Abstract
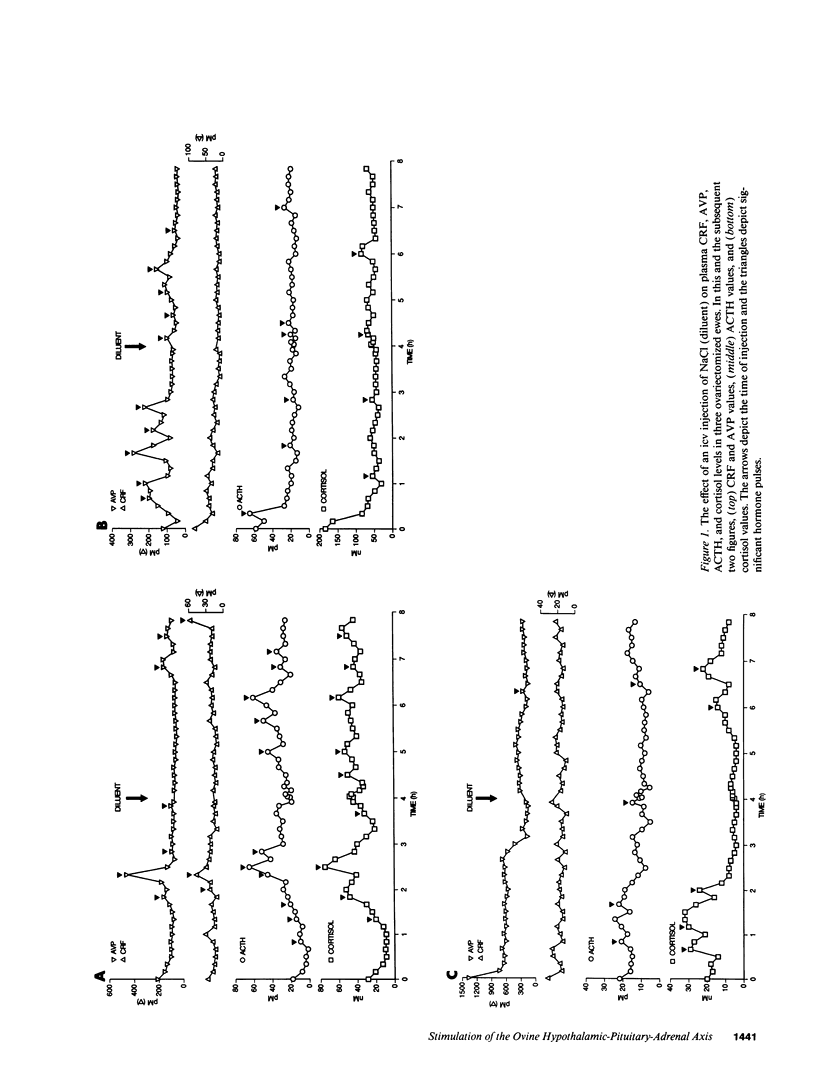
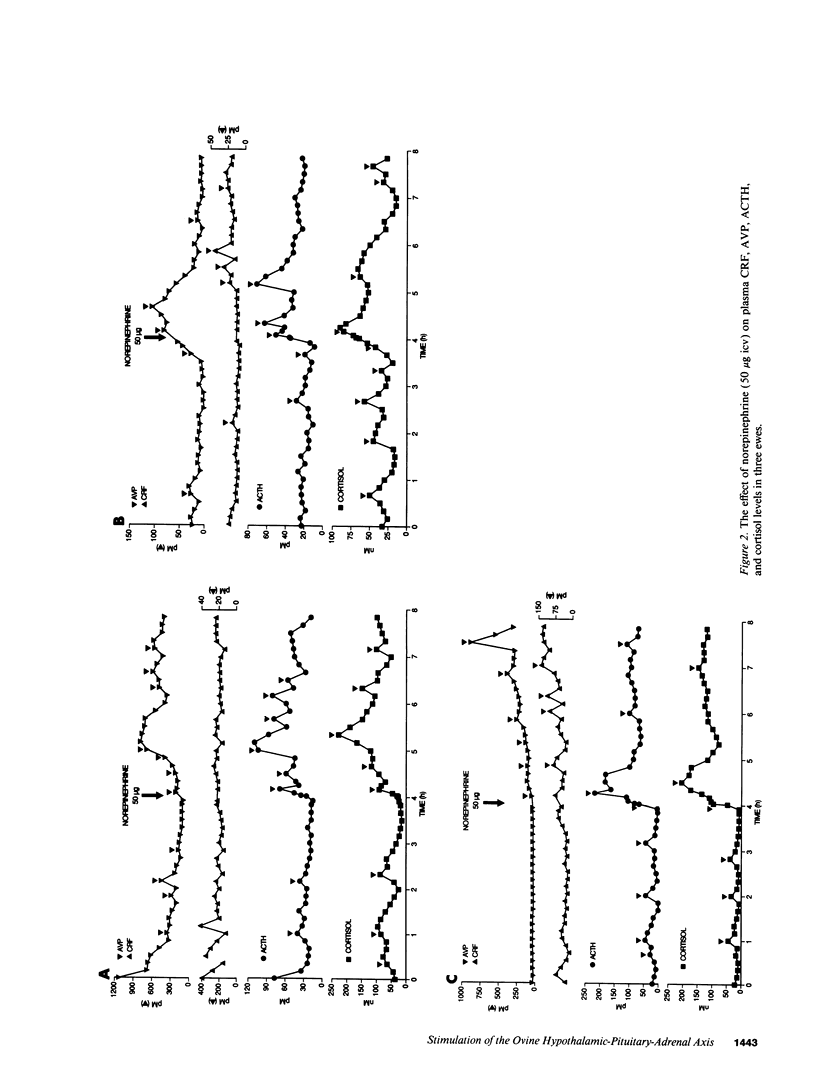
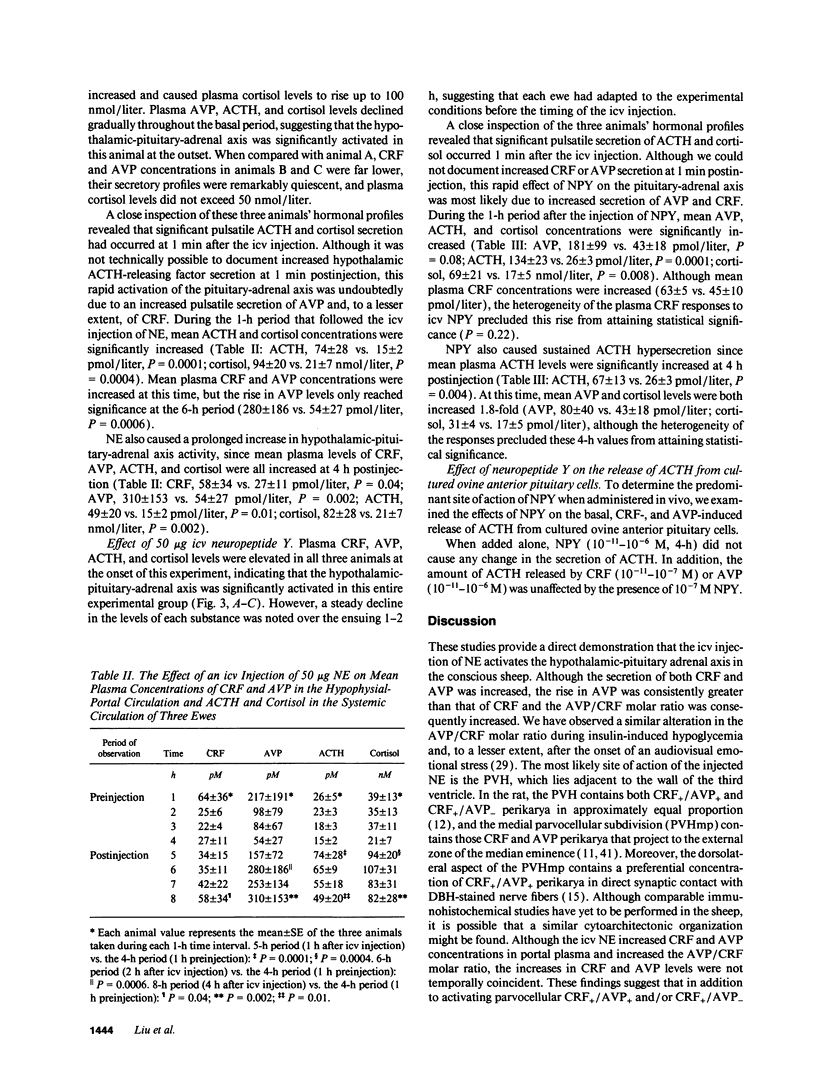
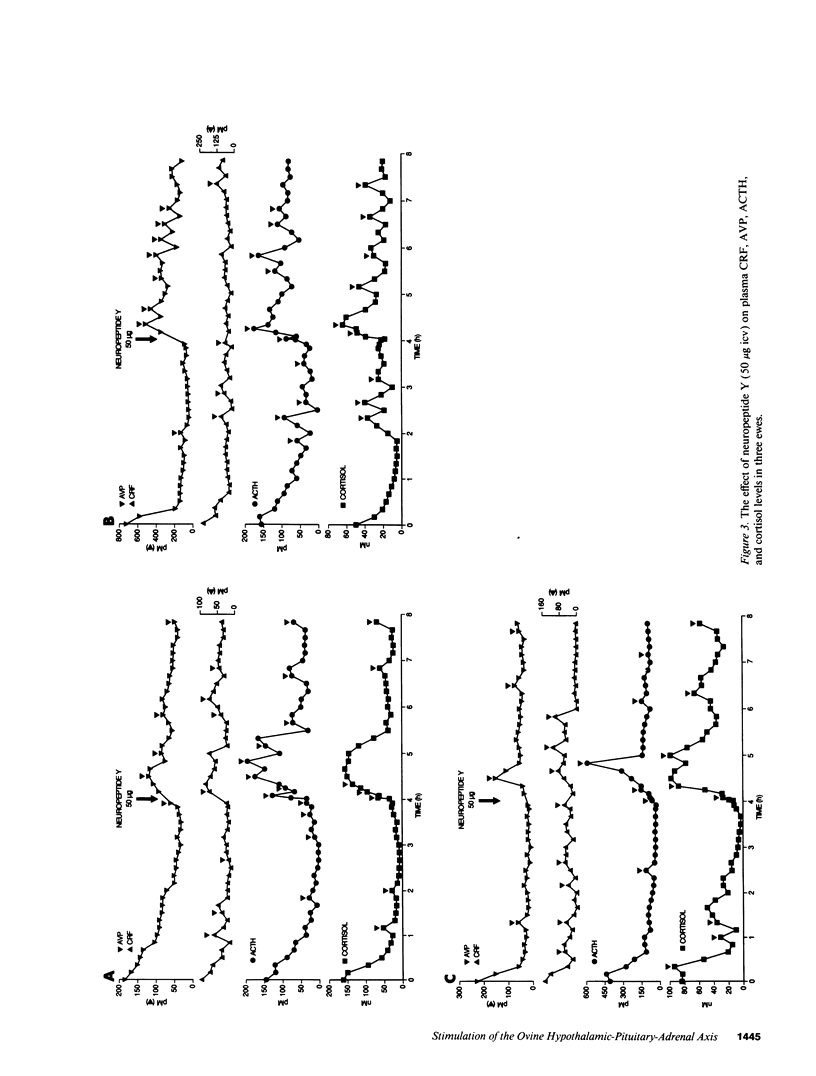
Studies were performed to determine the effects of intracerebroventricular norepinephrine (NE) or neuropeptide Y (NPY) on the ovine hypothalamic-pituitary-adrenal (HPA) axis. NE (50 micrograms) increased mean hypophysial-portal corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) levels (1 h, 1.3- and 2.9-fold; 4 h, 2.2- and 5.7-fold) and caused acute and sustained increases in mean plasma ACTH and cortisol. NPY (50 microgram) also increased mean CRF and AVP levels (1 h, 1.4- and 4.2-fold; 4 h, 1.1- and 1.9-fold), increased pituitary-adrenal activity at 1 h, and caused ACTH hypersecretion at 4 h. When added to cultured ovine anterior pituitary cells, NPY neither increased basal ACTH release nor augmented CRF- or AVP-induced ACTH release. We conclude that: (a) activation of either the central noradrenergic or NPY pathways causes an acute and sustained stimulation of the ovine HPA axis; (b) such activation increases the AVP/CRF ratio, suggesting a dominant role for AVP in the ovine stress response; and (c) the central noradrenergic or NPY systems may cause sustained HPA activation by attenuating or disrupting the glucocorticoid negative feedback on those brain areas concerned with regulation of the HPA axis. The possible roles of the central noradrenergic and NPY systems in the etiology of the hypercortisolemia of endogenous depression and anorexia nervosa are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Allen J. M., Bloom S. R., Ghatei M. A., Rossor M. N., Roberts G. W., Crow T. J., Tatemoto K., Polak J. M. Neuropeptide Y distribution in human brain. Nature. 1983 Dec 8;306(5943):584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Yu Z. Y., Härfstrand A., Okret S., Wikström A. C., Goldstein M., Zoli M., Vale W., Gustafsson J. A. Morphometrical analysis of the distribution of corticotrophin releasing factor, glucocorticoid receptor and phenylethanolamine-N-methyltransferase immunoreactive structures in the paraventricular hypothalamic nucleus of the rat. Neurosci Lett. 1985 Mar 15;54(2-3):147–152. doi: 10.1016/s0304-3940(85)80070-7. [DOI] [PubMed] [Google Scholar]
