Abstract
As axons myelinate, establish a stable neurofilament network, and expand in caliber, neurofilament proteins are extensively phosphorylated along their C-terminal tails, which is recognized by the monoclonal antibody, RT-97. Here, we demonstrate in vivo that RT-97 immunureactivity is generated by phosphorylation at KSPXK or KSPXXXK motifs and requires flanking lysines at specific positions. ERK1,2 and pERK1,2 levels increase in parallel with phosphorylation at the RT-97 epitope during early post-natal brain development. Purified ERK1,2 generated RT-97 on both KSP motifs on recombinant NF-H tail domain proteins, while cdk5 phosphorylated only KSPXK motifs. RT-97 epitope generation in primary hippocampal neurons was regulated by extensive crosstalk among ERK1,2, JNK1,2 and cdk5. Inhibition of both ERK1,2 and JNK1,2 completely blocked RT-97 generation. Cdk5 influenced RT-97 generation indirectly by modulating JNK activation. In mice, cdk5 gene deletion did not significantly alter RT-97 IR or ERK1,2 and JNK activation. In mice lacking the cdk5 activator P35, the partial suppression of cdk5 activity increased RT-97 IR by activating ERK1,2. Thus, cdk5 influences RT-97 epitope generation partly by modulating ERKs and JNKs, which are the two principal kinases regulating neurofilament phosphorylation. The regulation of a single target by multiple protein kinases underscores the importance of monitoring other relevant kinases when the activity of a particular one is blocked.
Keywords: phosphorylation, neurofilament, kinases, RT-97 epitope, crosstalk
Introduction
Neurofilaments (NF), the major cytoskeletal component of large myelinated axons, are heteropolymers that vary in subunit composition depending on neuron type and stage of development. In the mature CNS, neurofilaments contain four subunits, NF- H, NF- M, NF- L and α-internexin (Yuan et al. 2006) while, in the PNS, NF-H, NF-M and NFL subunits are associated with a fourth intermediate filament protein, peripherin (Lee and Cleveland 1996). Each subunit has a highly conserved central rod domain flanked by a variable N-terminal head domain and a C-terminal tail domain that varies in length depending on the subunit (Nixon and Sihag 1991; Pant and Veeranna 1995). A regionally specialized neurofilament network serves as a structural framework for maintaining neuronal shape and axon caliber and provides a scaffold for organizing and regulating functions of proteins and organelles (Fuchs and Cleveland 1998).
Neurofilaments are extensively regulated by phosphorylation. Among the most phosphorylated proteins, NF-H and NF-M contain 52 and 5 serine/ threonine residues, respectively in mouse and 44 and 13 serine / threonine residues in the NF-H and NF-M of human respectively, on their long carboxyl terminal tails which exist in conserved KS/TPmotifs differing in flanking sequence: KSPXK and KSPXXXK (Shetty et al. 1993; Nixon 1998; Pant et al. 2000). These sites and additional phosphorylation sites on the head domains of NFL and NFM are regulated spatially and temporally within neurons (Sihag et al. 2007). The major phospho-epitopes on the NF-M and NF-H tail domains, recognized by the monoclonal antibodies SMI-31, SMI-34 and RT-97 (Probst et al. 1983; Sternberger and Sternberger 1983; Lawson et al. 1984), appear sequentially during neuronal maturation on axonal neurofilaments (Sanchez et al. 1996; Sanchez et al. 2000). Phosphorylation on these domains influences the susceptibility of neurofilaments to calcium activated neutral proteases, and modulates the kinetic behaviors of neurofilaments (Pant 1988; Nixon et al. 1994; Ackerley et al. 2000) and their interactions with other cytoskeleton elements and specific receptors (Carden et al. 1985; Nixon et al. 1986; Pant 1988; Hisanaga et al. 1991; Miyasaka et al. 1993). Appearance of the RT-97 phospho-epitope late in axon maturation is triggered by myelinating glial cells (Sanchez et al. 1996; Sanchez et al. 2000) and is associated with the elaboration of an extensive stationary neurofilament network essential for expansion of axonal caliber and proper electrical conductance (Gasser and Grundfest 1939; Nixon and Lewis 1986; Nixon and Logvinenko 1986). The increased abundance of RT-97 on neurofilament and the microtubule-associated protein tau in affected neuronal perikarya accompanies the pathological accumulation of these cytoskeletal proteins in neurodegenerative diseases (Anderton et al. 1982; Sternberger et al. 1985; Veeranna et al. 2004; Kesavapany et al. 2007). The identity and regulation of NF phospho-epitopes, however, has not been clarified.
Ser / Thr kinases have been implicated in the phosphorylation of KSP repeats on neurofilament tail domains, including cdk5 (Wang et al. 1992; Shetty et al. 1993), MAPKs such as ERKs (Roder et al. 1993; Veeranna et al. 1998), JNKs (Giasson and Mushynski 1997; Ackerley et al. 2000) and P38 kinases (Ackerley et al. 2004); and additional kinases, including GSK3 β, Cdc2 and 115 kDa NF kinase (Xiao and Monteiro 1994; Starr et al. 1996; Sun et al. 1996; Bajaj and Miller 1997; Sasaki et al. 2002). Cdk5 phosphorylates KSPXK sites (Shetty et al. 1993), while ERK2 phosphorylates neurofilaments at both KSPXK and KSPXXXK repeats (Veeranna et al. 1998; Veeranna et al. 2004).
Here, we have investigated the structural determinants for generation of the RT-97 phospho-epitope. In addition, we modulated protein kinases pharmacologically and genetically to investigate their roles in generating the RT-97 phospho-epitopes in primary hippocampal neurons. The modulation of kinase activities revealed that crosstalk is extensive among signaling pathways that regulate neurofilament sidearm phosphorylation.
Materials and Methods
Antibodies, peptides and other reagents
We obtained the following antibodies commercially: monoclonal antibodies to β-tubulin (Amersham Life Sciences, Chicago, IL) SMI-31, and SMI 33 against phospho-and dephospho-epitopes, respectively, on NF-H and NF-M (Sternburger Monoclonal Inc. Lutherville, Baltimore MD); Polyclonal antibodies against GSK3-β, ERK 1,2, P35 and cdk5 (SantaCruz Biotechnology Inc. CA); phospho-ERK1,2 and phospho-independent ERK1,2 phospho-MEK 1,2, Phospho JNK 1,2 and Total JNK 1,2 and inactive GSK3-β (Cell Signaling. Boston, MA); anti calnexin polyclonal antibody (Stressgen, Victoria, BC, Canada); anti-mouse and anti-rabbit secondary antibodies conjugated to alkaline phosphatase (Promega, Madison, WI); or horseradish peroxidase conjugated anti-mouse and anti-rabbit secondary antibodies (Jackson Immunochemical Laboratories Baltimore MD); or Alexa 488 and Alexa 568 (Invitrogen, Gaithersburg, MD). A rabbit polyclonal antibody against NF-L was made in this laboratory. The RT-97 monoclonal antibody clone was a kind gift from Brian Anderton (Institute of Psychiatry London). Recombinant ERK2 and MEK1 proteins were kind gifts from Dr. N.G Ahn (University of Colorado, Boulder, CO). Additional commercial reagents included microcystin LR (Calbiochem, San Diego, CA), (gamma-32P) ATP (Perkin Elmer Life Sciences, Boston, MA), ECL kit (Amersham Biotech, IL), immobilon membranes (Millipore, Bedford, MA), dialysis tubing (Spectrum, Los Angeles, CA), myelin basic protein and histone H1 (Sigma Chemical Company, St. Louis, MO), Synthetic peptides derived from the mouse NF-H tail domain sequence were custom synthesized at the Advanced Protein Technology unit at the Hospital for Sick Children (Toronto, On, Canada). Block Ace (Serotec, Raleigh, NC). All cell culture reagents were purchased from Invitrogen. (Gaithersburg, MD).
Primary neuronal cultures and treatment with kinase inhibitors
Primary hippocampal neurons were established from day-19 Sprague Dawley rat embryos (Charles River Labs, NY). 19 day timed pregnant rat was euthanized using CO2 and pups were removed, decapitated and hippocampal regions were dissected from cerebral cortices in Hibernate-E media (Brain Bits LLC, IL). Dissociated hippocampal neurons were obtained by incubating the hippocampi in Hibernate-E containing 15units/ml of papain (Worthington Biochemicals, NJ) for 15 min at 37°C before triturating in neurobasal medium containing 20% fetal bovine serum (Hyclone, UT), DNAse (0.2 mg/ml) and 0.1M MgSO4. Undissociated neurons were removed from the cell suspension by passing the cell suspension through a 40µm cell strainer (Fisher Scientific, NY). Neurons were centrifuged at 200 × g for 3 min at 20°C and the pellet was resuspended in neurobasal medium supplemented with B27, penicillin (100 U/ml), streptomycin (100 U/ml) and L-glutamine (0.5mM, InVitrogen, NY). Neurons were then plated at a density of 150,000 cells/ml on circular glass coverslips and 6-well tissue culture dishes, coated with poly-d-lysine (50 µg/ml, Sigma, MO), and incubated in a humidified atmosphere containing 5% CO2: 95% O2 at 37°C. The following drugs (at 10 µM), U-0126, roscovitine and SP600125 to specifically inhibit ERK1,2, cdk5, or JNKs respectively, were added to the media at 4hr, 24hr and 48hr after plating. Neurons were harvested after 72hrs (3 days) in culture.
