Abstract
Curcumin or diferuloylmethane is a yellow polyphenol extracted from the rhizome of turmeric (Curcuma longa). A large volume of published reports (numbering in several 100s) has established the anti-cancer and chemopreventative properties of curcumin in preclinical models of every known major cancer type. Nevertheless, the clinical translation of curcumin has been significantly hampered due to its poor systemic bioavailability, which mandates that patients consume up to 8-10 grams of the free drug orally each day, in order for detectable levels in the circulation. We have engineered a polymeric nanoparticle encapsulated curcumin formulation (NanoCurc™), which demonstrates remarkably higher systemic bioavailability in plasma and tissues compared to free curcumin upon parenteral administration. In xenograft models of human pancreatic cancer established in athymic mice, administration of parenteral NanoCurc™ significantly inhibits primary tumor growth in both subcutaneous and orthotopic settings. The combination of parenteral NanoCurc™ with gemcitabine results in enhanced tumor growth inhibition versus either single agent, suggesting an additive therapeutic influence in vivo. Furthermore, this combination completely abrogates systemic metastases in orthotopic pancreatic cancer xenograft models. Tumor growth inhibition is accompanied by significant reduction in activation of nuclear factor κ B (NFκB), as well as significant reduction in expression of matrix metalloproteinase MMP-9 and cyclin D1, in xenografts treated with NanoCurc™ and gemcitabine. NanoCurc™ is a promising new formulation that is able to overcome a major impediment for the clinical translation of curcumin to cancer patients by improving systemic bioavailability, and by extension, therapeutic efficacy.
Keywords: curcumin, polymeric nanoparticle, NanoCurc™, bioavailability, pancreatic cancer, metastases, orthotopic, NFκB, matrix metalloproteinase
Introduction
Curcumin or diferuloylmethane is a yellow polyphenol extracted from the rhizome of turmeric (Curcuma longa), a plant grown tropical Southeast Asia (1). Enthusiasm for curcumin as an anti-cancer agent evolved based on the wealth of epidemiological evidence suggesting a correlation between dietary turmeric and low incidence of gastrointestinal mucosal cancers in South East Asian populations (2). A large volume of experimental data has established the therapeutic efficacy of curcumin in preclinical models (principally, in cell lines) derived from a variety of solid tumors like pancreatic, colorectal, lung, breast, prostate and hepatocellular carcinoma, amongst others [selected reviews include (3-5)]. Equally important, free curcumin was shown not to be cytotoxic to normal cells, including hepatocytes, mammary epithelial cells, kidney epithelial cells, lymphocytes, and fibroblasts, at the dosages required for therapeutic efficacy against cancer cell lines; these in vitro findings are underscored by the limited human clinical trials performed with oral curcumin, wherein doses up to 8 grams per day have had minimal adverse effects, even to the highly exposed gastrointestinal mucosa (6, 7). In addition to a possible role in the therapy of established tumors, studies in numerous experimental (chemical) carcinogenesis models have confirmed that curcumin can ameliorate the progression to cancer in a variety of organ sites, reiterating this agent's potential for chemoprevention (8, 9).
Despite these encouraging results, the promise of curcumin in the clinic has never been fully realized. The single most important reason for this “benchside to bedside” disconnect has been the poor bioavailability of curcumin, such that its therapeutic effects are essentially limited to the tubular lower GI tract (i.e., colorectum) (10, 11). In a Phase I clinical trial, patients with hepatic colorectal cancer metastases were administered 3600mg of oral curcumin daily, and curcumin and its glucoronide and sulphate conjugates were detected in low nano-molar concentrations in the peripheral blood or portal circulation (12). In another Phase I study, patients were required to partake 8000mg of free curcumin orally per day, in order to achieve detectable systemic levels; beyond this dose, tolerability of the formulation was unacceptable to patients (13). In the few curcumin clinical trials that are currently active for visceral cancers (http://www.clinicaltrials.gov), patients have to partake as much as 8 – 10 grams of oral curcumin per day. There is little doubt that, despite the absence of dose limiting toxicity, such high doses severely impact upon patient compliance due to a metallic after-taste and associated GI discomfort. In view of these issues, there has been a considerable interest in developing formulations that allow for improved systemic bioavailability. We envisioned that nanoparticle-mediated drug delivery could be useful for harnessing the full potential of curcumin in the clinical arena.
In our previous proof-of-principle report, we demonstrated the comparable efficacy of curcumin loaded within polymeric nanoparticles (NanoCurc™) to that of free curcumin in vitro, thereby confirming that the biological activities of curcumin are retained upon nano-encapsulation (14). Here, we have assessed the bioavailability, toxicity, and in vivo therapeutic efficacy of parenteral NanoCurc™, either as a single agent, or upon combination with the anti-metabolite gemcitabine, in xenograft models of pancreatic cancer. We selected pancreatic cancer as our disease model in light of the uniformly dismal prognosis of this malignancy and the dire need for developing more effective therapies, particularly for combating systemic metastases that are present in the overwhelming majority of patients (15). With a median survival of ∼5-6 months for most individuals with advanced pancreatic cancer, current chemotherapeutic modalities (including gemcitabine, the standard-of-care agent) have had minimal success in ameliorating the poor survival outcomes for this disease. Our results confirm that parenteral NanoCurc™ significantly reduces primary tumor growth, as well as systemic metastases, and potentiates the effects of gemcitabine in both subcutaneous and orthotopic xenograft models. Further, we demonstrate that the potent in vivo effects of NanoCurc™ are observed at dosages that are ∼20-fold lower than that previously published with free curcumin for antitumor efficacy in pancreatic cancer xenograft models (1 gram/kg per day, albeit administered through the oral route) (16), underscoring the translational relevance of this novel nano-formulation for cancer therapy.