- Akana S. F., Dallman M. F., Bradbury M. J., Scribner K. A., Strack A. M., Walker C. D. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992 Jul;131(1):57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- Allen Y. S., Adrian T. E., Allen J. M., Tatemoto K., Crow T. J., Bloom S. R., Polak J. M. Neuropeptide Y distribution in the rat brain. Science. 1983 Aug 26;221(4613):877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Antoni F. A., Fink G., Sheward W. J. Corticotrophin-releasing peptides in rat hypophysial portal blood after paraventricular lesions: a marked reduction in the concentration of corticotrophin-releasing factor-41, but no change in vasopressin. J Endocrinol. 1990 May;125(2):175–183. doi: 10.1677/joe.0.1250175. [DOI] [PubMed] [Google Scholar]
- Antoni F. A. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986 Nov;7(4):351–378. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- Autelitano D. J., Blum M., Lopingco M., Allen R. G., Roberts J. L. Corticotropin-releasing factor differentially regulates anterior and intermediate pituitary lobe proopiomelanocortin gene transcription, nuclear precursor RNA and mature mRNA in vivo. Neuroendocrinology. 1990 Feb;51(2):123–130. doi: 10.1159/000125327. [DOI] [PubMed] [Google Scholar]
- Bai F. L., Yamano M., Shiotani Y., Emson P. C., Smith A. D., Powell J. F., Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res. 1985 Apr 1;331(1):172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- Bruhn T. O., Sutton R. E., Rivier C. L., Vale W. W. Corticotropin-releasing factor regulates proopiomelanocortin messenger ribonucleic acid levels in vivo. Neuroendocrinology. 1984 Aug;39(2):170–175. doi: 10.1159/000123974. [DOI] [PubMed] [Google Scholar]
- Buma P., Nieuwenhuys R. Ultrastructural characterization of exocytotic release sites in different layers of the median eminence of the rat. Cell Tissue Res. 1988 Apr;252(1):107–114. doi: 10.1007/BF00213831. [DOI] [PubMed] [Google Scholar]
- Burger H. G., Lee V. W., Rennie G. C. A generalized computer program for the treatment of data from competitive protein-binding assays including radioimmunoassays. J Lab Clin Med. 1972 Aug;80(2):302–312. [PubMed] [Google Scholar]