Immunocytochemistry of hippocampal neurons
Hippocampal neurons fixed in 4% paraformaldehyde were permeabilized for 20 minutes in 0.2% Triton X-100, blocked in 10% horse serum in PBS for one hour, and incubated with primary antibodies (diluted in 4% horse serum/phosphate buffered saline 0.05% Tween-20) against phospho-specific monoclonal antibody against NF-H (RT-97) or with polyclonal antibodies to p-ERK1,2, cdk5, p-MEK1,2 or p-JNK 1,2 (Cell signaling) for 1 hour at RT. After three washes in blocking solution, the neurons were incubated with anti-rabbit and anti-mouse secondary antibodies, Alexa 488 and Alexa 568, respectively (Molecular Probes, Eugene, OR). Secondary antibodies were diluted in the same buffer as the primary antibodies and incubated for 1 hour at RT. Cells were washed and mounted on the cover slips and analyzed by laser confocal microscopy (Leica, Wetzlar, Germany) using a TCS software program. Imaging was performed using a Zeiss 100X objective on an Axiovert 200M microscope (Zeiss Germany).
Tissue extraction and immuno-precipitation of protein kinases
Brains from C57 BL/6 wild type mice aged 6 months or older were dissected and frozen in dry ice and stored at –80°C until further use. A separate set of spinal cords were harvested from C57 BL/6 wild type mice aged 1– 15 days and frozen at –80°C until assayed for neurofilament proteins and for protein kinase activities and protein levels. Frozen brains were homogenized in a tissue extraction buffer (50 mM Tris/HCl pH 7.4 containing 100 mM Na Cl, 5mM EDTA and 1% Triton X-100, 20 mM B-glycerophosphate, 20 mM sodium fluoride, 1 mM sodium vanadate, and a mixture of protease inhibitors containing 5µg/ml each of leupeptin, antipain and pepstatin (all from Sigma) in the ratio of 1:10 (W/V) using a hand-held battery operated tissue homogenizer. The samples were centrifuged at 15,000 rpm (30,000 × g) for 30 minutes.
Immunoprecipitation of cdk5 and ERK 1,2 from mouse brain was performed essentially as described earlier (Veeranna et al. 1996; Veeranna et al. 1998). In brief, protein A plus G agarose beads (60 µl packed volume) were washed in 1X PBS three times, suspended in 500µl of 1X PBS, and incubated at RT for 30 minutes with 60µl of primary antibodies to ERK 1,2 and supernatants obtained from brain spinal cord, sciatic nerve and optic nerve homogenates (equivalent to 50µg of protein) were incubated with Protein A plus G agarose beads coupled to ERK 1,2 and cdk5 polyclonal antibodies for 2 hours at 4°C. The beads were washed in buffer (50 mM Tris/HCl pH 7.4 containing 5mM EDTA, 10 mM NaF, 10 mM β-glycerophosphate, and 0.01 % Triton X-100) and suspended in 100 µl of 1X kinase buffer.
Expression and purification of KSPXXXK and KSPXK fusion proteins
A rat NF-H tail fragment with 24 KSPXXXK repeats, and another fragment derived from human NF-H with 14 KSPXK repeats, both tagged to GST fusion protein were expressed and purified as described previously (Pant et al. 1997; Veeranna et al. 1998). Full length NF-H was expressed as described earlier (Takahashi et al. 1995).
Phosphorylation of Recombinant NF-H and NF-H fusion proteins
Phosphorylation of the bacterially expressed full length NF-H and NF-H tail domain GST fusion proteins was carried out as described earlier (Veeranna et al., 1995 and Veeranna et al. 1998) using the immuno-precipitates of cdk5, phospho ERK 1,2 or recombinant ERK2 or a cdk5 / p25. complex. Briefly, 10 µl of the immunoprecipitates of ERK1,2, or cdk5 were mixed in a kinase reaction mixture containing 50 mM Tris /HCl, pH 7.5, with 1mM each of EDTA, EGTA, DTT and orthovanadate, 1µM microcystin LR, 5 ug of recombinant NF-H or KSPXK or KSPXXXK GST fusion protein and gamma (γ32P) ATP (50 µM) in a total volume of 100 ul. The reaction was initiated by the addition of (γ32P) ATP, incubated at 30°C for 2 hours, and terminated by transferring an aliquot of 20 ul of the kinase reaction mixture at 0, 0.5, 1, 1.5 and 2hrs to a tube containing 5 ul of 5X Laemmli buffer and analyzed by SDS-PAGE and Western blot analysis using RT-97 monoclonal antibody or gels were silver stained after SDS-PAGE and dried and subjected to autoradiography using Kodak X-ray film.
Purification and analysis of neurofilament triplet protein
The neurofilament triplets were prepared from mouse spinal cords and NF-H protein was purified on a DE-52 column using an FPLC system as described earlier (Veeranna et al., 1995; 1998). NF-H and NF-M were further purified by SDS-PAGE using a 7% 20×20 cm preparative gel, followed by zinc staining (Dzandu et al., 1988) and electro-elution, dialysed against 0.1 M ammonium bicarbonate, and used as an antigen and to generate tryptic digests for further analysis by dot blot assay.
SDS-PAGE and Western blot analysis
Protein concentration in each fraction was assayed by the BCA method (Pierce, IL), and protein aliquots (10–20 µg) were loaded for each lane on a 7% or 10% minigel for electrophoresis unless otherwise indicated. Proteins were electro-transferred from gels to PVDF or nitrocellulose membranes using a Genie blotter (Idea Scientific, Minneapolis, MN) and blocked using 3% nonfat dry milk (Biorad) in Tris-buffered saline (20mM Tris-HCl, pH 7.4,150 mM NaCl, 0.2% Tween 20; TBS-T) for at least 2 hours. The membranes were incubated overnight at 4°C with primary antibodies at appropriate dilutions. Blots were developed with alkaline phosphatase-conjugated secondary antibodies using the chromogenic substrate BCIP/NBT (Promega Madison, WI) or with chemiluminescence based CDP star (Tropix, Bedford, MA) phosphatase substrate or peroxidase conjugated secondary antibodies using the ECL kit (Amersham Biotech, Chicago, IL).
Enzyme linked immunosorbent assay (ELISA)
The RT-97 IR against synthetic phosphopeptides was determined by direct ELISA. Briefly, we added 100 ul of peptide solution, (peptides serially diluted in carbonate buffer (30mM NaHCO3, 70mM Na2 CO3,0.05% NaN3 pH 9.6) ranging from 1 mM to 0.001mM unless otherwise mentioned and incubated overnight at 4°C. Unbound peptides were removed by washing with PBS twice using a programmed ELISA washer MRW ™ Dynex Inc. Chantilly VA). The vacant sites on the polystyrene plates were quenched by adding 200ul of blocking solution (1 % Block Ace, 0.05% Na N3, in PBS pH 7.4) to each well and incubated at RT for 4–6 hours. 100 ul aliquot of primary antibody (RT-97) of 1: 2000 dilution in antibody dilution buffer (3 mM NaH2PO4, 17mM Na2HPO4, 2 mM EDTA, 400mM NaCl, 1% BSA pH 7.0) was added to each well and incubated overnight at 4°C. Unbound antibody was removed by washing twice with PBST and once with PBS using a programmed ELISA washer. A 100 ul aliquot of labeled secondary antibody (Goat anti mouse peroxidase conjugated IgG) was added to each well and incubated for 2 hours at room temperature and unbound secondary antibody was removed by washing the plates with PBST twice and once with PBS using the ELISA washer and developed by addition of 100ul of substrate solution (TMB 2-Component Micro well Peroxidase substrate kit provided by Kirkegaard and Perry Laboratories, Gaithersburg MD). Incubation at RT was continued until the desired intensity of color developed. The reaction was stopped by adding 100ul of 5.6% orthophosphoric acid and color was measured as optical density at 450 nm.
Mass spectrometric analysis of neurofilament phosphopeptides bound to RT-97 antibody
For MS analysis, an immunoaffinity ultrafiltration technique was employed (Wieboldt et al. 1997). NF-H (100 µg) was dissolved in 100µl 8M urea in 0.4M ammonium bicarbonate, heated for 30 min at 50°C with 20ul 0.45 M DTT. After cooling to RT, 20 µl 0.1 M iodoacetamide was added and allowed to react for 30 min. After dilution to 2M urea/ 0.1 M ammonium bicarbonate, 5 µg trypsin (Promega, Madison WI) was added, and the reduced and alkylated protein was digested overnight at 37°C. An aliquot (25 µl) of the digest was mixed with 425 µl of 1 × PBS and 50 µl of the RT-97 antibody in a 30K Microcon ultrafiltration device (Millipore, Bedford, MA) and incubated at 37°C for 2 hours. The reaction mixture was spun down to 20µl and washed with 500 µl of 1X PBS and eluted with 300µl of 2 % heptafluorobutyric acid. The released phosphopeptides were concentrated in a speedvac (Savant, Framingham, NY) and analyzed by ESI/MS/MS as previously described (Jaffe et al. 1998a; Jaffe et al. 1998b).
Results
Identification of the RT-97 phospho-epitope on mouse and human NF-H by mass spectrometry
To identify the RT-97 phosphoepitope of NF-H, we digested mouse NF-H with trypsin, which yielded phosphopeptides that bound to RT-97 antibody in a 30K immunoaffinity ultrafiltration device. Subsequent mass spectrometric analysis (Fig. 1 A, B) identified two distinct KSPXK peptides bound to the RT-97 antibody (Fig. 1C, D). One of these, identified as KAKS*PVKEDIKPPAEAKS*PEK (789–809), is shown in Figure 1B. KSPXXXK peptides were not detected in this analysis because the KSP core of mouse NF-H containing these motifs is resistant to proteolysis conferred by endogenous phosphorylation (Elhanany et al. 1994). Therefore, we subsequently analysed tryptic digests of human NF-H that contains a structurally different KSP region completely cleaved by trypsin. Mass spectrometry of tryptic digests yielded multiple peptides with either KSPXK and KSPXXXK motifs that bound RT–97 (Fig. 1C, D).