Materials and Methods
Materials
Ultra-pure curcumin (>99% diferuloylmethane) was purchased from Sabinsa Corporration (Piscataway, NJ); this source of curcumin has been used for both pre-clinical and clinical studies in the past (7, 12). Monomers for polymer nanoparticle synthesis - specifically N-isopropylacrylamide (NIPAAM), vinylpyrrolidone (VP), and acrylic acid (AA) - were obtained from Sigma Aldrich (St. Louis, MO). Reagents for the polymerization step, including NN′ methylene-bis-acrylamide (MBA), ammonium persulfate (APS), and ferrous sulfate (FeSO4) were also procured from Sigma. Gemcitabine (NetQem LLC, Research Triangle Park, NC) was stored at 4°C and dissolved in sterile NaCl (0.9% w/v) on the day of use. Reagents, used for western blot and immunohistochemistry were obtained from Invitrogen (Carlsbad, CA). Polyclonal antibodies against the p65 subunit of nuclear factor κ B (NFκB), cyclin-D1, and matrix metalloproteinase MMP-9 were obtained from Cell signaling (Beverly, MA). Anti β-actin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). In vivo xenograft studies were conducted using the low-passage metastatic human pancreatic cancer cell line, Pa03C (a.k.a. LZ10.7) (17). This cell line was part of the pancreas cancer genome sequencing effort, and harbors somatic mutations of both TP53 and KRAS2 (18).
Synthesis of NanoCurc™
Polymer nanoparticles comprised of NIPAAM, VP and AA were synthesized via free radical mechanism, according to the detailed synthesis method we have previously described (14, 19). The predistilled monomers of NIPAAM, VP and AA are mixed together in a molar ratio of 60:20:20, respectively, hence the acronym “NVA622” for the resulting polymer. Polymerization was performed for 24 hours at 30°C under an inert (nitrogen) atmosphere, using APS and FeSO4 as initiator and activator, respectively. After complete polymerization, the total aqueous solution of polymer was purified using dialysis, and then lyophilized for post loading of curcumin, as described (14). Typically, a 10 ml stock solution of polymeric nanoparticles (100 mg) was slowly mixed with 150μl of curcumin solution in chloroform (10 mg/ml), and gently stirred for 15-20 minutes on low heating, in order to load curcumin and evaporate chloroform simultaneously. The resulting solution, corresponding to 1.5% (w/w) loading of curcumin in nanoparticles, was then snap frozen on a dry ice/acetone bath, and lyophilized. The lyophilized NanoCurc™ powder is stored at 4°C until further use, whereupon simple reconstitution in an aqueous phase is required prior to parenteral administration.
Pharmacokinetic analyses of parenteral NanoCurc™ compared to free curcumin
In order to compare the in vivo pharmacokinetics of NanoCurc™ versus free curcumin suspended in corn oil, two cohorts of four mice each were administered a single intraperitoneal injection of either formulation, at an equivalent curcumin dose of 25 mg/kg. Pharmacokinetics data were analyzed by non-compartmental methods (WinNonlin Professional, version 5.2 software, Pharsight Corporation). Additional methodological details are available in Supplementary Data.
Establishment and treatment of Pa03C subcutaneous xenografts
All small animal (mouse) experiments described here conformed to the guidelines of the Animal Care and Use Committee of Johns Hopkins University. Mice were maintained in accordance to the guidelines of the American Association of Laboratory Animal Care. In order to generate subcutaneous Pa03C xenografts, flanks of 5-6 weeks old male athymic nu/nu mice (Harlan Laboratories, Indianapolis, IN) were injected with 2.5 × 106 Pa03C cells suspended in a total volume of 200 μl [PBS/Matrigel (BD Biosciences), 1:1 (v/v), prechilled to 4°C]. One week after the injection of tumor cells, subcutaneous tumor volumes (V) were measured with digital calipers (Fisher Scientific) and calculated using the formula V = 1/2(ab2), where a is the biggest and b is the smallest orthogonal tumor diameter (17, 20). Twenty mice with successfully engrafted Pa03C xenografts were then randomized into four cohorts of five animals each and administered one the following regimens (Supplementary Figure 1a): (i) void NVA622 polymeric nanoparticles, or (ii) single agent NanoCurc™ at a dose of 25 mg/kg i.p. twice daily, or (iii) single agent gemcitabine at a dose of 20 mg/kg i.p. twice weekly, or (iv) the combination of NanoCurc™ (25mg/kg i.p. twice daily) and gemcitabine (20 mg/kg i.p. twice weekly). The daily dose of NanoCurc was selected based on maximal tolerated volume of intraperitoneal injection over 24 hour period in mice. Gemcitabine was administered for a cycle of two weeks (i.e., total of four doses of the drug), with one additional week of NanoCurc™ in both single-agent and combination arms. Tumor size and body weight were measured once weekly. At the culmination of treatment, visceral organs and tumor tissues were harvested and either preserved in 10% neutral buffered formalin for histology and immunohistochemical studies, or snap frozen for pharmacokinetics and pharmacodynamic analyses (see Supplementary Methods). Intra-tumoral curcumin concentrations were estimated by liquid chromatography-tandem mass spectrometry (LC-MS/MS), as previously described (21).