- Butler P. D., Weiss J. M., Stout J. C., Nemeroff C. B. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990 Jan;10(1):176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero A. E., Gallucci W. T., Chrousos G. P., Gold P. W. Catecholamine effects upon rat hypothalamic corticotropin-releasing hormone secretion in vitro. J Clin Invest. 1988 Sep;82(3):839–846. doi: 10.1172/JCI113687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A., Grino M., Locatelli A., Guillaume V., Boudouresque F., Conte-Devolx B., Oliver C. Insulin-induced hypoglycemia stimulates corticotropin-releasing factor and arginine vasopressin secretion into hypophysial portal blood of conscious, unrestrained rams. J Clin Invest. 1990 Jun;85(6):1716–1721. doi: 10.1172/JCI114626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A., Grino M., Locatelli A., Oliver C. Secretion of corticotropin releasing factor (CRF) and vasopressin (AVP) into the hypophysial portal blood of conscious, unrestrained rams. Biochem Biophys Res Commun. 1988 Sep 15;155(2):841–849. doi: 10.1016/s0006-291x(88)80572-2. [DOI] [PubMed] [Google Scholar]
- Carroll B. J., Feinberg M., Greden J. F., Tarika J., Albala A. A., Haskett R. F., James N. M., Kronfol Z., Lohr N., Steiner M. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry. 1981 Jan;38(1):15–22. doi: 10.1001/archpsyc.1981.01780260017001. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M., DiMaggio D. A., Massari V. J., Pickel V. M., Ruggiero D. A., O'Donohue T. L. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985 Aug;15(4):1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- Clarke I. J., Cummins J. T. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982 Nov;111(5):1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- Emanuel R. L., Girard D. M., Thull D. L., Majzoub J. A. Second messengers involved in the regulation of corticotropin-releasing hormone mRNA and peptide in cultured rat fetal hypothalamic primary cultures. Endocrinology. 1990 Jun;126(6):3016–3021. doi: 10.1210/endo-126-6-3016. [DOI] [PubMed] [Google Scholar]
- Engler D., Pham T., Fullerton M. J., Clarke I. J., Funder J. W. Evidence for an ultradian secretion of adrenocorticotropin, beta-endorphin and alpha-melanocyte-stimulating hormone by the ovine anterior and intermediate pituitary. Neuroendocrinology. 1989 Apr;49(4):349–360. doi: 10.1159/000125139. [DOI] [PubMed] [Google Scholar]
- Engler D., Pham T., Fullerton M. J., Ooi G., Funder J. W., Clarke I. J. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. I. Effect of an audiovisual stimulus and insulin-induced hypoglycemia. Neuroendocrinology. 1989 Apr;49(4):367–381. doi: 10.1159/000125141. [DOI] [PubMed] [Google Scholar]
