals of stimulus intensity. This paradigm was repeated at least 3 times. The detection thresholds for these two monkeys were within 5 dB of those from monkeys aged 10 and 14 years for clicks and 500 msec duration tonal stimuli from 500 Hz to 16 kHz, indicating that there was no significant hearing deficit of these aged monkeys over this frequency range.
The gap stimuli consisted of two main classes for each of two different types of stimulus durations. The first class was broadband noise presented at 65 dB SPL. The second class was a tone-in-noise stimulus, where a tonal stimulus at the characteristic frequency of the neuron at 65 dB SPL was presented with a broadband noise at 45 dB SPL. The first type of stimulus was 512 msec in total duration, with gaps imposed in the middle of this period at durations of 0 (no-gap), 1, 2, 4, 6, 8, 12, 16, 24, 32, 48, 64, 96, 128, 192 and 256 msec. The second type of stimulus was a control stimulus that was equivalent to the duration of the pre-gap stimulus at durations of 256 (no-gap), 255.5, 255, 254, etc. to 128 msec.
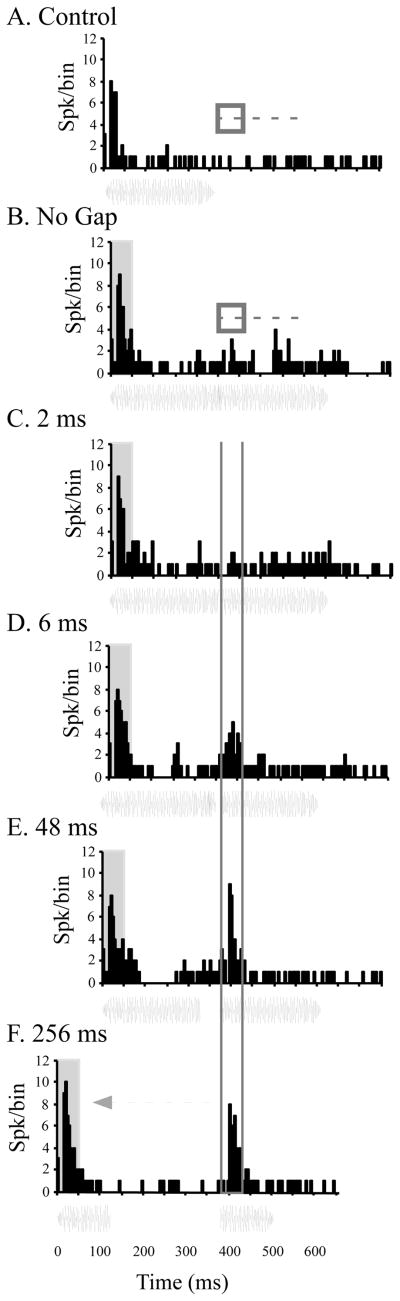
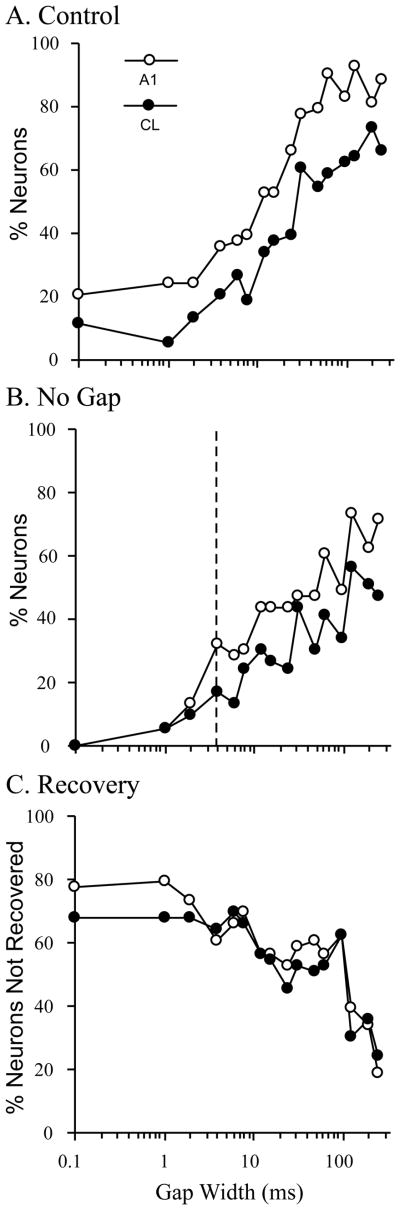
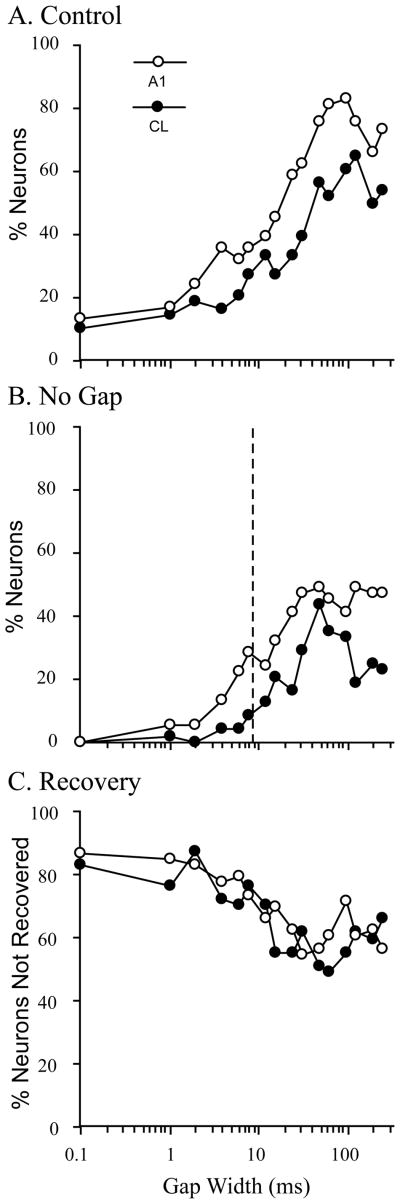
We recorded from 84 neurons in A1 and 60 neurons in CL from these two monkeys. These neurons were then classified into phasic or tonic responders based on the ratio of the response to the last 96 msec of the 192 msec control stimulus (late), divided by the response to the first 96 msec of the same stimulus (early). This resulted in 53 A1 neurons and 53 CL neurons that were considered ‘phasic’, where the late/early ratio was less than 0.50 (Recanzone, 2000). Figure 7A shows the response of a typical phasic neuron to the control stimulus and Figure 7B shows the response to the no-gap stimulus. The next four panels show the response to increasing gap sizes. As can be seen in this example, there was essentially no response after the gap for the 2-msec gap condition. However, there was a clear phasic response after gaps of 6 msec (within the thin vertical gray lines) that continued to grow in magnitude with increasing gap duration, but had not yet reached the same magnitude as the response to the onset of the stimulus even with a gap size of 256 msec. In order to test the temporal processing abilities of A1 and CL neurons, we compare how well these responses related to those to the control stimulus using an ROC analysis described previously (see Petkov et al., 2007; see also Sutter et al., this volume). This analysis takes into account the response variance between trials and gives an indication of whether an ideal observer could discriminate the gap from a continuous noise stimulus. If the responses to the two stimuli are equivalent, the ROC value is 0.50, and if the two stimuli are completely different the value goes to either 0.0 or 1.0. For comparisons between the post-gap onset and the equivalent period of time after the offset of the single control stimulus, we chose a threshold