Figure 1. Identification of RT-97 epitope sites on NF-H.
(A) Neutral Loss Chromatogram (−32.7) of the HPLC ESI/MS/MS analysis of Mouse NF-H digest bound to RT-97. (B) MS/MS spectrum of the triply charged diphosphopeptide eluting at 28.48 min (Figure- 2A) identified as KAKS*PVKEDIKPPEAK S*PEK. Most abundant doubly charged series are labeled. (C) A schematic representation of NF-H showing the location of RT-97 bound KSP peptides in the tail domain of mouse and human NF-H. (D) Mouse and human NF-H tail domain sequence containing RT-97 binding peptides.
Structural requirements for RT-97 phosphoepitope generation
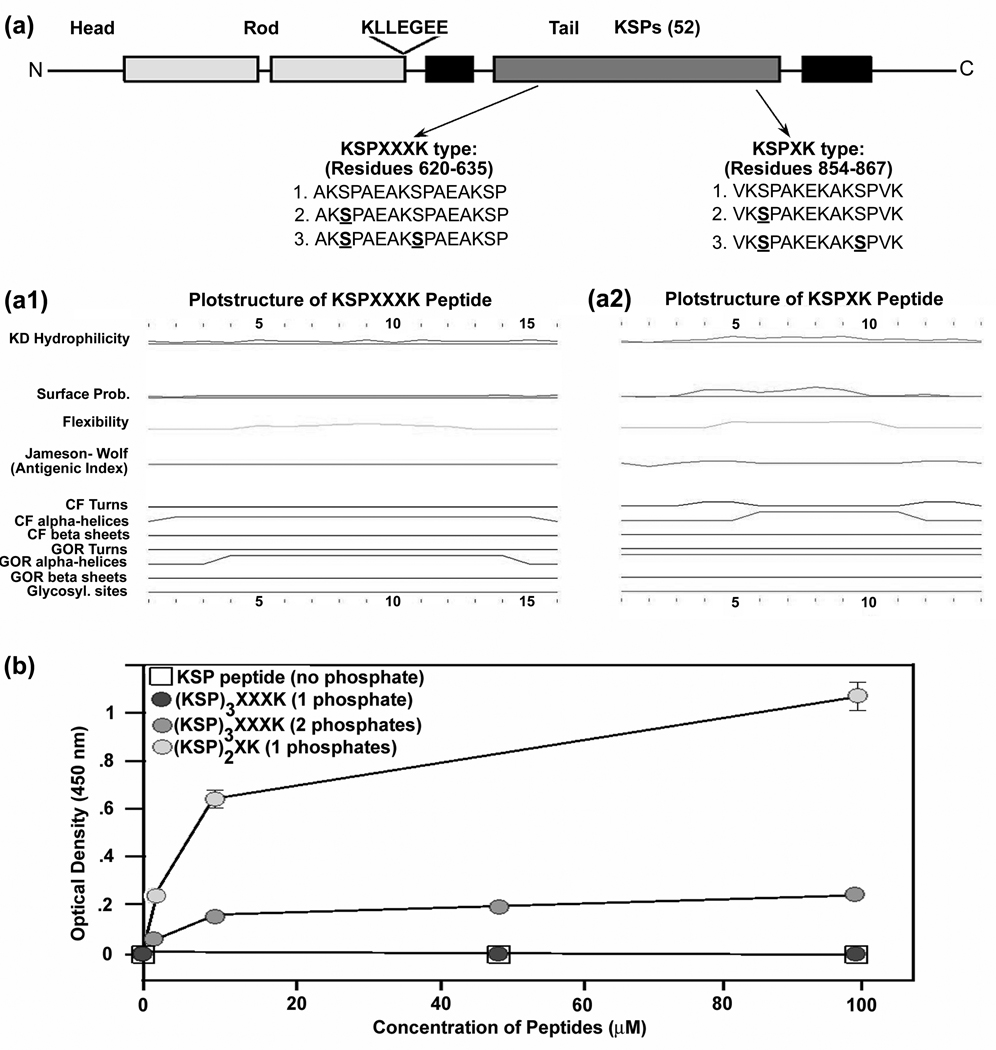
To investigate the relative affinity of the RT-97 antibody for KSPXK and KSPXXXK motifs, we used synthetic phosphopeptides derived from the sequences of the mouse NF-H tail domain containing either KSPXK (amino acid residues 854–867) or KSPXXXK (amino acid residues 620–635) motifs (Fig. 2A). Based on the peptide structure prediction using a standard program, these two peptides differed in hydrophilicity, surface probability, chain flexibility turns, and alpha helices. (Fig. 2A1, 2A2). Although there are limitations in the reliability of these structural predictions, they are consistent with differences evident from sequence differences alone. The RT-97 immuno-reactivity of these peptides was analyzed either by ELISA (Fig. 2B) or dot blot assay (not shown). As shown in Figure 2B, a single phosphate on KSPXK peptides was necessary and sufficient for RT-97 IR. By contrast, RT-97 IR generation on the KSPXXXK repeat peptide required at least two phosphates and the RT-97 IR was 2 fold lower compared to that of the singly phosphorylated KSPXK peptide. These data suggested that the sequence of the peptide and the position of K(lysine) on the C-terminus of the peptides plays a key role in RT-97 IR generation.
Figure 2. RT-97 Immunoreactivity of synthetic mouse NF-H tail phosphopeptides.
(A) Schematic representation of mouse NF-H showing the sequences of synthetic phospho-peptides derived from mouse NF-H tail domain sequence. (A1) Representation of the predicted peptide structure of KSPXXXK peptide in (A). (A2) Representation of the predicted peptide structure of KSPXK peptide in (A). (B) Line graph of ELISA showing the RT-97 immunoreactivity against KSPXXXK and KSPXK peptides with and without phosphate (direct ELISAs were performed by coating the peptides onto ELISA plates).
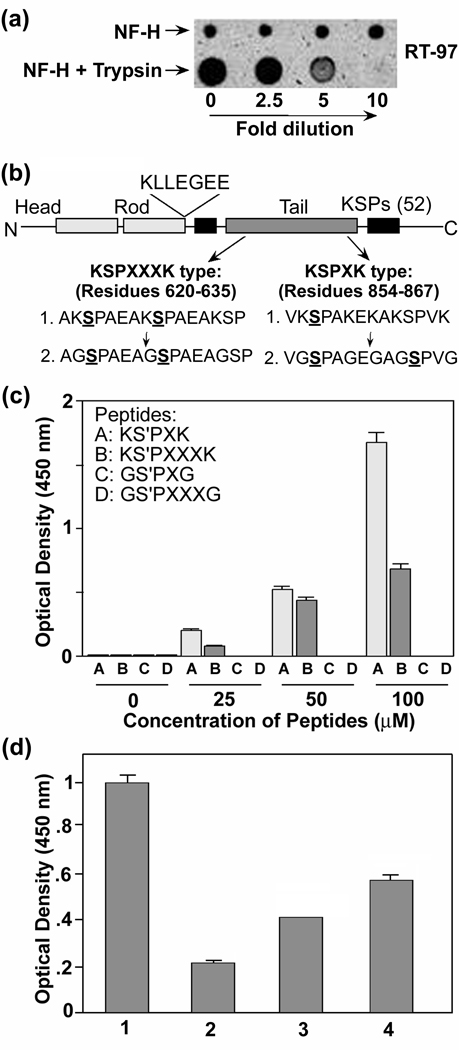
We and others showed that tryptic digestion reduces RT-97 IR on NF-H in vitro (Fig. 3A) and in tissue sections (Ulrich et al. 1987). Since trypsin cleaves at the C-terminus of lysine (Keil-Dlouha et al. 1971; Olsen et al. 2004), the possibility exists that the N-terminal lysine may be important for RT-97 IR in addition to lysine in the C-terminal side of the peptide. To test this hypothesis, we generated phospho-variants of wild type (KSP) and mutant (GSP) peptides derived from the mouse NF-H sequence, in which glycine replaced lysine in the KSP motifs (Fig 3B). The data presented in Figure 3C show that N-terminal lysine also affects the RT-97 IR. This replacement completely eliminated RT-97 IR on phosphopeptides containing either KSPXK or KSPXXXK motifs, thus explaining the effects of trypsin digestion on RT-97 IR (Fig. 3B, C) and establishing the importance of N-terminal lysine for RT-97 recognition. Because both N and C terminal lysines influence RT-97 IR, we analyzed RT-97 IR of a series of synthetic 8mer KSP phosphopeptides with N-terminal lysine with or without a C-terminal lysine varying in its position within the peptide. The presence and position of the C-terminal lysine significantly influenced RT-97 IR (Fig. 3D): The peptide K(S-P)AAVGS lacking a second lysine on the C-terminus was less RT-97 immunoreactive than the peptides, K(S-P)PAAVGK and K(S-P)PAKAVS, with C-terminal lysine. Among the latter, positioning of the second lysine as the 8th residue from the N-terminal lysine was less immunoreactive than positioning at the 5th residue. Moreover, further sequence extension at the C-terminal end of the KSPXK peptide (14 mer) (Fig. 3B), without any additional phosphate, increased RT-97 IR suggesting that additional aspects of peptide structure influence affinity of RT-97.
Figure 3. Trypsin abolishes RT-97 IR in vitro.