- Esler M., Jennings G., Lambert G., Meredith I., Horne M., Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990 Oct;70(4):963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Hökfelt T., Terenius L., Tatemoto K., Mutt V., Goldstein M. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984 Feb;11(2):443–462. doi: 10.1016/0306-4522(84)90036-8. [DOI] [PubMed] [Google Scholar]
- Familari M., Smith A. I., Smith R., Funder J. W. Arginine vasopressin is a much more potent stimulus to ACTH release from ovine anterior pituitary cells than ovine corticotropin-releasing factor. 1. In vitro studies. Neuroendocrinology. 1989 Aug;50(2):152–157. doi: 10.1159/000125214. [DOI] [PubMed] [Google Scholar]
- Gehlert D. R., Chronwall B. M., Schafer M. P., O'Donohue T. L. Localization of neuropeptide Y messenger ribonucleic acid in rat and mouse brain by in situ hybridization. Synapse. 1987;1(1):25–31. doi: 10.1002/syn.890010106. [DOI] [PubMed] [Google Scholar]
- Gibbs D. M., Vale W. Presence of corticotropin releasing factor-like immunoreactivity in hypophysial portal blood. Endocrinology. 1982 Oct;111(4):1418–1420. doi: 10.1210/endo-111-4-1418. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Loriaux D. L., Roy A., Kling M. A., Calabrese J. R., Kellner C. H., Nieman L. K., Post R. M., Pickar D., Gallucci W. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986 May 22;314(21):1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- Hotta M., Shibasaki T., Masuda A., Imaki T., Demura H., Ling N., Shizume K. The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. J Clin Endocrinol Metab. 1986 Feb;62(2):319–324. doi: 10.1210/jcem-62-2-319. [DOI] [PubMed] [Google Scholar]
- Inui A., Inoue T., Nakajima M., Okita M., Sakatani N., Okimura Y., Chihara K., Baba S. Brain neuropeptide Y in the control of adrenocorticotropic hormone secretion in the dog. Brain Res. 1990 Mar 5;510(2):211–215. doi: 10.1016/0006-8993(90)91369-r. [DOI] [PubMed] [Google Scholar]
- Kalra S. P., Dube M. G., Sahu A., Phelps C. P., Kalra P. S. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W. H., Berrettini W., Gwirtsman H., George D. T. Altered cerebrospinal fluid neuropeptide Y and peptide YY immunoreactivity in anorexia and bulimia nervosa. Arch Gen Psychiatry. 1990 Jun;47(6):548–556. doi: 10.1001/archpsyc.1990.01810180048008. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M. E., Dallman M. F. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984 Winter;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Liposits Z., Phelix C., Paull W. K. Adrenergic innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A combined light and electron microscopic immunocytochemical study. Histochemistry. 1986;84(3):201–205. doi: 10.1007/BF00495783. [DOI] [PubMed] [Google Scholar]