of 0.75 to indicate that the neuron could discriminate between the two stimuli. The results of this analysis are shown in Figure 8 for A1 (open symbols) and CL (closed symbols) neurons. Note the logarithmic x-axis. Panel A shows the results when comparing the post-gap response relative to the same time period following the control stimulus (which was limited in duration to the that of the pre-gap stimulus). It is not until gap sizes of 12 msec in A1 and 32 msec in CL until the ideal observer could discriminate this difference between the onset of the post-gap stimulus and silence based on the responses of half of the neurons. Panel B shows the comparison between the post-gap stimuli and the same time period when no gap is presented. The dashed vertical line indicates the gap detection thresholds for young monkeys using a broadband noise stimulus taken from Petkov et al. (2003). In this case, the population of neurons where the ideal observer could discriminate between the two conditions was not as great as when the gap and control stimuli were compared, but again neurons in A1 are much better at discriminating this difference across all gap widths compared to neurons in CL. Finally, we determined when the response to the post-gap stimulus was fully recovered. In this analysis, neurons that had a ROC value between 0.25 and 0.75 were considered similar in magnitude, i.e. the ideal observer could not discriminate between the initial onset of the stimulus and the post-gap response, and were therefore fully recovered. ROC values less than 0.25 would indicate that the response to the post-gap stimulus was different enough (lower) to be discriminated as different from the initial stimulus onset. Figure 8C shows the percentage of neurons that have not fully recovered as a function of the gap duration. Interestingly, A1 and CL neurons had similar recovery functions. The percent of cells that did recover was quite small compared to the psychophysical thresholds measured in different, younger monkeys studied previously (see Petkov et al. 2003; Sutter et al., this volume). Taken together, these three sets of analyses indicate that A1 neurons are better able to encode gap information across different gap widths. There were neurons encountered that were as good as or better than the psychophysically measured detection thresholds, but the vast majority in A1 and CL neurons were much poorer than the behavioral measure, which could account for decreased temporal processing abilities in the aged. For neurons that detected a gap, population analysis of neural gap thresholds revealed that CL neurons (mean rank = 33.50) had similar gap detection thresholds to A1 neurons (mean rank = 30.24) (Kruskal Wallis, χ2 = .48, df = 1, p >.05). Finally, in spite of the 256 msec gap, there was a significant proportion of neurons that were unable to encode any gap compared to the continuous stimulus, or a shorter stimulus where no stimulus was presented during the same time period.
