Abstract
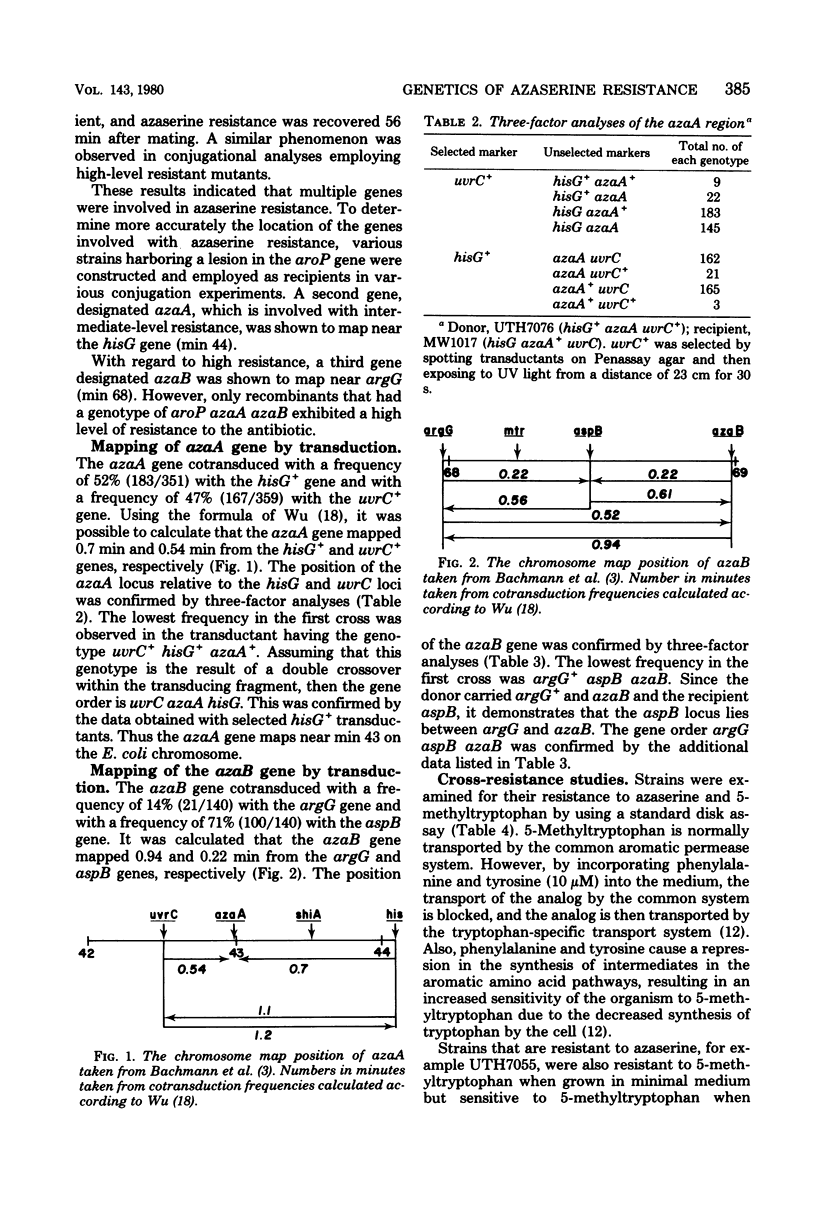
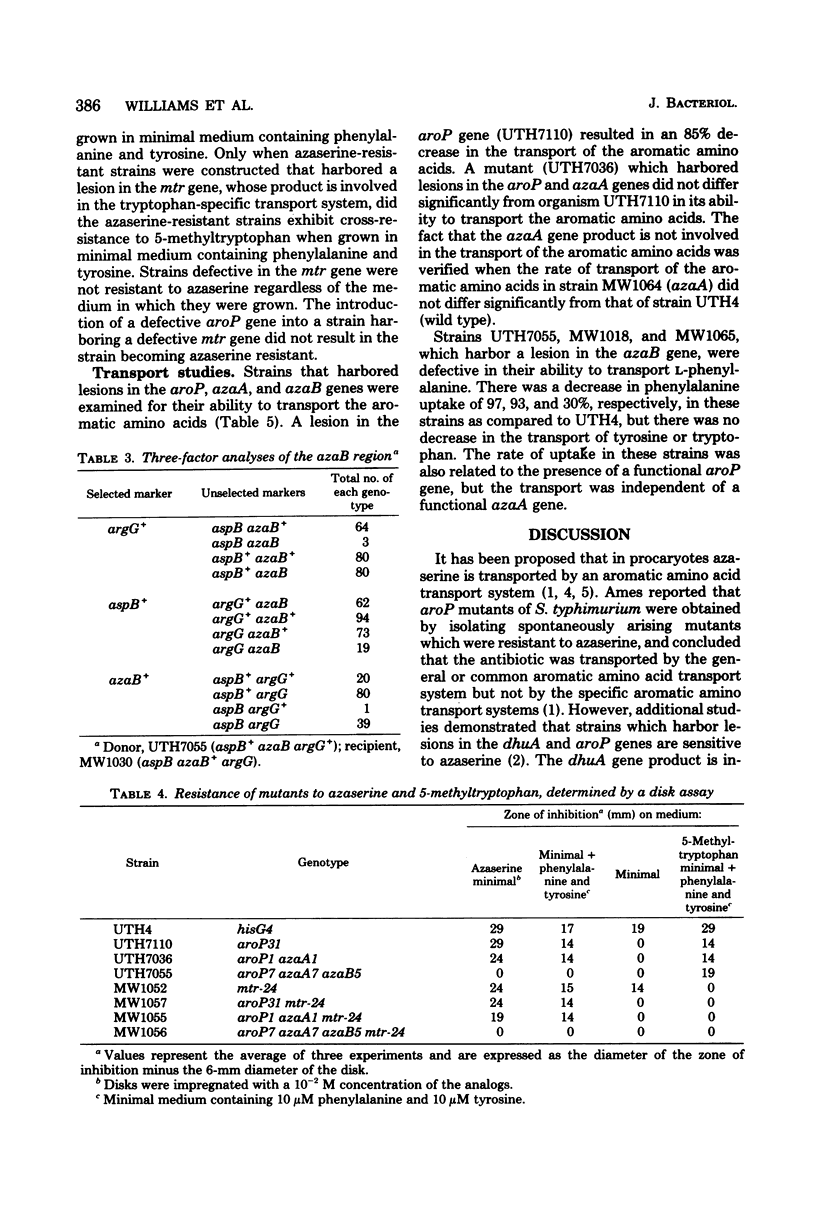
Resistance to azaserine in Escherichia coli is the result of mutations in at least three different loci. All spontaneously arising azaserine-resistant mutants harbor a lesion in the aroP gene. However, a lesion in this gene is not solely responsible for resistance. All spontaneously arising intermediate-level azaserine-resistant mutants also harbor a lesion in a gene designated azaA, which lies near min 43 on the chromosome. High-level resistant mutants harbor lesions in the aroP and azaA genes and in a third gene designated azaB, which lies near min 69 on the chromosome. Transport studies demonstrate that mutants harboring lesions in the azaA gene are not defective in the transport of the aromatic amino acids, but that mutants which harbor lesions in the azaB gene are defective in phenylalanine transport but not in tyrosine or tryptophan transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Lever J. E. The histidine-binding protein J is a component of histidine transport. Identification of its structural gene, hisJ. J Biol Chem. 1972 Jul 10;247(13):4309–4316. [PubMed] [Google Scholar]
- BENNETT L. L., Jr, SCHABEL F. M., Jr, SKIPPER H. E. Studies on the mode of action of azaserine. Arch Biochem Biophys. 1956 Oct;64(2):423–436. doi: 10.1016/0003-9861(56)90286-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Brock M. L. REVERSAL OF AZASERINE BY PHENYLALANINE. J Bacteriol. 