(A) 20 µg of NF-H was trypsinized overnight as described in the text and a 2µl aliquot of different dilutions was spotted on a nitrocellulose membrane as shown in the figure. Corresponding dilutions of NF-H without trypsinization also were spotted and the membrane was air dried at room temperature for 30 min. and immunostained with RT-97 monoclonal antibody. (B) Schematic representation of mouse NF-H showing the sequences of synthetic phospho-peptides derived from mouse NF-H tail domain with and without replacement of lysine with glycine. (C) Bar graph of ELISA using synthetic KSPXXXK and KSPXK phospho-peptides with and without replacement of lysine with glycine. (D) Bar graph of ELISA using 100µM synthetic 8mer KSP phospho-peptides with and without C-terminal lysine at different positions as shown. Bar 1 represents a KSPXK 14mer peptide with a single phosphate and five lysine residues (Fig. 3B). Bar 2,3 and 4 represent 8mer KSP phospho-peptides with no second lysine residue K(S-P)PAAVGS (2), with second lysine at 8th position K(S-P)PAAVGK (3) and with second lysine at 5th position K(S-P)PAKAVS.).(n= 4, P= <0.001)
Cdk5 and ERK 1,2 generate the RT-97 phospho-epitope with different specificities for KSP motifs
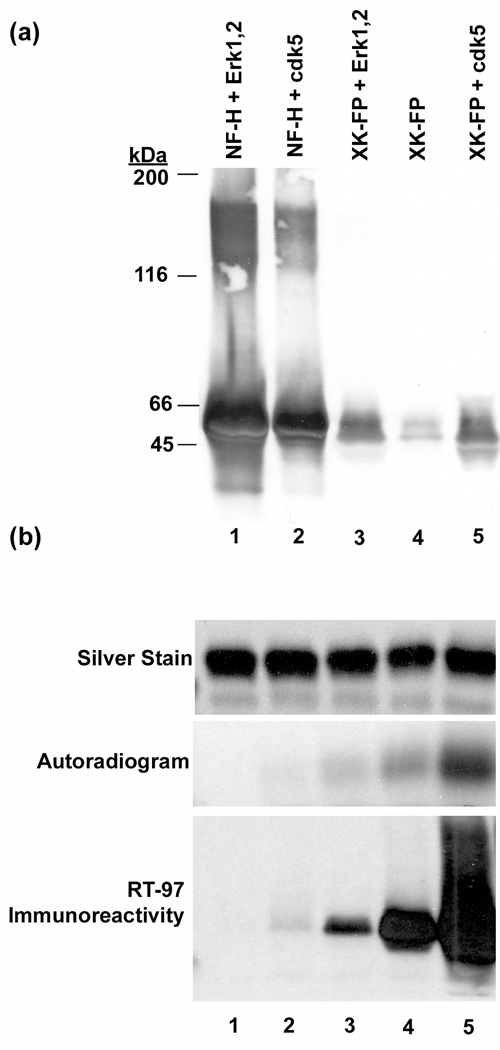
Among the various proline-directed kinases, ERK1,2, cdk5 and JNKs are known to be prominent in phosphorylating NF-H tail domain KSP motifs (Roder et al. 1993; Shetty et al. 1993; Giasson and Mushynski 1997; Veeranna et al. 1998). To examine RT-97 IR generation after phosphorylation by ERK1,2 and cdk5, we purified bacterially expressed rat NF-H and GST fusion proteins carrying KSP repeats. ERK1, 2 immunoprecipitated from mouse brain generated the RT-97 epitope on GST fusion proteins derived from human NF-H containing 14 KSPXK repeats or GST fusion protein derived from rat NF-H containing 24 KSPXXXK repeats. By contrast, cdk5 generated the RT-97 epitope only on the KSPXK fusion protein in vitro, consistent with its more restricted sequence specificity (Fig. 4A). Both kinases immunoprecipitated from mouse brain were able to generate the RT-97 epitope on recombinant full length NF-H which contains both motifs (Fig. 4A). RT-97 IR paralleled the degree of phosphorylation of KSPXXXK fusion protein by recombinant ERK2 and was accompanied by a concomitant retardation in electrophoretic mobility of the phosphoprotein (Fig. 4B), as seen for full length NFH after phosphorylation in vitro and in vivo.
Figure 4. Phosphorylation of NF-H and NF-H tail domain fusion proteins by proline directed kinases.
(A) Both cdk5 and ERK 1,2 phosphorylate recombinant NF-H and and generate the RT-97 phosphoepitope. Western blot analysis of full length bacterially expressed NF-H immunostained with RT-97 after it was phosphorylated using the immunoprecipitate (IP) of cdk5 as enzyme source (lane 2) and ERK 1,2 (lane 1) and lane 3 represents a 14 mer KSPXK repeat fusion protein derived from human NF-H tail sequence, immunostained with RT-97 after phosphorylation using immunoprecipitate of ERK1,2 and IP of cdk5 (lane 5) and lane 4 represents the fusion protein alone. (B) ERK2 phosphorylation KSPXXXK fusion protein elicits RT-97 IR: 10 µg of 24 KSPXXXK repeat fusion protein was phosphorylated with a mixture of recombinant ERK2 and Mek1 in a time course reaction ranging from 0–2 hours (0, 0.5, 1, 1.5 and 2 hrs represented in lanes 1 through 5 respectively) in triplicate. Equal aliquots were removed from the reaction mixture and were electrophoresed on 7% SDS gels followed by electrotransfer onto nitrocellulose membrane in order to immunostain with RT-97 monoclonal antibody (lower panel). One gel was stained with silver to discern the protein (top panel) dried and subjected to autoradiography to visualize phosphorylation (middle panel).
Inhibition of ERK 1,2, JNK1,2 or cdk5 alter RT-97 immunoreactivity in hippocampal neurons
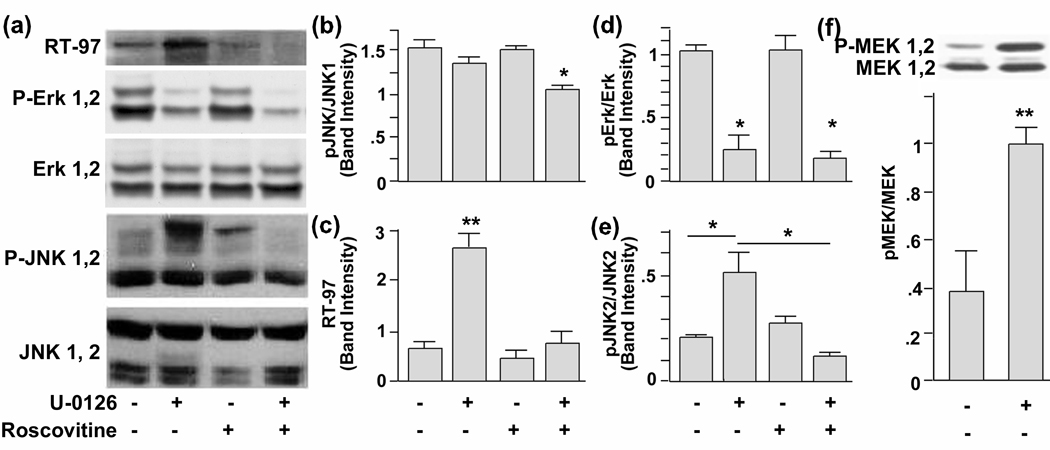
To investigate the regulation of the RT-97 phospho epitope in vivo, we used primary hippocampal neurons in culture and modulated different kinase activities using specific inhibitors. We compared RT-97 IR by quantitative Western blot analysis in untreated hippocampal primary neurons in culture relative to those treated with the ERK 1/2 inhibitor U-0126 (10 µM), a cell permeable inhibitor of MEK, or with the cell permeable cdk5 inhibitor roscovitine (10 µM), or with both inhibitors. Unexpectedly, U-0126 treatment, which significantly reduced pERK 1,2 levels (Fig. 5A, D), greatly increased RT-97 IR over control levels (Fig. 4A, C). This response was explained, however, by a concomitant increase in the levels of active JNK2 (Fig. 5A, E), a downstream target of phospho MEK1,2 (Adler et al. 2005), levels of which also rose after this treatment (Fig. 5F). and could reflect activation of a Raf or other MEKK that would not inhibited by U-0126. Supporting this conclusion, roscovitine addition to U-0126 treated cells blocked JNK2 activation (Fig. 5A, E) and reduced RT-97 IR to close to normal baseline levels. Roscovitine treatment alone did not alter ERK or JNK phosphorylation and only modestly lowered RT-97 IR (Fig. 5A, C).
Figure 5. Western blot analysis of lysates of hippocampal neurons treated with kinase inhibitors.
(A) 10µM each of U-0126 (lane −2), roscovitine (lane 3) and U-0126 together with Roscovitine (lane 4). Lane 1 represents the lysates from neurons treated with vehicle (DMSO). The neurons were treated for 72 hours and lysed in MPER (Pierce) with added protease and phosphatase inhibitors. Quantities of protein (25 µg/ lane) were electrophoresced on a 8% SDS-Tris –glycine gel and immunoblot analysis was performed with antibodies against neurofilament kinases and phosphoneurofilament antibodies. (B) Relative band intensities of JNK1(p-46), (C) phosphoneurofilaments, (D) P-ERK 1,2, (E) p-Jnk 2 (p-54), and (F) p-MEK1, 2.