Figure 7.
Representative example of a single neuron responding to the noise stimuli. Panel A shows the response to the control stimulus, which is just the pre-gap stimulus. Panel B shows the response to the full duration no-gap stimulus. Panels C – F show the response to stimuli with increasing gap sizes. Comparisons were made between the response to the 50 msec post-gap stimulus (vertical lines in panels C-F) and (i) the same period following the offset of the control stimulus. This varied with the gap duration that was being tested, and is represented by the box and dashed horizontal line in panel A. The post-gap stimulus response was also compared to (ii) the same period during the no-gap stimulus. This also varied with the gap duration that was being tested and is represented by the box and dashed line in panel B. Finally this post-gap stimulus response was compared to (iii) the onset of the gap stimulus, represented by the shaded region at the start of the PSTH in panels B-F. This neuron had no clear gap-response until 6 msec, but did not fully recover even with a gap size of 256 msec. PSTHs are offset in order to align the gap-response between panels B – F.
Figure 8.
Discriminability of the post-gap response. A. Percent neurons that the ROC analysis could reliably discriminate the difference between the gap stimulus and the control stimulus (ROC > 0.75). A1 neurons were much better at making this discrimination than CL neurons, where the ideal observer was able to make this discrimination on only about 60% of CL neurons. B. Analysis comparing the post-gap response to the no-gap response. Again, A1 neurons were better able to make this discrimination than CL neurons. The dashed vertical line shows the psychophysical threshold taken from a different set of monkeys (Petkov et al., 2003). C. Analysis comparing the response to the post-gap stimulus to the response to the stimulus onset. The y-axis shows the percent of neurons that were not fully recovered based on an ROC values between 0.25 and 0.75 (no difference between the gap response and the control response). A1 and CL neurons were equivalent in this recovery. Even at 256 msec gap durations, about 20% of neurons were not yet fully recovered (see Fig. 7).
We also made the same comparisons in these same neurons using a tone-in-noise stimulus (Figure 9). This allowed us to test the responses of these neurons to stimuli that had the most energy in the tone frequency but prevented the detection of spectral splatter at the onset and offset of the tone during the gap. Given that A1 neurons have a much better response to tone stimuli compared to noise stimuli in contrast to CL neurons (e.g. Recanzone et al., 2000a) it may be that recovery functions would occur earlier for these stimuli with narrower spectral bandwidth. In this case, the basic finding was the same, namely that A1 neurons were better able to encode the gap stimulus compared to CL neurons. For neurons that detected a gap, population analysis of neural gap thresholds revealed that A1 neurons (mean rank = 18.97) had significantly shorter gap detection thresholds than CL neurons (mean rank = 29.83) for these stimuli (Kruskal Wallis, χ2 = 6.48, df = 1, p <.01). There was also the same relatively low percentage of neurons that were as good as, or better, than the psychophysically determined thresholds, in this case a 2 kHz stimulus (Petkov et al., 2003). Finally, the percentage of neurons that fully recovered was also relatively small even at the largest gap durations.
Figure 9.
Discriminability of the post-gap response to tone-in-noise stimuli. The same analysis as shown in Figure 8 for noise stimuli is shown here for gaps in a 65 dB SPL tone stimulus at the CF of the neuron under study, embedded in a background of 45 dB SPL broadband noise stimuli. The basic features of these neuronal responses are similar to those for noise stimuli. Conventions as in Figure 8.