Establishment and treatment of Pa03C orthotopic xenografts
The generation of orthotopic Pa03C human pancreatic cancer xenografts by surgical implantation in athymic mice has been described previously by our group (17, 20). Briefly, subcutaneous xenograft tumors (Pa03C) were harvested under sterile conditions and minced into 1mm3 cubes for orthotopic implantation. A small pocket was prepared inside the pancreas, into which one of the previously prepared fresh tumor chunks was inserted. Three weeks after surgical orthotopic implantation, the presence of “primary” tumors was confirmed by ultrasound scan (Vevo660™, VisualSonics) and measured in three orthogonal axes, a, b, and c; tumor volumes were determined as V = (abc) / 2, as described (17, 20). Twenty-eight mice with demonstrable “primary” xenografts were then randomized into four cohorts, with seven mice / arm, as follows (Supplementary Figure 1b): (i) void NVA622 polymeric nanoparticles, or (ii) single agent NanoCurc™ at a dose of 25 mg/kg i.p. twice daily, or (iii) single agent gemcitabine at a dose of 20 mg/kg i.p. twice weekly, or (iv) the combination of NanoCurc™ (25mg/kg i.p. twice daily) and gemcitabine (20 mg/kg i.p. twice weekly). Both NanoCurc™ and gemcitabine were administered for a period of three weeks.
At the culmination of therapy, the mice were euthanized; spleen, liver, kidneys, intestine, peritoneum, and lungs were inspected for grossly visible metastases. In addition to macroscopic inspection, visceral organs (including any enlarged lymph nodes) and tumor tissues were harvested and preserved in 10% neutral buffered formalin for histology and immunohistochemical studies, including a rigorous effort to detect any organ-specific micrometastases. Additionally, tumor tissues were snap-frozen for pharmacokinetics and pharmacodynamic analyses (see Supplementary Methods). Intra-tumoral curcumin concentrations were estimated by LC-MS/MS (21).
Results
NanoCurc™ readily overcomes the bioavailability pitfall of free curcumin in vivo
To improve the systemic bioavailability of free curcumin, the free compound was encapsulated in NVA622 polymeric nanoparticles (henceforth, referred to as NanoCurc™). We compared the bioavailability of NanoCurc™ to free curcumin dissolved in corn oil, following administration of a single dose of either formulation (equivalent to 25mg/kg of curcumin) through the intraperitoneal route in non-tumor bearing mice (Figure 1a). Relevant pharmacokinetic parameters, including Cmax, Tmax and area under the curve (AUC) were calculated and compared for both formulations (see Supplementary Methods). As evident from the figure, marked differences in the bioavailability of curcumin were observed. NanoCurc™ showed sustained plasma concentrations of curcumin with a Tmax of 2.75±1.50 hours, and Cmax of 17176±5176ng/mL, whereas mean plasma levels of free curcumin in corn oil were barely detectable above the LC-MS/MS limit of quantitation. A large difference in the AUC values were also observed, with NanoCurc™ having an AUC of 153865 ± 30445ng*hr/mL (N = 4) and free curcumin in corn oil an AUC of 445ng*hr/mL (N = 1; in the remaining three mice, curcumin concentrations were below the detection limit). In addition to this single dose study, we also determined steady state levels of curcumin in plasma, as well as in tissue samples, following the administration of parenteral NanoCurc™ (25 mg/kg equivalent curcumin, twice daily i.p.) to non-tumor bearing mice for 4 weeks (Figure 1b). Curcumin was readily detectable in all visceral tissues, as well as in plasma at the end of four weeks of therapy. Body weights were also monitored on a weekly basis to observe for any signs of systemic toxicity (Figure 1c). No evidence of loss in body weight, or behavioral abnormalities, was found during the course of treatment. Histological evaluation of major visceral organs (kidneys, pancreas, lungs, liver, spleen, and intestines) did not demonstrate any overt microscopic abnormalities (Figure 1d). These pilot experiments confirmed the enhanced systemic bioavailability of NanoCurc™ compared to free curcumin, as well as the absence of overt toxicity in mice, allowing us to proceed to in vivo therapeutic efficacy trials in xenograft models of pancreatic cancer.
Figure 1. In vivo pharmacokinetics, biodistribution and toxicity analysis of NanoCurc™.
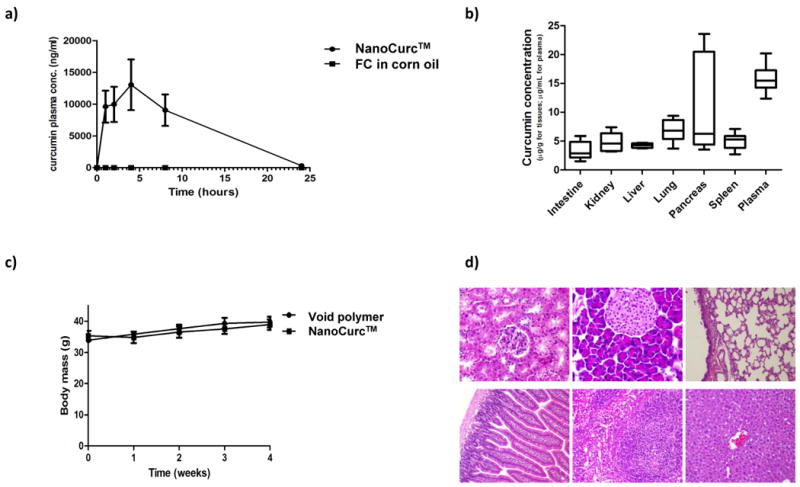
(a) Comparative pharmacokinetics of parenteral NanoCurc™ versus free curcumin in corn oil, administered in non-tumor bearing mice. Plasma concentrations of curcumin (ng/mL) were determined at 1, 2, 4, 8 and 24 hours after single intraperitoneal administration of either formulation, at an equivalent dose of 25 mg/kg curcumin. See text for analytical details.
(b) Tissue and plasma levels of curcumin assessed following necropsy in non-tumor bearing mice receiving 4 weeks of parenteral NanoCurc™. Curcumin levels were measured by LC-MS/MS, and are expressed as μg/g for tissues, and in μg/mL for plasma. Horizontal line indicates mean of levels measured in three mice. The anatomic site is indicated on the x-axis.