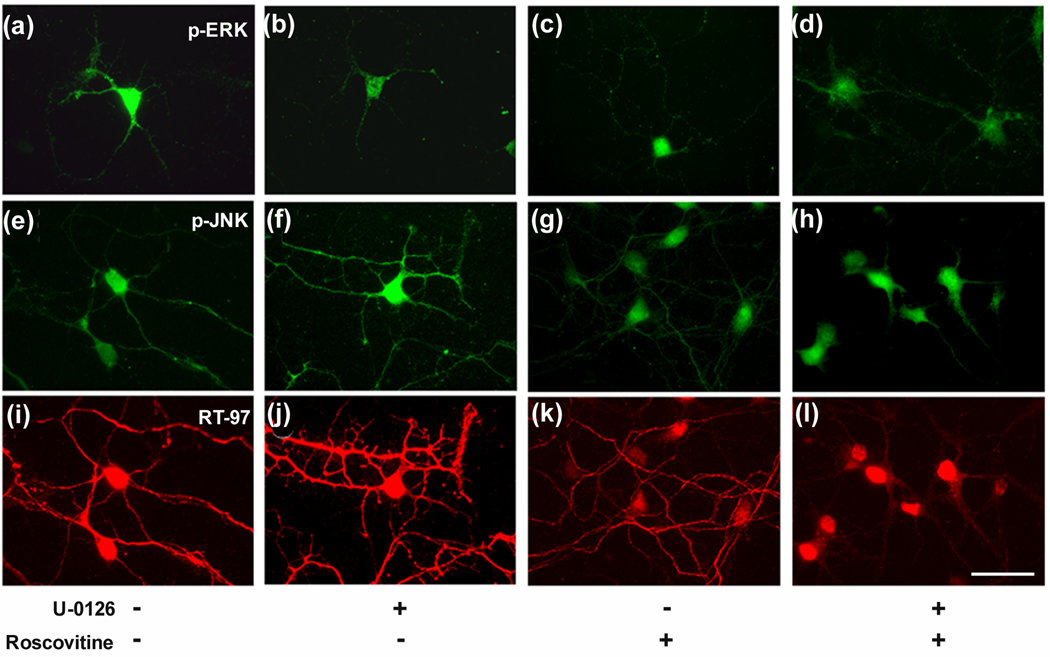
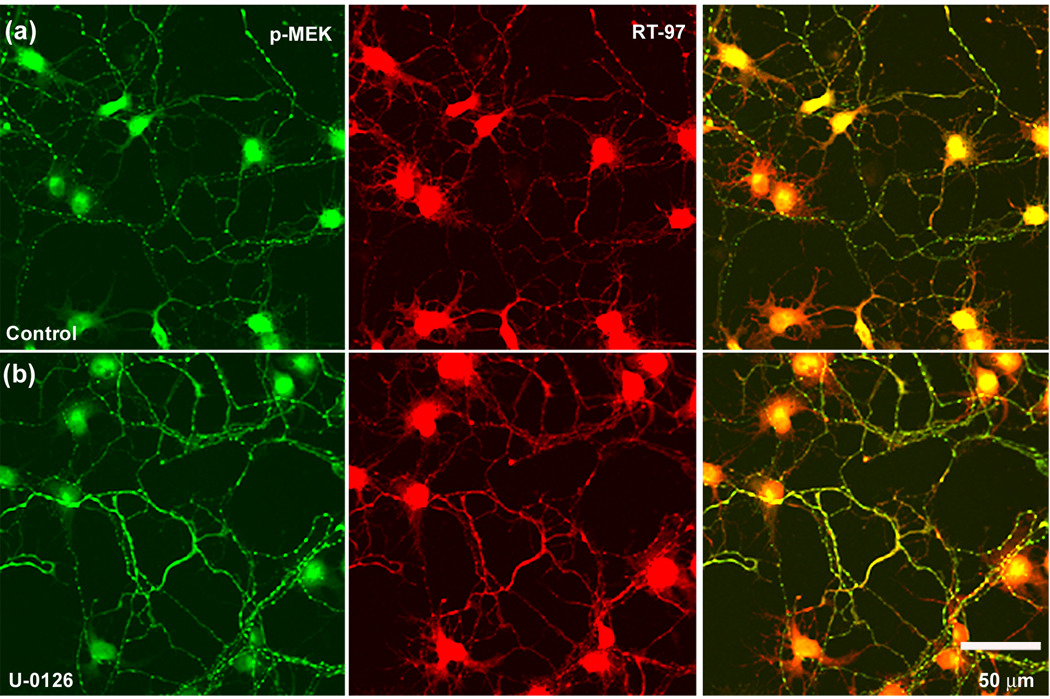
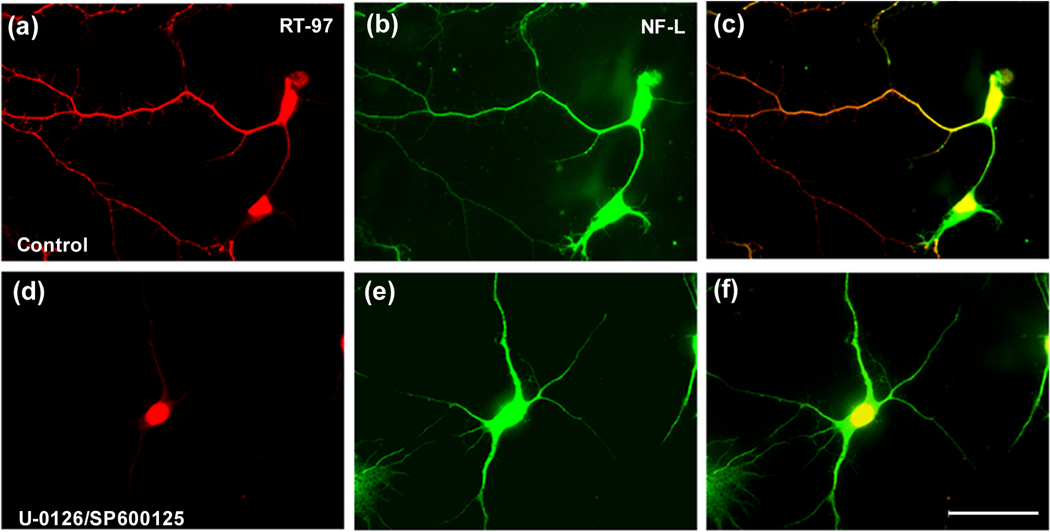
Confirming these Western blot studies, immunofluorescence labeling analyses of hippocampal neurons showed that U-0126 treatment decreased pERK 1,2 immunoreactivity (Fig. 6B) yet greatly increased RT-97 staining (Fig. 6, J and Fig. 7), which was accompanied by increased pJNK IR (Fig. 6. F) and p MEK1,2 IR (Fig. 7) Roscovitine partially reversed the U-0126-induced increase in RT-97 labeling (Fig. 6. K) and pJNK IR whereas, by itself, it only marginally reduced RT-97 IR compared to control. Similarly, SP600125, an inhibitor of JNKs (Bennett et al. 2001), markedly reduced RT-97 staining of processes from U-0126 –treated neurons but only modestly lowered RT-97 IR when administered alone. These data established the importance of both ERK 1,2 and JNKs in regulating the RT-97 epitope in neurons (Fig. 8. D, E).
Figure 6. Inhibition of ERK 1,2 by U-0126 leads to enhanced RT-97 IR.
The hippocampal neurons grown in culture, four hours after plating on polylysine-coated glass coverslips were treated with vehicle (A, E, I) or with U-0126 (10 µM) (B, F, J) or roscovitine (10 µM) (C, G, K) or roscovitine together with U-0126 (D, H, L) for 72 hours and fixed in 4% paraformaldehyde and then subjected to double immunostaining using monoclonal antibody RT-97 together with polyclonal antibodies to either pERK1,2 or pJNK. A mixture of anti-mouse and anti-rabbit secondary antibodies tagged to fluorophores Alexa 568 red and Alexa 488 green respectively, were used to develop and visualize the neurons in this and subsequent images of Fig 5.2 and 5.3.
Figure 7. RT-97 immuno-reactivity and phospho-MEK 1,2 levels are augmented in hippocampal neurons treated with U-0126.
Hippocampal neurons in culture following treatment with the Mek1,2 inhibitor, U-0126 (10 µM) for 72 hours fixed in 4% paraformaldehyde along with untreated control neurons and immunostained using a polyclonal antibody raised p-MEK 1,2 and a monoclonal antibody RT-97 that recognizes the phosphorylated tail domain of NF-H.
Figure 8. RT-97 immuno-reactivity is reduced in hippocampal neurons when both ERK 1,2 and JNKs are inhibited.
The hippocampal neurons in culture following treatment with, the Mek1,2 inhibitor, U-0126 (10 µM) together with JNK inhibitor SP 600125 (10 µM) for 72 hours (D,E), fixed in 4% paraformaldehyde along with untreated control neurons (A,B) and immunostained using a polyclonal antibody raised against NF-L and a monoclonal antibody RT-97 that recognizes the phosphorylated tail domain of NF-H.