These results show that the ability of some auditory cortical neurons to encode gap stimuli in aged monkeys is similar to the gap detection thresholds noted in younger animals. In our hands, there was a small but significant fraction of neurons that could discriminate differences between the gap and no-gap stimuli, about 20 – 30%, near the psychophysically measured threshold of younger animals. However, very few of the neurons showed complete recovery, even out to gap widths of up to 256 msec, which is nearly an order of magnitude greater than the behavioral detection thresholds. A clear caveat is that the gap detection thresholds were not measured in these aged animals, nor were neural gap thresholds measured in younger animals, so it is not clear if these thresholds were compromised, as is commonly but not universally seen in humans (e.g. Schneider et al., 1994; Schneider and Hamstra, 1999; Snell and Frisina, 2000). One possibility is that the neural signal up to the level of A1 is relatively intact, yet the refinement of this signal at higher order cortical stations is not accomplished in older animals. A deficit in the serial cortical processing of sensory information in the aged would be consistent with a variety of perceptual deficits encountered in geriatric populations. Future studies will be necessary to tease out whether this type of recovery is consistent with the psychophysical results, and which aspect of the recovery functions are best correlated with the perception.
Summary
In summary, single neurons in the macaque auditory cortex carry little information with respect to the spatial location of a stimulus. However, the firing rates of populations of single neurons do carry that information for contralateral space, with neurons in the cortical field CL containing enough information in their firing rate to account for sound localization performance in human subjects. Presumably this information is passed onward through the spatial processing regions of the macaque, which can ultimately lead to the animal utilizing this information in order to make a behavioral response. The ability of auditory cortical neurons to encode different gap stimuli in their firing rate is better in A1 than in CL, however the recovery of the response to the gap in aged animals is much longer than the perceptual threshold seen in younger animals. It remains to be seen which aspects of the neural responses are best correlated with the detection of the gap.
Acknowledgments
The authors would like to thank the California National Primate Research Center, R. Oates-O’Brien, and G. Martin for excellent animal care. Funding provided by NIH grants AG024372, DC-02371 and DC-00442.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Altshuler MW, Comalli PE. Effect of stimulus intensity and frequency on median horizontal plane sound localization. J Auditory Res. 1975;15:262–265. [Google Scholar]
- Beitel RE, Kaas JH. Effects of bilateral and unilateral ablation of auditory cortex in cats on the unconditioned head orienting response to acoustic stimuli. J Neurophysiol. 1993;70:351–369. doi: 10.1152/jn.1993.70.1.351. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. Neural response properties of primary, rostral, and rostrotemporal core fields in the auditory cortex of marmoset monkeys. J Neurophysiol. 2008;100:888–906. doi: 10.1152/jn.00884.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Davis RT, Miller JM. Demonstration of presbycusis across repeated measures in a nonhuman primate species. Behav Neurosci. 1983;97:602–607. doi: 10.1037//0735-7044.97.4.602. [DOI] [PubMed] [Google Scholar]
- Blauert J. The Psychophysics of Human Sound Localization. 2. MIT Press; 1997. Spatial Hearing. [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol. 1995;30:349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age- related changes in GABA-A receptor subunit composition and function in rat auditory system. Neurosci. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Comalli PE, Altshuler MW. Effect of stimulus intensity, frequency, and unilateral hearing loss on sound localization. J Auditory Res. 1976;16:275–279. [Google Scholar]
- Eisenman LM. Neural encoding of sound location: An electrophysiological study in auditory cortex (AI) of the cat using free field stimuli. Brain Res. 1974;75:203–214. doi: 10.1016/0006-8993(74)90742-2. [DOI] [PubMed] [Google Scholar]
- Fowler CG, Torre P, Kemnitz JW. Effects of caloric restriction and aging on the auditory function of rhesus monkeys (Macaca mulatta): the University of Wisconsin study. Hear Res. 2002;169:24–35. doi: 10.1016/s0378-5955(02)00335-0. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res l. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Xu L, Middlebrooks JC. Coding of sound-source location by enxembles of cortical neurons. J Neurosci. 2000;20:1216–1228. doi: 10.1523/JNEUROSCI.20-03-01216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Sources of age-related recognition difficulty for time- compressed speech. J Speech Lang Hear Res. 2001;44:709–719. doi: 10.1044/1092-4388(2001/056). [DOI] [PubMed] [Google Scholar]