(c) No significant alterations in body mass (grams) are observed in cohorts of non-tumor bearing mice (N=3) receiving either NanoCurc™ or void NVA622 polymer for four weeks.
(d) Histopathological assessment of visceral tissues (clockwise from top left, kidney, pancreas, lung, liver, spleen, and intestine) obtained at necropsy from mice receiving 4 weeks of NanoCurc™ demonstrate no abnormalities.
NanoCurc™ inhibits the growth of subcutaneous Pa03C xenografts, and potentiates the effects of gemcitabine in this setting
The in vivo therapeutic efficacy of NanoCurc™ on tumor growth was assessed either alone, or in combination with gemcitabine, in subcutaneous Pa03C xenografts. When compared to animals treated with void NVA622 polymer, there was significant tumor growth inhibition in mice receiving single-agent NanoCurc™ (∼50% reduction in mean tumor volume, P<0.01, Figure 2a and b). Single-agent gemcitabine was very effective in inhibiting Pa03C tumor growth in the subcutaneous milieu, and superfluously, there was no significant difference in mean tumor volumes between this cohort versus mice receiving combination therapy with NanoCurc™ and gemcitabine. However, a readout of “mean tumor volume” per se greatly underestimated the effects of combination therapy, as four of five xenografts underwent complete histological regression in this cohort (see the inset with magnified Y-axis in Figure 2b, highlighting the onset of tumor regression in the combination therapy cohort during week 3). Thus, only a single residual xenograft “nubbin” was observed at the culmination of therapy in the combination group, illustrated in the photomicrograph panel on the right (Figure 2a). Plasma curcumin measured using samples obtained at necropsy confirmed comparable levels of drug between single-agent and combination arms (Supplementary Figure 2).
Figure 2. Parenteral NanoCurc™ significantly inhibits the growth of subcutaneous pancreatic cancer xenografts, and therapeutic efficacy is further potentiated by the combination with gemcitabine.
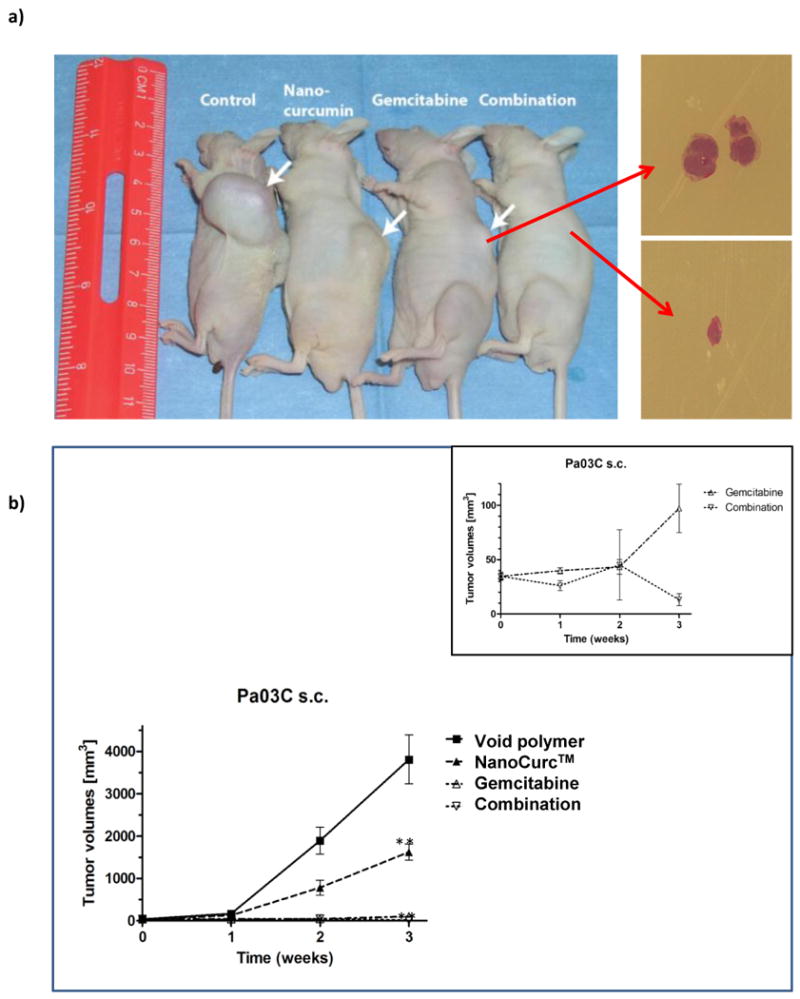
(a) Subcutaneous xenografts were established using the low-passage Pa03C (a.k.a. LZ10.7) human pancreatic cancer cell line, and mice were randomized to four arms, including (i) control (void NVA622 polymer), (ii) NanoCurc™, (iii) gemcitabine, and (iv) combination of NanoCurc™ and gemcitabine. Treatment was culminated at three weeks. Representative xenografted mice from each of the four arms are illustrated. While single-agent NanoCurc™ significantly blocked tumor growth compared to void NVA622 arm (see panel b), the results were even more striking upon administration with gemcitabine, wherein four of five xenografts demonstrated complete regression in the combination arm. Photomicrographs to the right depict a representative xenograft from the gemcitabine arm, and the single residual “nubbin” from the combination therapy group.