Importance of ERK-mediated phosphorylation of neurofilament tail domains during early postnatal brain development and in gene knock-out models
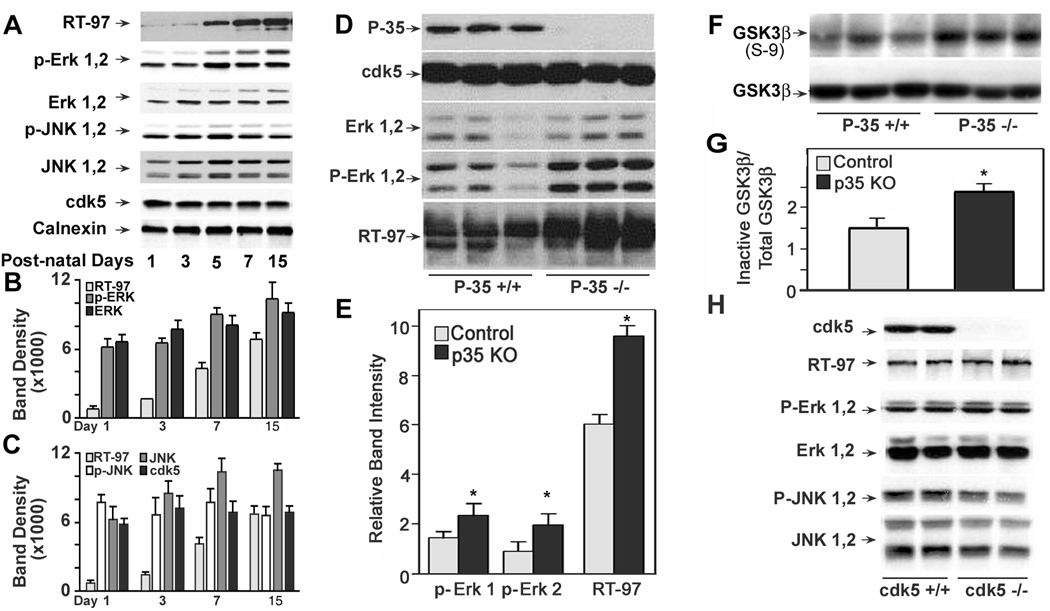
To investigate the functional role of specific protein kinases in RT97 generation during brain, western blot analyses were performed using whole homogenates of mouse spinal cord from wild-type mice at postnatal days 1, 3, 5, 7, and 15. These analyses revealed that RT-97 IR increases with maturation and strongly correlated with the rise in immuno-reactivities of both pERK 1,2 and ERK 1,2 (Pearson r value of 0.99 and 0.95 respectively) (Fig 9 A, B). By contrast, the immuno-reactivities of JNK 1,2 (r =.78), pJNK 1,2 (r = 0.24) and cdk5 (r = 0.11), did not show a significant correlation with RT-97 IR during this period of development ( Fig 9 A, C)
Figure 9. Analysis of immunoreactivities of RT-97 and kinases in spinal cord of developing mice, p35 knock out mice, and brains from cdk5 knockout mice.
A: RT-97, ERK1,2 and pERK 1,2 immuno-reactivities rise in parallel during early postnatal development of mouse spinal cord: Western blot analysis was carried out on whole homogenates of four sets of spinal cord from mice at postnatal days 1, 3, 5 and 15 using RT-97 monoclonal antibody and polyclonal antibodies to the neurofilament kinases, phospho and total ERK 1,2, JNK 1,2 , cdk5. Calnexin was measured to confirm equal protein loading. Blots were developed using an HRP based chemiluminescence (ECL kit from Amersham) B and C: Graphic representation of RT-97 and kinase immunoreactivities: The films were scanned using HP Scan Jet 4070 and band densities were measured using NIH image J 1.37 V software, and analyzed using Graph Pad Prism-4 software. D–H: RT-97 IR is normal in mice lacking cdk5 and enhanced in P35 knock out mice: Western blot analysis was carried out on whole homogenates of three sets of spinal cord from p-35 knock out and control mice using monoclonal antibodies to phospho-neurofilament H and polyclonal antibodies to neurofilament kinases ERK 1,2 and cdk5/p-35. Blots were developed using an HRP based chemiluminescence (ECL kit from Amersham) E: The band densities presented in the bar graph were measured using NIH Image J 1.3 V software after scanning the films using HP Scan. Jet 4070. F: Western blot analysis of samples used in figure 9 D, using polyclonal antibodies to inactive GSK3-β (phosphorylated at serine 9) and total GSK3-β. G: The band densities presented in the bar graph were measured using NIH Image J 1.3 V after scanning the films using HP Scanjet 4070. H: Western blot analysis of whole homogenates of brain from four sets of E-16.5 cdk5 knock out and control mouse embryos, using polyclonal antibodies against cdk5, P-ERK 1,2, ERK 1,2, P-JNK 1,2 and JNK 1,2.
Given that cdk5 inhibition only modestly lowered RT-97 IR levels in neurons, we analyzed its possible role further in vivo by investigating mice lacking P35 (P35 KO), the protein activator of cdk5. The cdk5 activity of brain and spinal cord from P35 knockout mice was 10–20 % of wild type mice, suggesting that P35 is the major activator of cdk5 in these tissues (Ohshima et al. 2001). Despite markedly reduced cdk5 activity in these mice, phosphorylation at the RT-97 epitope was actually increased by 40% (p<.05) (Fig. 9 D, E) in parallel to elevations in the levels of ERK 1,2 (Fig. 9 D, E). JNK 46/54 levels remained unaltered in P35 KO mice (data not shown). The inactive form of GSK3-β which is phosphorylated on serine −9 was elevated in the spinal cord of P35 KO mice (Fig. 9F, G) suggesting that its role in the regulation of phosphorylation at RT-97 epitope under these conditions was minimal. In cdk5 knock out mice, RT-97 IR was not significantly different from control levels (Fig. 9 H) despite the total absence of cdk5. Active ERK1,2 and JNK1 were present at normal levels that are sufficient to account for the normal levels of RT-97 IR seen in these mice. Interestingly we observed only active JNK p 46 although inactive isoforms of both p46 and p54 are present (Fig. 9 H).
Discussion
The appearance of the RT-97 phosphoepitope on NFH and NFM represents a functionally critical phosphorylation event that marks a late stage in the maturation of axons in vivo (Sanchez et al. 2000). This stage of maturation is associated with prolonged detachment of neurofilaments from the axonal transport machinery and marked expansion of axonal caliber in response to signals from myelinating glial cells (Sanchez et al. 2000). Whether the RT-97 phosphoepitope directly mediates these effects on neurofilament dynamic behaviors or plays a role after these events have occurred is not yet established. Here, we have established the molecular determinants of RT-97 epitope and defined the roles of specific protein kinases in its regulation.
Mass spectrometric analysis of human NF-H peptides isolated by RT-97 immunoaffinity ultrafiltration after tryptic digestion have demonstrated, for the first time, that RT-97 epitopes are generated by phosphorylation at both KSPXK and KSPXXXK motifs in vivo, extending the previous observation that tail domains of neurofilament subunits are phosphorylated in vivo (Elhanany et al. 1994; Pant and Veeranna 1995; Jaffe et al. 1998; Jaffe et al. 1998). Our studies establish that flanking lysines at both the N and C-terminus are essential determinants for RT-97 generation, extending evidence that KSP motif phosphorylation generates RT-97 IR (Lee et al. 1988; Coleman and Anderton 1990). We also observed that positioning of the C-terminal lysine, which determined the relative specificity of cdk5 and ERK 1,2 for NF-H and NF-M, as previously shown (Shetty et al. 1993), influences the affinity of the RT-97 antibody for the phosphorylated KSP motif. The lower RT-97 IR exhibited by synthetic KSPXXXK phospho- peptide relative to that by KSPXK phospho-peptide parallels the immunogenicity of these two peptides and suggests the importance of peptide structure or conformation. The predicted structures of these peptides revealed clear differences providing a basis for the different antibody affinities.
RT-97 IR was generated on bacterially expressed NF-H and fusion proteins derived from the NF-H tail after phosphorylation on KSPXK motifs by cdk5, and phosphorylation on either KSPXP or KSPXXXK motifs by ERK 1,2. These results are consistent with the established consensus sequences for these kinases (Kemp and Pearson 1991; Kennelly and Krebs 1991). Selective inhibition of these kinases in hippocampal neurons in culture in our studies revealed that levels of RT-97 IR are regulated by extensive crosstalk among kinase pathways (Cowan and Storey 2003). U-0126, a specific inhibitor of MEK 1,2-mediated ERK phosphorylation, leads to an increase MEK1,2 phosphorylation by mechanisms that are not fully understood, as previously reported(Favata et al. 1998). Despite U-0126 suppression of ERK 1,2 activation (Veeranna et al. 2004), RT-97 generation was increased due to JNK2 activation. JNKs are known to phosphorylate KSP repeats (Giasson and Mushynski 1997) and in the presence of U-0126, MEK 1,2 has been reported to activate JNK2 in Xenopus oocytes (Adler et al. 2005). We observed a similar increase in RT-97 immunoreactivity after treating the mouse brain slices in culture with 10 µM U-0126 (unpublished observation). Although MEK activation of JNK2 is the likely basis for the increased RT97 IR after U-0126, we cannot exclude the possibility that stress induced by the inhibitor activates another upstream kinase or inactivates a phosphatase acting on the RT97 epitope.. Consistent with its role as a stress-activated protein kinase, JNK had relatively little activity in generating RT-97 IR in untreated cells but was activated in response to ERK inhibition and this activation was blocked by either a JNK inhibitor (SP600125) or by inhibiting cdk5 (Havlícek et al. 1997). Combined ERK 1,2 and JNK 2 inhibition markedly lowered RT-97 levels, nearly completely eliminating its presence in neurites and underscoring the importance of these two kinases as generators of RT-97 IR.
Previous studies have shown that overexpression of cdk5 and NF-H in non-neural cells generates RT-97 on the NF-H tail domain (Ackerley et al. 2000). We confirmed that cdk5 generates RT-97 on the NF-H tail derived GST fusion protein containing KSPXK repeats in vitro, however, surprisingly, cdk5 is not required for maintaining normal levels of RT-97 in neurons as evidenced by the presence of normal levels of RT-97 IR in cdk5 knock out mice. RT-97 IR in these mice is generated by other established NF kinases, viz active ERK 1,2 and active JNK1,2 (Giasson and Mushynski 1997; Veeranna et al. 1998). It should be noted that the intensity of RT-97 IR in E-16.5 embryo brains is much lower compared to that seen in P 35 knock out mouse spinal cord consistent with its developmental regulation (Sanchez et al., 2000). Further, RT-97 IR levels were actually increased in P35 knockout mice where cdk5 activity is reduced by 80–90% (Ohshima et al. 2001; Takahashi et al. 2003). In these mice, rises in RT-97 levels coincided with increased ERK 1,2 activation (Sharma et al. 2002; Hallows et al. 2003; Zeng et al. 2006) while JNK activity remained unaltered, providing further evidence for the role of ERK 1, 2 in RT-97 generation. The elevated levels of the serine-9 phosphorylated inactive isoform of GSK3-β in the spinal cord of P35 KO mice suggest that its role in regulating the phosphorylation at RT-97 epitope under these conditions is minimal. GSK3-β might play a role in other circumstances by indirectly modulating cdk5. Moreover, cdk5 influences GSK3-β activity by altering the activity of protein phosphatase-1 through phosphorylation of inhibitor 1 and 2. (Plattner et al., 1998; Huang and Paudel, 2000; Agarwal-Mawal and Paudel 2001; Morfini et al,. 2006;). Although cdk5 was modestly or minimally involved in regulating RT-97 IR on mouse NF-H, it is possible that its role is more important in regulating human NF-H because the majority of KSPs are of the KSPXK type, which are targets of cdk5 phosphorylation (Shetty et al. 1993).