- Liposits Z., Sievers L., Paull W. K. Neuropeptide-Y and ACTH-immunoreactive innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamus of the rat. An immunocytochemical analysis at the light and electron microscopic levels. Histochemistry. 1988;88(3-6):227–234. doi: 10.1007/BF00570278. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Clarke I. J., Funder J. W., Engler D. Evidence that the central noradrenergic and adrenergic pathways activate the hypothalamic-pituitary-adrenal axis in the sheep. Endocrinology. 1991 Jul;129(1):200–209. doi: 10.1210/endo-129-1-200. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Robinson P. J., Funder J. W., Engler D. The biosynthesis and secretion of adrenocorticotropin by the ovine anterior pituitary is predominantly regulated by arginine vasopressin (AVP). Evidence that protein kinase C mediates the action of AVP. J Biol Chem. 1990 Aug 25;265(24):14136–14142. [PubMed] [Google Scholar]
- Mizuno Y., Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984 Jul 30;307(1-2):109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- Morley J. E., Levine A. S., Gosnell B. A., Kneip J., Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol. 1987 Mar;252(3 Pt 2):R599–R609. doi: 10.1152/ajpregu.1987.252.3.R599. [DOI] [PubMed] [Google Scholar]
- Mortola J. F., Liu J. H., Gillin J. C., Rasmussen D. D., Yen S. S. Pulsatile rhythms of adrenocorticotropin (ACTH) and cortisol in women with endogenous depression: evidence for increased ACTH pulse frequency. J Clin Endocrinol Metab. 1987 Nov;65(5):962–968. doi: 10.1210/jcem-65-5-962. [DOI] [PubMed] [Google Scholar]
- Ono T., Nishino H., Fukuda M., Sasaki K., Muramoto K., Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res. 1982 Jan 28;232(2):494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ooyama H., Sugimori M., Nakamura T., Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974 Feb 1;247(5439):284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Plotsky P. M., Bruhn T. O., Vale W. Hypophysiotropic regulation of adrenocorticotropin secretion in response to insulin-induced hypoglycemia. Endocrinology. 1985 Jul;117(1):323–329. doi: 10.1210/endo-117-1-323. [DOI] [PubMed] [Google Scholar]