- Hackett TA, Preuss TM, Kaas JH. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol. 2001;441:197–222. doi: 10.1002/cne.1407. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Miller JM, Rouse RC, Davis JA, Rarey K. Inner ear histopathology in aging rhesus monkeys (Macaca mulatta) In: Davis RT, Leathers CW, editors. Behavior and Pathology of Aging in Rhesus Monkeys. Alan R. Liss; New York: 1985. pp. 137–154. [Google Scholar]
- Heffner HE, Heffner RS. Effects of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Irons WA, Samson FR. Single-unit selectivity to azimuthal direction and sound pressure level of noise bursts in cat high-frequency primary auditory cortex. J Neurophysiol. 1990;63:1448–66. doi: 10.1152/jn.1990.63.6.1448. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, Movshon JA. Optimal representation of sensory information by neuronal populations. Nat Neurosci. 2006;5:690–696. doi: 10.1038/nn1691. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- Jennings CR, Jones NS. Presbyacusis. J Laryngol & Otol. 2001;115:1710178. doi: 10.1258/0022215011906984. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–9. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazee AM, West NR. Preservation of synapses on principal cells of the central nucleus of the inferior colliculus with aging in the CBA mouse. Hear Res. 1999;133:98–106. doi: 10.1016/s0378-5955(99)00058-1. [DOI] [PubMed] [Google Scholar]
- Kiani R, Esteky H, Mirpour K, Tanaka K. Object category structure in response patterns of neuronal population in monkey inferior temporal cortex. J Neurophysiol. 2007;97:4296–4309. doi: 10.1152/jn.00024.2007. [DOI] [PubMed] [Google Scholar]
- King AJ, Hutchings ME. Spatial response properties of acoustically responsive neurons in the inferior colliculus of the ferret: a map of auditory space. J Neurophysiol. 1987;57:596–624. doi: 10.1152/jn.1987.57.2.596. [DOI] [PubMed] [Google Scholar]
- Mazelová J, Popelar J, Kyka J. Auditory function in presbycusis: peripheral vs. central changes Exp Gerentol. 2003;38:87–94. doi: 10.1016/s0531-5565(02)00155-9. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Pettigrew JD. Functional classes of neurons in primary auditory cortex of the cat distinguished by sensitivity to sound location. J Neurosci. 1981;1:107–20. doi: 10.1523/JNEUROSCI.01-01-00107.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Knudsen EI. A neural code for auditory space in the cat’s superior colliculus. J Neurosci. 1984;4:2621–2634. doi: 10.1523/JNEUROSCI.04-10-02621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Recanzone GH. Populations of auditory cortical neurons can accurately encode acoustic space across stimulus intensity. Proc Natl Acad Sci U S A. 2009;106:5931–5. doi: 10.1073/pnas.0901023106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, King AJ, Schnupp JWH. Encoding of virtual acoustic space stimuli by neurons in ferret primary auditory cortex. J Neurophysiol. 2005;93:3489–3503. doi: 10.1152/jn.00748.2004. [DOI] [PubMed] [Google Scholar]
- Nelson EG, Hinojosa R. Presbycusis: A human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Larygoscope. 2006;116(Suppl 112):1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Age-related hearing loss: the status of Schuknecht’s typology. Curr Opin Otolaryngol Head Neck Surg. 2004;12:439–443. doi: 10.1097/01.moo.0000134450.99615.22. [DOI] [PubMed] [Google Scholar]
- Palmer AR, King AJ. The representation of auditory space in the mammalian superior colliculus. Nature. 1982;299:248–249. doi: 10.1038/299248a0. [DOI] [PubMed] [Google Scholar]
- Petkov CI, O’Connor KN, Sutter ML. Illusory sound perception in macaque monkeys. J Neurosci. 2003;8:9155–9161. doi: 10.1523/JNEUROSCI.23-27-09155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, O’Connor KN, Sutter ML. Encoding of illusory continuity in primate auditory cortex. Neuron. 2007;54:153–165. doi: 10.1016/j.neuron.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R, Aitkin LM, Irvine DRF, McKay J. Azimuthal sensitivity of neurons in primary auditory cortex of cats. I Types of sensitivity and the effects of variations in stimulus parameters J Neurophysiol. 1990a;64:872–887. doi: 10.1152/jn.1990.64.3.872. [DOI] [PubMed] [Google Scholar]