Taken together, our results demonstrate that multiple proline-directed kinases, which cross talk extensively, regulate the NF-tail domain phosphorylation that generates the RT-97 phosphoepitope. The strong correlation between selective rises in RT-97 IR and pERK 1,2 and ERK 1,2 immuno-reactivities during postnatal development of mouse spinal cord and in P35 knock-out mice underscore the importance of this kinase in regulating the RT-97 epitope in vivo. However, the lack of correlation between RT-97 IR and immuno-reactivities of cdk5, JNKs and pJNKs does not exclude their possible role under other conditions. The age dependent increase in levels of the RT-97 epitope is consistent with our earlier work on the SMI-31 epitope in developing rat spinal cord and cerebellum (Bush and Gordon-Weeks, 1994; Veeranna et al, 1997). Furthermore, RT-97 immunoreactivity is greatly influenced by the presence and positioning of flanking lysines and the structure of the peptide. These studies provide a foundation for understanding how phosphorylation leading to RT-97 epitope generation influences neurofilament function.
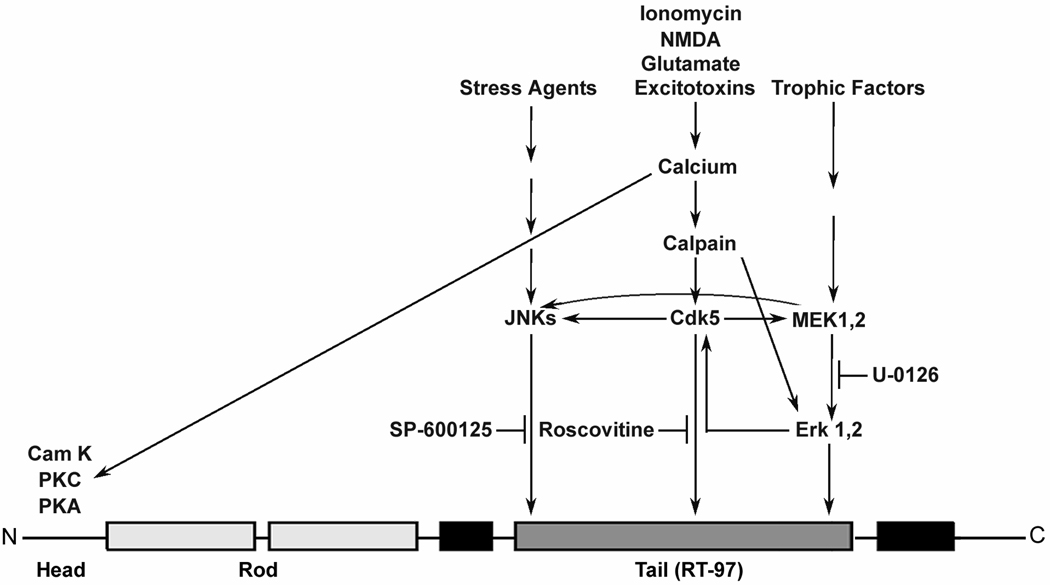
Figure 10. Schematic representation of cross talk among kinases resulting in NF-H phosphorylation at RT-97 epitopes.
Acknowledgements
The authors thank Ramaswamy Neelakantan for useful discussions, Corrinne Peterhoff for assistance with figures, and Nicole Piorkowski for assistance with manuscript preparation. This work was supported by NIA AG05604, NIA P01A02219 and NIH intramural program.
References
- Ackerley S, Grierson AJ, Brownlees J, Thornhill P, Anderton BH, Leigh PN, Shaw CE, Miller CC. Glutamate slows axonal transport of neurofilaments in transfected neurons. J. Cell Biol. 2000;150:165–176. doi: 10.1083/jcb.150.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley S, Grierson AJ, Banner S, Perkinton MS, Brownlees J, Byers HL, Ward M, Thornhill P, Hussain K, Waby JS, et al. p38alpha stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis. Moll. Cell Neurosci. 2004;26:354–364. doi: 10.1016/j.mcn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Adler V, Qu Y, Smith SJ, Izotova L, Pestka S, Kung HF, Lin M, Friedman FK, Chie L, Chung D, et al. Functional interactiosn of Raf and MEK with Jun-N-terminal kinase (JNK) result in a positive feedback loop on the oncogenic Ras signaling pathway. Biochemistry. 2005;44:10784–10795. doi: 10.1021/bi050619j. [DOI] [PubMed] [Google Scholar]
- Agarwal-Mawal A, Paudel HK. Neuronal Cdc2-like protein kinase (Cdk5/p25) is associated with protein phosphatase 1 and phosphorylates inhibitor-2. J. Biol. Chem. 2001;276:23712–23718. doi: 10.1074/jbc.M010002200. [DOI] [PubMed] [Google Scholar]
- Anderton BH, Breinburg D, Downes MJ, Green PJ, Tomlinson BE, Ulrich J, Wood JN, Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982;298:84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Bajaj NP, Miller CC. Phosphorylation of neurofilament heavy-chain side-arm fragments by cyclin-dependent kinase-5 and glycogen synthase kinase-3alpha in transfected cells. J. Neurochem. 1997;69:737–743. doi: 10.1046/j.1471-4159.1997.69020737.x. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlees J, Yates A, Bajaj NP, Davis D, Anderton BH, Leigh PN, Shaw CE, Miller CC. Phosphorylation of neurofilament heavy chain side-arms by stress activated protein kinase-1b/Jun N-terminal kinase-3. J. Cell Sci. 2000;113:401–407. doi: 10.1242/jcs.113.3.401. [DOI] [PubMed] [Google Scholar]
- Bush MS, Gordon-Weeks PR. Distribution and expression of developmentally regulated phosphorylation epitopes on MAP 1B and neurofilament proteins in the developing rat spinal cord. J. Neurocytol. 1994;23:682–698. doi: 10.1007/BF01181643. [DOI] [PubMed] [Google Scholar]
- Carden MJ, Schlaepfer WW, Lee VM. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J. Biol. Chem. 1985;260:9805–9817. [PubMed] [Google Scholar]
- Coleman MP, Anderton BH. Phosphate-dependent monoclonal antibodies to neurofilaments and Alzheimer neurofibrillary tangles recognize a synthetic phosphopeptide. J. Neurochem. 1990;54:1548–1555. doi: 10.1111/j.1471-4159.1990.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 2003;206(Pt 7):1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- Dzandu JK, Johnson JF, Wise GE. Sodium dodecyl sulfate-gel electrophoresis: staining of polypeptides using heavy metal salts. Anal. Biochem. 1988;174:157–167. doi: 10.1016/0003-2697(88)90531-3. [DOI] [PubMed] [Google Scholar]
- Elhanany E, Jaffe H, Link WT, Sheeley DM, Gainer H, Pant HC. Identification of endogenously phosphorylated KSP sites in the high-molecular-weight rat neurofilament protein. J. Neurochem. 1994;63:2324–2335. doi: 10.1046/j.1471-4159.1994.63062324.x. [DOI] [PubMed] [Google Scholar]
- Elhanany E, Jaffe H, Link WT, Sheeley DM, Gainer H, Pant HC. Identification of endogenously phosphorylated KSP sites in the high-molecular-weight rat neurofilament protein. J. Neurochem. 1994;63:2324–2335. doi: 10.1046/j.1471-4159.1994.63062324.x. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- Gasser HS, Grundfest H. Axondiameters in relation to the spike dimensions and conduction velocity in mammalian A fibers. Am. J. Physiol. 1939;127:393–414. [Google Scholar]
- Giasson BI, Mushynski WE. Study of proline-directed protein kinases involved in phosphorylation of the heavy neurofilament subunit. J. Neurosci. 1997;17:9466–9472. doi: 10.1523/JNEUROSCI.17-24-09466.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidato S, Tsai LH, Woodgett J, Miller CC. Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J. Neurochem. 1996;66:1698–1706. doi: 10.1046/j.1471-4159.1996.66041698.x. [DOI] [PubMed] [Google Scholar]
- Hallows JL, Chen K, DePinho RA, Vincent I. Decreased cyclin-dependent kinase 5 (cdk5) activity is accompanied by redistribution of cdk5 and cytoskeletal proteins and increased cytoskeletal protein phosphorylation in p35 null mice. J. Neurosci. 2003;23:10633–10644. doi: 10.1523/JNEUROSCI.23-33-10633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlícek L, Hanus J, Veselý J, Leclerc S, Meijer L, Shaw G, Strnad M. Cytokinin-derived cyclin-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J. Med. Chem. 1997;40:408–412. doi: 10.1021/jm960666x. [DOI] [PubMed] [Google Scholar]
- Hisanaga S, Kusubata M, Okumura E, Kishimoto T. Phosphorylation of neurofilament H subunit at the tail domain by CDC2 kinase dissociates the association to microtubules. J. Biol. Chem. 1991;266:21798–21803. [PubMed] [Google Scholar]
- Huang KX, Paudel HK. Ser67-phosphorylated inhibitor 1 is a potent protein phosphatase 1 inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5824–5829. doi: 10.1073/pnas.100460897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H, Veeranna, Pant HC. Characterization of serine and threonine phosphorylation sites in beta-elimination/ethanethiol addition-modified proteins by electrospray tandem mass spectrometry and database searching. Biochemistry. 1998a;37:16211–16224. doi: 10.1021/bi981264p. [DOI] [PubMed] [Google Scholar]
- Jaffe H, Veeranna, Shetty KT, Pant HC. Characterization of the phosphorylation sites of human high molecular weight neurofilament protein by electrospray ionization tandem mass spectrometry and database searching. Biochemistry. 1998b;37:3931–3940. doi: 10.1021/bi972518u. [DOI] [PubMed] [Google Scholar]
- Keil-Dlouha VV, Zylber N, Tong N, Keil B. Cleavage of glucagon by alpha- and beta-trypsin. FEBS Lett. 1971;16:287–290. doi: 10.1016/0014-5793(71)80372-1. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Pearson RB. Design and use of peptide substrates for protein kinases. Methods Enzymol. 1991;200:121–134. doi: 10.1016/0076-6879(91)00134-i. [DOI] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- Kesavapany S, Patel V, Zheng YL, Pareek TK, Bjelogrlic M, Albers W, Amin N, Jaffe H, Gutkind JS, Strong MJ, et al. Inhibition of Pin1 reduces glutamate-induced perikaryal accumulation of phosphorylated neurofilament-H in neurons. Mol. Cell Biol. 2007;18:3645–3655. doi: 10.1091/mbc.E07-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J. Comp. Neurol. 1984;228:263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- Lee VM, Otvos LJ, Schmidt ML, Trojanowski JQ. Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc. Natl. Acad. Sci. USA. 1988;85:7384–7388. doi: 10.1073/pnas.85.19.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu. Rev. Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- Miyasaka H, Okabe S, Ishiguro K, Uchida T, Hirokawa N. Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by tau protein kinase II. J. Biol. Chem. 1993;268:22695–22702. [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Lewis SE. Differential turnover of phosphate groups on neurofilament subunits in mammalian neurons in vivo. J. Biol. Chem. 1986;261:16298–16301. [PubMed] [Google Scholar]