1961 Feb;81(2):212–217. doi: 10.1128/jb.81.2.212-217.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Maintenance and exchange of the aromatic amino acid pool in Escherichia coli. J Bacteriol. 1971 Apr;106(1):70–81. doi: 10.1128/jb.106.1.70-81.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- MATNEY T. S., ACHENBACH N. E. New uses for membrane filters III. Bacterial mating procedure. J Bacteriol. 1962 Oct;84:874–875. doi: 10.1128/jb.84.4.874-875.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne G. M., Corwin L. M. Mutations affecting aromatic amino acid transport in Escherichia coli and Salmonella typhimurium. J Gen Microbiol. 1975 Oct;90(2):203–216. doi: 10.1099/00221287-90-2-203. [DOI] [PubMed] [Google Scholar]
- Whipp M. J., Pittard A. J. Regulation of aromatic amino acid transport systems in Escherichia coli K-12. J Bacteriol. 1977 Nov;132(2):453–461. doi: 10.1128/jb.132.2.453-461.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. V., Rowe J. J., Kerr T. J., Tritz G. J. Studies on the modes of action of azaserine in Escherichia coli. Mechanism of resistance to azaserine. Microbios. 1977;19(77-78):181–190. [PubMed] [Google Scholar]
- Williams M. V., Tritz G. J. Studies on the modes of action of azaserine inhibition of Escherichia coli. Potentiation of phenylalanine reversal. J Antimicrob Chemother. 1977 Jan;3(1):65–77. doi: 10.1093/jac/3.1.65. [DOI] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