- Rajan R, Aitkin LM, Irvine DR. Azimuthal sensitivity of neurons in primary auditory cortex of cats. II Organization along frequency-band strips J Neurophysiol. 1990b;64:888–902. doi: 10.1152/jn.1990.64.3.888. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–4. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Pons T, Mishkin M. Serial and parallel processing in rhesus monkey auditory cortex. J Comp Neurol. 1997;382:89–103. [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–6. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Processing of band-passed noise in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;91:2578–2589. doi: 10.1152/jn.00834.2003. [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 2000;150:104–118. doi: 10.1016/s0378-5955(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, Phan ML. Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. J Neurophysiol. 2000a;83:2315–2331. doi: 10.1152/jn.2000.83.4.2315. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, Phan ML, Su TK. Correlation between the activity of single auditory cortical neurons and sound localization behavior in the macaque monkey. J Neurophysiol. 2000b;83:2723–2739. doi: 10.1152/jn.2000.83.5.2723. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Beckerman NS. Effects of intensity and location on sound location discrimination in macaque monkeys. Hear Res. 2004;198:116–124. doi: 10.1016/j.heares.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Representation of con-specific vocalizations in the core and belt areas of the auditory cortex in the alert macaque monkey. J Neurosci. 2008;28:13184–13193. doi: 10.1523/JNEUROSCI.3619-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–6. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AT, Macpherson EA, Middlebrooks JC. Human sound localization at near-threshold levels. Hear Res. 2005;199:124–134. doi: 10.1016/j.heares.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK, Kowalchuk D, Lamb M. Gap detection and the precendence effect in young and old adults. J Acoust Soc Amer. 1994;95:980–991. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Hamstra SJ. Gap detection thresholds as a function of tonal duration for younger and older listeners. J Acoust Soc Amer. 1999;106:371–380. doi: 10.1121/1.427062. [DOI] [PubMed] [Google Scholar]
- Schwartz IR, Keh A, Hsu G. Morphology, GluR1 and GRIP-C loclaization differ in octopus cells of C57BL6 and B6Cast mice. Hear Res. 2002;171:1–12. doi: 10.1016/s0378-5955(01)00396-3. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Amer. 2000;107:1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Snell KB, Mapes FM, Hickman ED, Frisina DR. Word recognition in competing babble and the effects of age, temporal processing, and absolute sensitiivty. J Acoust Soc Amer. 2002;112:720–727. doi: 10.1121/1.1487841. [DOI] [PubMed] [Google Scholar]
- Stecker GC, Harrington IA, Middlebrooks JC. Location coding by opponent neural populations in the auditory cortex. PLos Biol. 2005;3:520–528. doi: 10.1371/journal.pbio.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Amer. 1998;104:2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Su TK, Recanzone GH. Differential effect of near-threshold stimulus intensities on sound localization performance in azimuth and elevation in normal human subjects. J Assoc Res Otolaryngol. 2001;2:246–256. doi: 10.1007/s101620010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;92:2993–3013. doi: 10.1152/jn.00472.2003. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–3. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Versfeld NJ, Dreschler WA. The relationship between the intelligibility of time-compressed speech and speech in noise in young and elderly listeners. J Acoust Soc Amer. 2002;111:401–408. doi: 10.1121/1.1426376. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisnia RD, Ison JR, O’Neil WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol [A] 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, O’Neil WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Reiss U, Groh JM. A rate code for sound azimuth in monkey auditory cortex: implications for human neuroimaging studies. J Neurosci. 2009;28:3747–3758. doi: 10.1523/JNEUROSCI.5044-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Turner JG, Carlson S, Ding D, Broos LS, Falls WA. The BALB/c mouse as an animal model for progressive sensorineural hearing loss. Hear Res. 1998;115:162–174. doi: 10.1016/s0378-5955(97)00189-5. [DOI] [PubMed] [Google Scholar]
- Woods TM, Lopez SE, Long JH, Rahman JE, Recanzone GH. Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey. J Neurophysiol. 2006;96:3323–37. doi: 10.1152/jn.00392.2006. [DOI] [PubMed] [Google Scholar]
- Wiley TL, Cruickshanks KJ, Nondahl DM, Tweed TS, Klein R, Klein BEK. Aging and high-frequency hearing sensitivity. J Speech Lang Hear Res. 1998;41:1061–1072. doi: 10.1044/jslhr.4105.1061. [DOI] [PubMed] [Google Scholar]