(b) Graphical depiction of the tumor volumes in the four arms, over the three week time course of therapy. Single-agent NanoCurc™ demonstrates significant reduction in tumor volume compared to void NVA622 (control) arm at three weeks (double asterisk, P<0.01). No significant difference is observed in the tumor volumes between single-agent gemcitabine and combination arms; however, this data underestimates the effect of combination therapy, as four of the five xenografts had undergone complete histological regression (see panel a), and only a residual “nubbin” of tumor from a single xenograft was available for measurement. The inset demonstrates the comparative tumor volume data for gemcitabine and combination arms using a magnified Y-axis, which illustrates the clear separation in growth curves between the two arms during the third week.
NanoCurc™ inhibits the growth of Pa03C orthotopic xenografts, and abrogates systemic metastases upon combination with gemcitabine
While subcutaneous pancreatic cancer xenografts provide preliminary insights into therapeutic efficacy, they do not recapitulate one of the most critical features of the cognate human disease, namely, systemic metastases (17, 20). Moreover, the subcutaneous milieu may not accurately reflect the microenvironment of the pancreas, nor simulate the drug distribution kinetics observed in the specific organ (likely accounting for the observed efficacy of even single-agent gemcitabine in this setting, see above) (22). In this respect, intra-pancreatic orthotopic xenografts have been considered to be more biologically relevant (23, 24). Therefore, we investigated the efficacy of NanoCurc™ as single-agent, or in combination with gemcitabine, in orthotopically implanted Pa03C pancreatic cancer xenografts. Compared to void NVA622 polymer-treated mice, there was significant reduction (double asterisk = P<0.01) in growth of the “primary” tumor in mice receiving either NanoCurc™ or gemcitabine alone (Figures 3a and 3b); the effects of the two single agents were comparable and no significant difference was noted. In contrast, there was further significant accentuation of growth inhibition when the two agents were combined (double asterisk = P<0.01 compared to either single agent; triple asterisk = P<0.001 compared to control arm), confirming the prior observations that curcumin can have an additive effect with conventional chemotherapeutics in vivo (5, 25-28), including potentiating the effects of gemcitabine against pancreatic cancer cells (16).
Figure 3. Parenteral NanoCurc™ significantly inhibits the growth of orthotopic pancreatic cancer xenografts, including the abrogation of systemic metastases upon combination with gemcitabine.
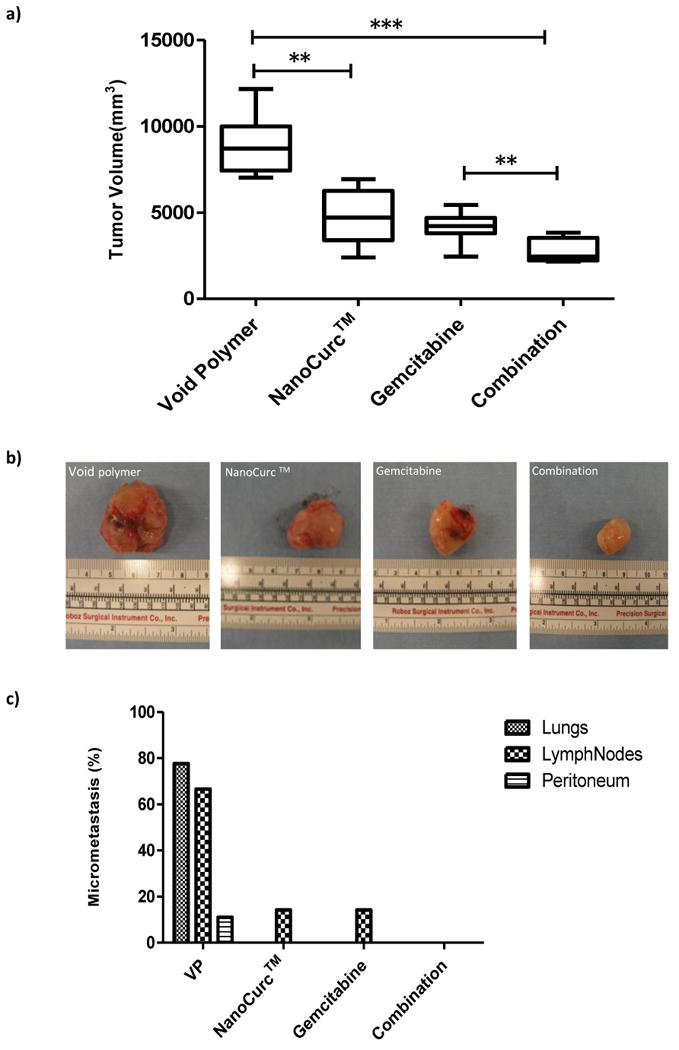
(a) Graphical illustration of tumor volumes for orthotopic Pa03C pancreatic cancer xenografts treated with NanoCurc™, gemcitabine, or the combination, compared to void NVA622 polymer. Single agent NanoCurc™ leads to significant inhibition of the “primary” tumor volume compared to void polymer (double asterisks), and the effect is further accentuated upon addition of gemcitabine (triple asterisks). Note that the combination also has a more significant effect on tumor volume compared to single-agent gemcitabine (double asterisks). All treatments were carried out for a period of three weeks; horizontal lines represent the average of measurements in seven mice per arm.
(b) Representative excised Pa03C xenografts from the control (far left) and three treatment arms (NanoCurc™, gemcitabine, and combination, respectively) are designated.
(c) Control mice receiving void polymer (VP) demonstrate extensive micrometastases to the lungs, lymph nodes and peritoneum (also see Supplementary Figure 3). Both single-agent therapy arms demonstrate considerable reduction in micrometastatic disease (albeit still present in the lymph nodes), while the combination arm demonstrates complete abrogation of micrometastatic disease in all examined viscera.