- Plotsky P. M. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987 Sep;121(3):924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- Pradier P., Davicco M. J., Safwate A., Tournaire C., Dalle M., Barlet J. P., Delost P. Plasma adrenocorticotrophin, cortisol and aldosterone responses to ovine corticotrophin-releasing factor and vasopressin in sheep. Acta Endocrinol (Copenh) 1986 Jan;111(1):93–100. doi: 10.1530/acta.0.1110093. [DOI] [PubMed] [Google Scholar]
- Recht L. D., Hoffman D. L., Haldar J., Silverman A. J., Zimmerman E. A. Vasopressin concentrations in hypophysial portal plasma: insignificant reduction following removal of the posterior pituitary gland. Neuroendocrinology. 1981 Aug;33(2):88–90. doi: 10.1159/000123208. [DOI] [PubMed] [Google Scholar]
- Redecker P. Ultrastructural demonstration of neurohaemal contacts in the internal zone of the median eminence of the Mongolian gerbil (Meriones unguiculatus): correlation with synaptophysin immunohistochemistry. Histochemistry. 1991;95(5):503–511. doi: 10.1007/BF00315747. [DOI] [PubMed] [Google Scholar]
- Reincke M., Allolio B., Würth G., Winkelmann W. The hypothalamic-pituitary-adrenal axis in critical illness: response to dexamethasone and corticotropin-releasing hormone. J Clin Endocrinol Metab. 1993 Jul;77(1):151–156. doi: 10.1210/jcem.77.1.8392081. [DOI] [PubMed] [Google Scholar]
- Roy A., Pickar D., De Jong J., Karoum F., Linnoila M. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine. Relationship to hypothalamic-pituitary-adrenal axis function in depression. Arch Gen Psychiatry. 1988 Sep;45(9):849–857. doi: 10.1001/archpsyc.1988.01800330081010. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Plotsky P. M. Hypercortisolism and its possible neural bases. Biol Psychiatry. 1990 May 1;27(9):937–952. doi: 10.1016/0006-3223(90)90032-w. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Grzanna R., Howe P. R., Bloom S. R., Polak J. M. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985 Nov 8;241(2):138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982 Nov;257(3):275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Vale W. Dissociation of the adrenocorticotropin secretory responses to corticotropin-releasing factor (CRF) and vasopressin or oxytocin by using a specific cytotoxic analog of CRF. Endocrinology. 