- Nixon RA, Logvinenko KB. Multiple fates of newly synthesized neurofilament proteins: evidence for a stationary neurofilament network distributed nonuniformly along axons of retinal ganglion cell neurons. J. Cell Biol. 1986;102:647–659. doi: 10.1083/jcb.102.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Sihag RK. Neurofilament phosphorylation: a new look at regulation and function. Trends Neurosci. 1991;14:501–506. doi: 10.1016/0166-2236(91)90062-y. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Quackenbush R, Vitto A. Multiple calcium-activated neutral proteinases (CANP) in mouse retinal ganglion cell neurons: specificities for endogenous neuronal substrates and comparison to purified brain CANP. J. Neurosci. 1986;6:1252–1263. doi: 10.1523/JNEUROSCI.06-05-01252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Lewis SE, Mercken M, Sihag RK. [32P]orthophosphate and [35S]methionine label separate pools of neurofilaments with markedly different axonal transport kinetics in mouse retinal ganglion cells in vivo. Neurochem. Res. 1994;19:1445–1453. doi: 10.1007/BF00972474. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM, Mathews PM, Mohan P, Golomb J. Sixth International Conference on Alzheimer's Disease. Amsterdam: 1998. Abnormalities of the endosomal-lysosomal system in Alzheimer's disease as potential therapeutic targets; p. S136. [Google Scholar]
- Ohshima T, Ogawa M, Veeranna, Hirasawa M, Longenecker G, Ishiguro K, Pant HC, Brady RO, Kulkarni AB, Mikoshiba K. Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain. Proc. Natl. Acad. Sci. USA. 2001;98:2764–2769. doi: 10.1073/pnas.051628498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Ong SE, Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell Proteomics. 2004;3:608–614. doi: 10.1074/mcp.T400003-MCP200. [DOI] [PubMed] [Google Scholar]
- Pant AC, Veeranna, Pant HC, Amin N. Phosphorylation of human high molecular weight neurofilament protein (hNF-H) by neuronal cyclin-dependent kinase 5 (cdk5) Brain Res. 1997;765:259–266. doi: 10.1016/s0006-8993(97)00561-1. [DOI] [PubMed] [Google Scholar]
- Pant HC. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 1988;256:665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant HC, Veeranna Neurofilament phosphorylation. Biochem. Cell Biol. 1995;73:575–592. doi: 10.1139/o95-063. [DOI] [PubMed] [Google Scholar]
- Pant HC, Veeranna, Grant P. Regulation of axonal neurofilament phosphorylation. Curr. Top Cell Regul. 2000;36:133–150. doi: 10.1016/s0070-2137(01)80006-6. [DOI] [PubMed] [Google Scholar]
- Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Probst A, Anderton BH, Ulrich J, Kohler R, Kahn J, Heitz PU. Pick's disease: an immunocytochemical study of neuronal changes. Monoclonal antibodies show that Pick bodies share antigenic determinants with neurofibrillary tangles and neurofilaments. Acta Neuropathol. 1983;60:175–182. doi: 10.1007/BF00691864. [DOI] [PubMed] [Google Scholar]
- Roder HM, Eden PA, Ingram VM. Brain protein kinase PK40ERK converts TAU into a PHF-like form as found in Alzheimer's disease. Biochem. Biophys. Res. Commun. 1993;193:639–647. doi: 10.1006/bbrc.1993.1672. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J. Neurosci. 1996;16:5095–5105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Sihag RK, Cleveland DW, Mohan P, Nixon RA. Local control of neurofilament accumulation during radial growth of myelinating axons in vivo. Selective role of site-specific phosphorylation. J. Cell Biol. 2000;151:1013–1024. doi: 10.1083/jcb.151.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Taoka M, Ishiguro K, Uchida A, Saito T, Isobe T, Hisanaga S. In vivo and in vitro phosphorylation at Ser-493 in the glutamate (E)-segment of neurofilament-H subunit by glycogen synthase kinase 3beta. J. Biol. Chem. 2002;277:36032–36039. doi: 10.1074/jbc.M206674200. [DOI] [PubMed] [Google Scholar]
- Sharma P, Veeranna, Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, Pant HC. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J. Biol. Chem. 2002;277:528–534. doi: 10.1074/jbc.M109324200. [DOI] [PubMed] [Google Scholar]
- Shetty KT, Link WT, Pant HC. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc. Natl. Acad. Sci. USA. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res. 2007;313:2098–2109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R, Hall FL, Monteiro MJ. A cdc2-like kinase distinct from cdk5 is associated with neurofilaments. J. Cell Sci. 1996;109:1565–1573. doi: 10.1242/jcs.109.6.1565. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl. Acad. Sci. USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger NH, Sternberger LA, Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1985;82:4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Leung CL, Liem RKH. Phosphorylation of the high molecular weight neurofilament protein (NF-H) by Cdk5 and p35. J. Biol. Chem. 1996;271:14245–14251. doi: 10.1074/jbc.271.24.14245. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Amin N, Grant P, Pant HC. P13suc1 associates with a cdc2-like kinase in a multimeric cytoskeletal complex in squid axoplasm. J. Neurosci. 1995;15:6222–6229. doi: 10.1523/JNEUROSCI.15-09-06222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Saito T, Hisanaga S, Pant HC, Kulkarni AB. Tau phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J. Biol. Chem. 2003;278:10506–10515. doi: 10.1074/jbc.M211964200. [DOI] [PubMed] [Google Scholar]
- Ulrich J, Haugh M, Anderton BH, Probst A, Lautenschlager C, His B. Alzheimer dementia and Pick's disease: neurofibrillary tangles and Pick bodies are associated with identical phosphorylated neurofilament epitopes. Acta. Neuropathol. 1987;73:240–246. doi: 10.1007/BF00686617. [DOI] [PubMed] [Google Scholar]
- Veeranna, Shetty KT, Amin N, Grant P, Albers RW, Pant HC. Inhibition of neuronal cyclin-dependent kinase-5 by staurosporine and purine analogs is independent of activation by Munc-18. Neurochem. Res. 1996;21:629–636. doi: 10.1007/BF02527763. [DOI] [PubMed] [Google Scholar]
- Veeranna, Grant P, Pant HC. Expression of p67 (Munc-18), Cdk5, P-NFH and syntaxin during development of the rat cerebellum. Dev Neurosci. 1997;19:172–183. doi: 10.1159/000111203. [DOI] [PubMed] [Google Scholar]
- Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (ERK1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J. Neurosci. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna, Kaji T, Boland B, Odrljin T, Mohan P, Basavarajappa BS, Peterhoff C, Cataldo A, Rudnicki A, Amin N, et al. Calpain mediates calcium-induced activation of the ERK1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer's disease. Am. J. Pathol. 2004;165:795–805. doi: 10.1016/S0002-9440(10)63342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FI, Hinton DR, Gilmore W, Trousdale MD, Fleming JO. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab. Invest. 1992;66:744–754. [PubMed] [Google Scholar]
- Wieboldt R, Zweigenbaum J, Henion J. Immunoaffinity ultrafiltration with ion spray HPLC/MS for screening small-molecule libraries. Anal. Chem. 1997;69:1683–1691. doi: 10.1021/ac9610265. [DOI] [PubMed] [Google Scholar]
- Xiao J, Monteiro MJ. Identification and characterization of a novel (115 kDa) neurofilament-associated kinase. J. Neurosci. 1994;14:1820–1833. doi: 10.1523/JNEUROSCI.14-03-01820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna, Liem RK, Eyer J, Peterson AC, Julien JP, Nixon RA. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J. Neurosci. 2006;26:10006–10019. doi: 10.1523/JNEUROSCI.2580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J. Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]