Apart from significantly inhibiting “primary” tumor growth, NanoCurc™ also showed profound effects on metastatic tumor spread (Figure 3c). In the control arm, 5 of 7 (71%) mice exhibited histologically confirmed metastases to the lungs and regional lymph nodes (Supplementary Figure 3), with 1 of 7 (14%) mice also demonstrating peritoneal metastases. Both NanoCurc™ and gemcitabine administered as single agents led to striking and comparable reduction in metastases, with only lymph node metastases seen in 1of 7 (14%) mice, and absence of lung or peritoneal disease. In the combination group, complete abrogation of systemic metastases was observed in all microscopically examined viscera. Of note, while our results mirror those previously observed by combining free curcumin with gemcitabine in preclinical models (16), the reduction in primary tumor growth and abrogation of systemic metastases was observed at a NanoCurc™ dose that was ∼20-fold lower than used previously with free curcumin (1gram/kg per day, albeit through the oral route).
NanoCurc™ downregulates activation of nuclear factor kappa B (NFκB) in subcutaneous and orthotopic pancreatic cancer xenograft models
NFkB is a transcription factor that activates cell survival pathways in cancer cells, and renders them resistant to conventional cytotoxic agents (29, 30). NFkB is constitutively active in human pancreatic cancer (31). Prior studies have established that free curcumin is a potent inhibitor of NFkB activity in cancer cells (25, 26, 32, 33), which was also confirmed previously by our group in vitro in pancreatic cancer cell lines using nanoparticulated curcumin (14). We now demonstrate that systemic NanoCurc™ also blocks NFkB activation in vivo, in both subcutaneous and orthotopic settings. Two different assay methods were used to enhance the stringency of our observations. In subcutaneous Pa03C xenografts, NFkB activity was assessed by quantitative immunohistochemistry for nuclear localization of p65, the principal subunit protein of NFkB. Quantification of nuclear p65 labeling demonstrated significant reduction in NanoCurc™-treated xenografts versus those treated with void NVA622 polymer (Figure 4a). Of note, we were only able to compare NanoCurc™-treated group with the control arm due to the lack of availability of sufficient tumor material in the combination group. The “gold standard” for assessing NFkB activity is to determine its DNA binding capacity in an electrophoretic mobility shift assay (EMSA) (34). We had sufficient tumor material from all four arms in the orthotopic P03C study to perform EMSA, and nuclear extracts were obtained from two representative xenografts in each cohort (see Supplementary methods); intra-tumoral curcumin concentrations were assessed to determine whether there was a dose-dependent effect of curcumin in the tumor milieu on NFκB activation. As seen in Figure 4b, there was downregulation in the DNA-binding capacity of NFkB in both xenografts receiving combination therapy (Lanes 5 and 6), and in one of two xenografts receiving single agent NanoCurc™ (Lane 3) - all three had robust intra-tumoral curcumin levels (>2.5μg/g of tissue). In contrast, the second xenograft administered single-agent NanoCurc™ (Lane 4), which retained NFκB activity, had ∼4-fold lower curcumin levels in the tumor than its counterpart. Thus, the anti-cancer effects of systemic NanoCurc™ appear to be mediated via the same major intracellular pathways as free curcumin.
Figure 4. NanoCurc™ inhibits nuclear factor kappa B (NFκB) activation in both subcutaneous and orthotopic pancreatic cancer xenografts.
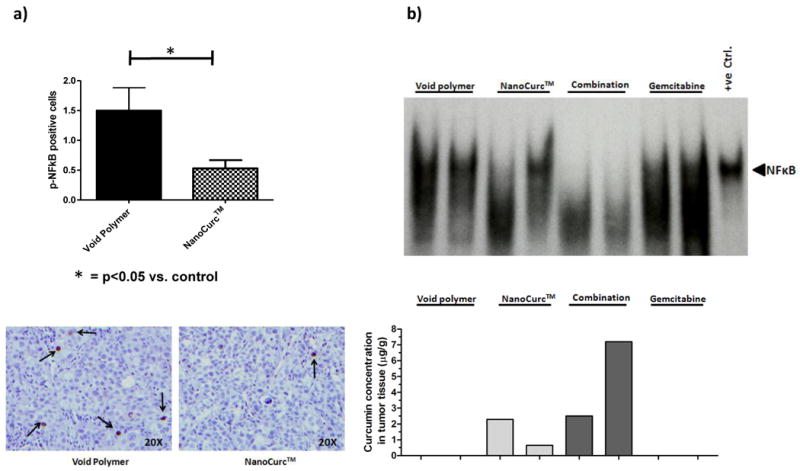
(a) In subcutaneous Pa03C xenografts, immunohistochemistry for nuclear p65 (active NFκB) demonstrates significant reduction in nuclear labeling in NanoCurc™-treated xenografts (hashed bar) compared to those receiving void NVA622 polymer (black bar). The Y-axis designates number of cells with nuclear p65 staining per high power filed (40X), over an average of 10 randomly selected fields. Bottom panel illustrates photomicrograph of p65 immunohistochemistry in the two arms, at the given (40X) objective. Please note that due to only a single residual “nubbin” of tumor in the combination therapy arm, immunohistochemistry could not be reliably performed in this case.
(b) Electrophoretic mobility shift assay (EMSA) was performed as a “gold standard” for NFκB activity in orthotopic Pa03C xenografts, treated with vehicle (Lanes 1 and 2), NanoCurc™ (Lanes 3 and 4), combination (Lanes 5 and 6) and gemcitabine (Lanes 7 and 8); Lane 9 is a Jurkat cell line nuclear lysate with activated NFκB (positive control). The intra-tumoral curcumin concentrations for each pair of xenografts in each arm are represented at the bottom (Y-axis equals concentration in μg/g of tissue). Reduction in DNA binding ability of NFκB (as indicated by gel shift) is observed in both xenografts in the combination arm (Lanes 5 and 6), as well as in one of two xenografts receiving single-agent NanoCurc™ (Lane 3), all three of which have robust levels of intra-tumoral curcumin (>2.5μg/g of tissue). In contrast, one of the two xenografts receiving single agent NanoCurc™ (Lane 4) with retained NFκB activity also demonstrates ∼4-fold lower intra-tumoral curcumin concentration (∼0.6μg/g). Expectedly, no reduction in DNA binding ability is seen for the xenografts treated with vehicle or single agent gemcitabine only.