1988 Apr;122(4):1695–1700. doi: 10.1210/endo-122-4-1695. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Smythe G. A., Grunstein H. S., Bradshaw J. E., Nicholson M. V., Compton P. J. Relationships between brain noradrenergic activity and blood glucose. Nature. 1984 Mar 1;308(5954):65–67. doi: 10.1038/308065a0. [DOI] [PubMed] [Google Scholar]
- Stanley B. G., Leibowitz S. F. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984 Dec 24;35(26):2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- Suda T., Tozawa F., Yamada M., Ushiyama T., Tomori N., Sumitomo T., Nakagami Y., Demura H., Shizume K. Insulin-induced hypoglycemia increases corticotropin-releasing factor messenger ribonucleic acid levels in rat hypothalamus. Endocrinology. 1988 Sep;123(3):1371–1375. doi: 10.1210/endo-123-3-1371. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A., Guillaume V., Conte-Devolx B., Alonso G., Malaval F., Pares-Herbuté N., Oliver C., Assenmacher I. Central catecholaminergic system stimulates secretion of CRH at different sites. Am J Physiol. 1988 Oct;255(4 Pt 1):E463–E468. doi: 10.1152/ajpendo.1988.255.4.E463. [DOI] [PubMed] [Google Scholar]
- Tannahill L. A., Dow R. C., Fairhall K. M., Robinson I. C., Fink G. Comparison of adrenocorticotropin control in Brattleboro, Long-Evans, and Wistar rats. Measurement of corticotropin-releasing factor, arginine vasopressin, and oxytocin in hypophysial portal blood. Neuroendocrinology. 1988 Dec;48(6):650–657. doi: 10.1159/000125077. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Carlquist M., Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982 Apr 15;296(5858):659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Tillet Y. Adrenergic neurons in sheep brain demonstrated by immunohistochemistry with antibodies to phenylethanolamine N-methyltransferase (PNMT) and dopamine-beta-hydroxylase (DBH): absence of the C1 cell group in the sheep brain. Neurosci Lett. 1988 Dec 19;95(1-3):107–112. doi: 10.1016/0304-3940(88)90641-6. [DOI] [PubMed] [Google Scholar]
- Tsagarakis S., Holly J. M., Rees L. H., Besser G. M., Grossman A. Acetylcholine and norepinephrine stimulate the release of corticotropin-releasing factor-41 from the rat hypothalamus in vitro. Endocrinology. 1988 Oct;123(4):1962–1969. doi: 10.1210/endo-123-4-1962. [DOI] [PubMed] [Google Scholar]