NanoCurc™ in combination with gemcitabine inhibits cyclin D1 and matrix metalloproteinase MMP-9 in orthotopic Pa03C xenografts
Curcumin has pleiotropic effects on cancer cells, one of which is inhibition of proliferation through downregulation of cyclin D1, as previously described (35, 36). In orthotopic Pa03C xenografts, we did not observe a significant difference in CCDN1 (gene encoding cyclin D1) transcripts measured by qRT-PCR in either single agent therapy arm compared to the void polymer-treated control xenografts (Supplementary Figure 4a); in contrast, however, we detected a significant reduction (P<0.01) in CCDN1 expression in xenografts administered combination NanoCurc™ and gemcitabine. We then assessed for expression of the matrix metalloproteinase MMP9 transcripts, as this has been previously identified as another bona fide target of curcumin in cancer cells (37-39). Comparable to CCDN1, we observed a significant reduction in MMP9 transcripts in the combination therapy arm (P<0.01), when compared to the control xenografts and either single agent tumor cohort (Supplementary Figure 4b). The mRNA data was confirmed at the protein level using representative xenografts from each of the four arms (Supplementary Figure 4c), and the results were particularly striking for MMP-9, where we observed near-total abrogation of this matrix metalloproteinase in the combination therapy group. It is unclear why the effects on curcumin targets is significantly different in the combination therapy arm compared to xenografts receiving single-agent NanoCurc™. It is possible that the enhanced MMP-9 downregulation represents an additive influence of gemcitabine itself, which has been shown to modestly reduce matrix metalloproteinase levels in pancreatic cancer xenografts when used as a single agent (40), and this effect could well be independent of NFkB. Similarly, gemcitabine as an anti-metabolite, likely has NFκB independent effects on cell cycling, and hence, on the expression of cyclin D1, which could explain the additive effects observed in combination therapy. Finally, we performed studies on proliferation and microvessel density in the treated xenografts, which are presented in the Supplementary Results (see Supplementary Figure 5).
Discussion
In this manuscript, we describe the in vivo application of a polymeric nanoparticle encapsulated formulation of curcumin (NanoCurc™) in preclinical models of pancreatic cancer. Parenteral NanoCurc™ is able to overcome one of the primary pitfalls of free curcumin, namely sub-optimal systemic bioavailability (10), without compromising the therapeutic efficacy of this promising natural compound. We demonstrate that single-agent NanoCurc™ inhibits the growth of pancreatic cancer xenografts in both subcutaneous and orthotopic settings, and in particular, we confirm the ability of NanoCurc™ in potentiating gemcitabine efficacy, as evidenced by histological regression in subcutaneous xenografts, and complete abrogation of metastases emanating from orthotopic tumors upon combination therapy. NanoCurc™ appears to function through blockade of the same major intracellular pathways (for example, NFκB) in cancer cells as free curcumin (1, 4). The readily demonstrable levels of tissue curcumin in most visceral organs suggest that this formulation could potentially be used for treatment of visceral malignancies, including metastases, beyond the tubular GI tract. While NanoCurc™ is not orally bioavailable and must be administered as an injectable formulation this would not impede its applicability to patients with established cancers, since the majority of chemotherapeutics are parenterally administered. In fact, one of the most widely utilized nanoparticle formulations of an anti-cancer agent (nab-paclitaxel or Abraxane®) is administered solely through the parenteral route (41). Our pilot toxicity experiment, albeit not “regulatory-grade” by any means, does provide some degree of reassurance that the formulation and dosing regimen used does not entail obvious systemic adverse effects. NanoCurc™ can be readily stored at room temperature as a lyophilized powder, and can also be transported as such, requiring only reconstitution in an aqueous phase at the point of destination, all of which should facilitate eventual application in a clinical setting.
While our prior report on polymeric nanoparticle-encapsulated curcumin was the first attempt at engineering a nanoparticle formulation of this compound (14), multiple investigators have synthesized a variety of curcumin nanoparticles since that time (42-50); of note, all but two (47, 50) have restricted their observations to the in vitro setting, and none have performed in vivo xenograft treatment studies. Thus, to the best of our knowledge, our study is the first to rigorously demonstrate that curcumin nano-encapsulation has tangible therapeutic effects in vivo, when used as either single agent, or in combination with a conventional anti-metabolite. We emphasize that our study neither demonstrates, nor claims superiority, of NanoCurc™ over any given curcumin nanoparticle formulation, and it is likely that many of the published platforms will have “niche” applications in cancer. For example, some of the recent curcumin nanoparticles have sustained release properties (45, 50), and could be applicable in the setting of chemoprevention (analogous to a “patch” or “depot” preparation). Most importantly, our study reinforces the emerging notion that nanotechnology provides a highly appropriate avenue for harnessing the full potential of promising, yet poorly bioavailable, natural anticancer agents like curcumin (10, 11).
Supplementary Material
Acknowledgments
Financial Support: Supported by R01CA113669 (A.M.); R01CA13767 (A.M.); P01CA134292 (A.M.); P30CA069773 (M.A.R.); Flight Attendants Medical Research Institute (FAMRI) (A.M. and M.A.R.); Johns Hopkins CTSA Institute for Clinical and Translational Research (UL1RR025005) (M.A.R.); The Sol Goldman Pancreatic Cancer Research Center (A.M.); Michael Rolfe Foundation for Pancreatic Cancer Research (A.M.); the German Academic Exchange (DAAD) postdoctoral program (G.F.); and SignPath Pharmaceuticals, Inc (A.M.).
Abbreviations
- NIPAAM
NVA622 = N-isopropylacrylamide
- VP
vinylpyrrolidone
- AA
acrylic acid, in a molar ratio of 60:20:20
Footnotes
Conflicts of Interest: NanoCurc™ is a registered trademark of SignPath Pharmaceuticals, Inc., Quakerstown, Pennsylvania. Dr. Maitra is a member of the scientific advisory board of SignPath Pharma, Inc., and any conflicts of interest under this arrangement are handled in accordance with the Johns Hopkins University Office of Policy Coordination (OPC) guidelines.
References
- 1.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 2.Mohandas KM, Desai DC. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol. 1999;18:118–21. [PubMed] [Google Scholar]
- 3.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Limtrakul P. Curcumin as chemosensitizer. Adv Exp Med Biol. 2007;595:269–300. doi: 10.1007/978-0-387-46401-5_12. [DOI] [PubMed] [Google Scholar]
- 6.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–45. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–9. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 9.Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–90. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Bisht S, Maitra A. Systemic Delivery of Curcumin: 21st Century Solutions for an Ancient Conundrum. Curr Drug Discov Technol. 2009 doi: 10.2174/157016309789054933. [DOI] [PubMed] [Google Scholar]
- 11.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 12.Garcea G, Jones DJ, Singh R, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer research. 2001;21:2895–900. [PubMed] [Google Scholar]
- 14.Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer research. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 17.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer research. 2007;67:2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisht S, Feldmann G, Koorstra JB, et al. In vivo characterization of a polymeric nanoparticle platform with potential oral drug delivery capabilities. Mol Cancer Ther. 2008;7:3878–88. doi: 10.1158/1535-7163.MCT-08-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmann G, Fendrich V, McGovern K, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7:2725–35. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Z, Shayeganpour A, Brocks DR, Lavasanifar A, Samuel J. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed Chromatogr. 2007;21:546–52. doi: 10.1002/bmc.795. [DOI] [PubMed] [Google Scholar]
- 22.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer biology & therapy. 2003;2:S134–9. [PubMed] [Google Scholar]
- 24.Troiani T, Schettino C, Martinelli E, Morgillo F, Tortora G, Ciardiello F. The use of xenograft models for the selection of cancer treatments with the EGFR as an example. Crit Rev Oncol Hematol. 2008;65:200–11. doi: 10.1016/j.critrevonc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–8. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 26.Deeb D, Jiang H, Gao X, et al. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004;3:803–12. [PubMed] [Google Scholar]
- 27.Chirnomas D, Taniguchi T, de la Vega M, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–61. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Deeb D, Jiang H, Liu YB, Dulchavsky SA, Gautam SC. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol. 2005;5:39–48. [PubMed] [Google Scholar]
- 29.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nature reviews. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 30.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res. 2004;119:139–73. doi: 10.1007/1-4020-7847-1_8. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 32.Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci. 2004;1030:434–41. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–62. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 34.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 35.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 36.Tomita M, Kawakami H, Uchihara JN, et al. Curcumin (diferuloylmethane) inhibits constitutive active NF-kappaB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006;118:765–72. doi: 10.1002/ijc.21389. [DOI] [PubMed] [Google Scholar]
- 37.Hong JH, Ahn KS, Bae E, Jeon SS, Choi HY. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006;9:147–52. doi: 10.1038/sj.pcan.4500856. [DOI] [PubMed] [Google Scholar]
- 38.Ohashi Y, Tsuchiya Y, Koizumi K, Sakurai H, Saiki I. Prevention of intrahepatic metastasis by curcumin in an orthotopic implantation model. Oncology. 2003;65:250–8. doi: 10.1159/000074478. [DOI] [PubMed] [Google Scholar]
- 39.Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma AV. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. The Journal of biological chemistry. 2005;280:9409–15. doi: 10.1074/jbc.M413398200. [DOI] [PubMed] [Google Scholar]
- 40.Haq M, Shafii A, Zervos EE, Rosemurgy AS. Addition of matrix metalloproteinase inhibition to conventional cytotoxic therapy reduces tumor implantation and prolongs survival in a murine model of human pancreatic cancer. Cancer research. 2000;60:3207–11. [PubMed] [Google Scholar]
- 41.Henderson IC, Bhatia V. Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert review of anticancer therapy. 2007;7:919–43. doi: 10.1586/14737140.7.7.919. [DOI] [PubMed] [Google Scholar]
- 42.Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4:1752–61. doi: 10.1016/j.actbio.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 2009 doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Koppolu B, Rahimi M, Nattama S, Wadajkar A, Nguyen KT. Development of multiple-layer polymeric particles for targeted and controlled drug delivery. Nanomedicine. 2009 doi: 10.1016/j.nano.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer research. 2009;29:3867–75. [PubMed] [Google Scholar]
- 46.Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine. 2009;4:115–22. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–30. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Shutava TG, Balkundi SS, Vangala P, et al. Layer-by-Layer-Coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols. ACS Nano. 2009 doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 49.Sou K, Oyajobi B, Goins B, Phillips WT, Tsuchida E. Characterization and cytotoxicity of self-organized assemblies of curcumin and amphiphatic poly(ethylene glycol) J Biomed Nanotechnol. 2009;5:202–8. doi: 10.1166/jbn.2009.1025. [DOI] [PubMed] [Google Scholar]
- 50.Anand P, Nair HB, Sung B, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2009;79:330–8. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