- Ur E., Dinan T. G., O'Keane V., Clare A. W., McLoughlin L., Rees L. H., Turner T. H., Grossman A., Besser G. M. Effect of metyrapone on the pituitary-adrenal axis in depression: relation to dexamethasone suppressor status. Neuroendocrinology. 1992 Oct;56(4):533–538. doi: 10.1159/000126271. [DOI] [PubMed] [Google Scholar]
- Vale W., Vaughan J., Smith M., Yamamoto G., Rivier J., Rivier C. Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, catecholamines, neurohypophysial peptides, and other substances on cultured corticotropic cells. Endocrinology. 1983 Sep;113(3):1121–1131. doi: 10.1210/endo-113-3-1121. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Foote S. L., Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983 Jul 4;270(2):363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K., De Mey J. The origin of the vasopressinergic and oxytocinergic fibres of the external region of the median eminence of the rat hypophysis. Cell Tissue Res. 1977 Jun 13;180(4):443–452. doi: 10.1007/BF00220167. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Skagerberg G., Ekman R., Heilig M., Sundler F., Håkanson R. Neuropeptide Y (NPY) in the area of the hypothalamic paraventricular nucleus activates the pituitary-adrenocortical axis in the rat. Brain Res. 1987 Aug 4;417(1):33–38. doi: 10.1016/0006-8993(87)90176-4. [DOI] [PubMed] [Google Scholar]
- Walsh B. T., Katz J. L., Levin J., Kream J., Fukushima D. K., Weiner H., Zumoff B. The production rate of cortisol declines during recovery from anorexia nervosa. J Clin Endocrinol Metab. 1981 Jul;53(1):203–205. doi: 10.1210/jcem-53-1-203. [DOI] [PubMed] [Google Scholar]
- Whitnall M. H. Distributions of pro-vasopressin expressing and pro-vasopressin deficient CRH neurons in the paraventricular hypothalamic nucleus of colchicine-treated normal and adrenalectomized rats. J Comp Neurol. 1988 Sep 1;275(1):13–28. doi: 10.1002/cne.902750103. [DOI] [PubMed] [Google Scholar]
- Widmaier E. P., Lim A. T., Vale W. Secretion of corticotropin-releasing factor from cultured rat hypothalamic cells: effects of catecholamines. Endocrinology. 1989 Feb;124(2):583–590. doi: 10.1210/endo-124-2-583. [DOI] [PubMed] [Google Scholar]
- Widmaier E. P., Plotsky P. M., Sutton S. W., Vale W. W. Regulation of corticotropin-releasing factor secretion in vitro by glucose. Am J Physiol. 1988 Sep;255(3 Pt 1):E287–E292. doi: 10.1152/ajpendo.1988.255.3.E287. [DOI] [PubMed] [Google Scholar]


