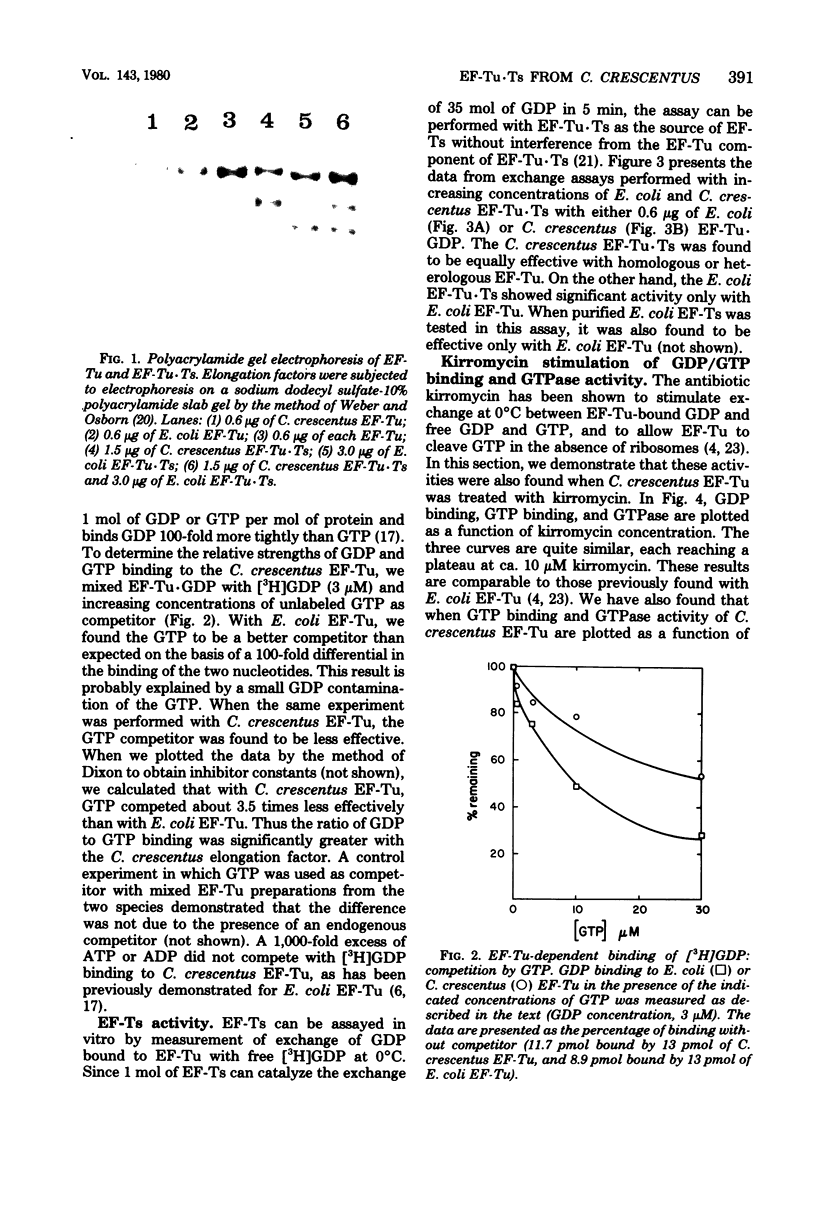
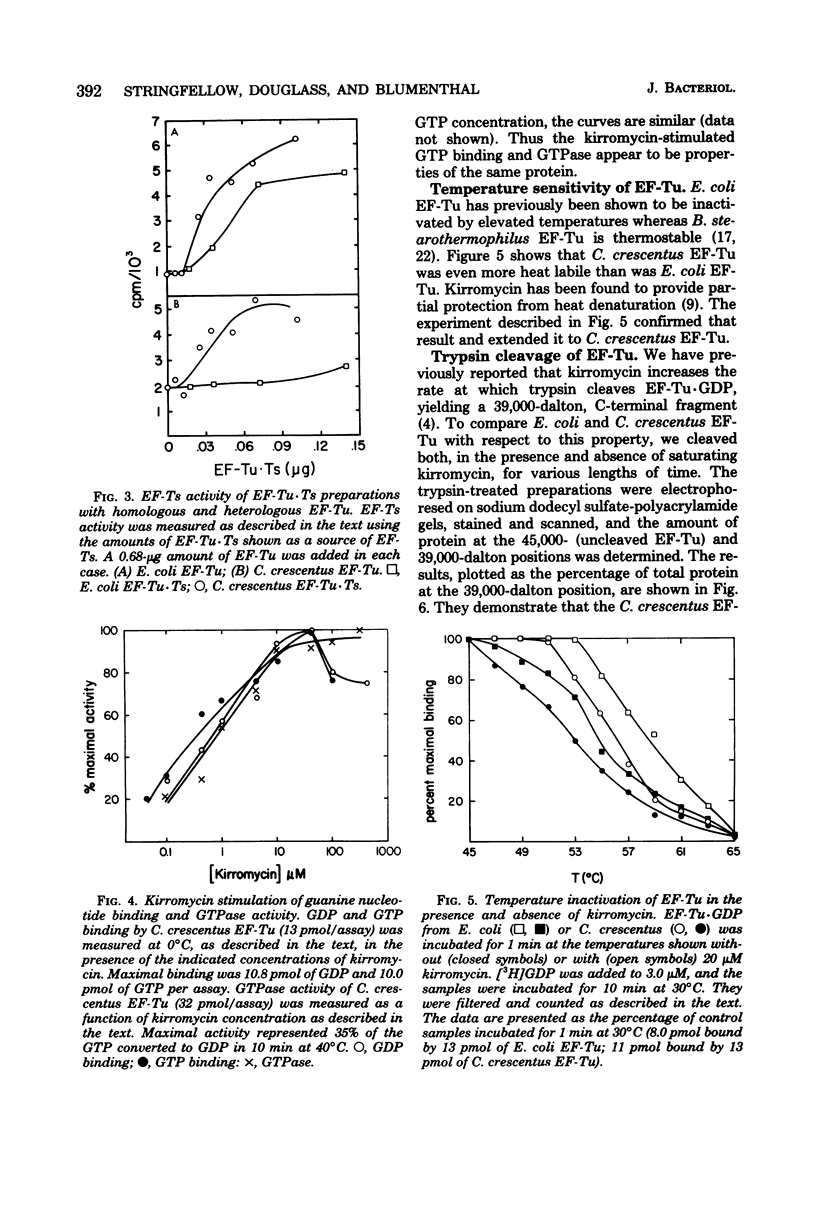
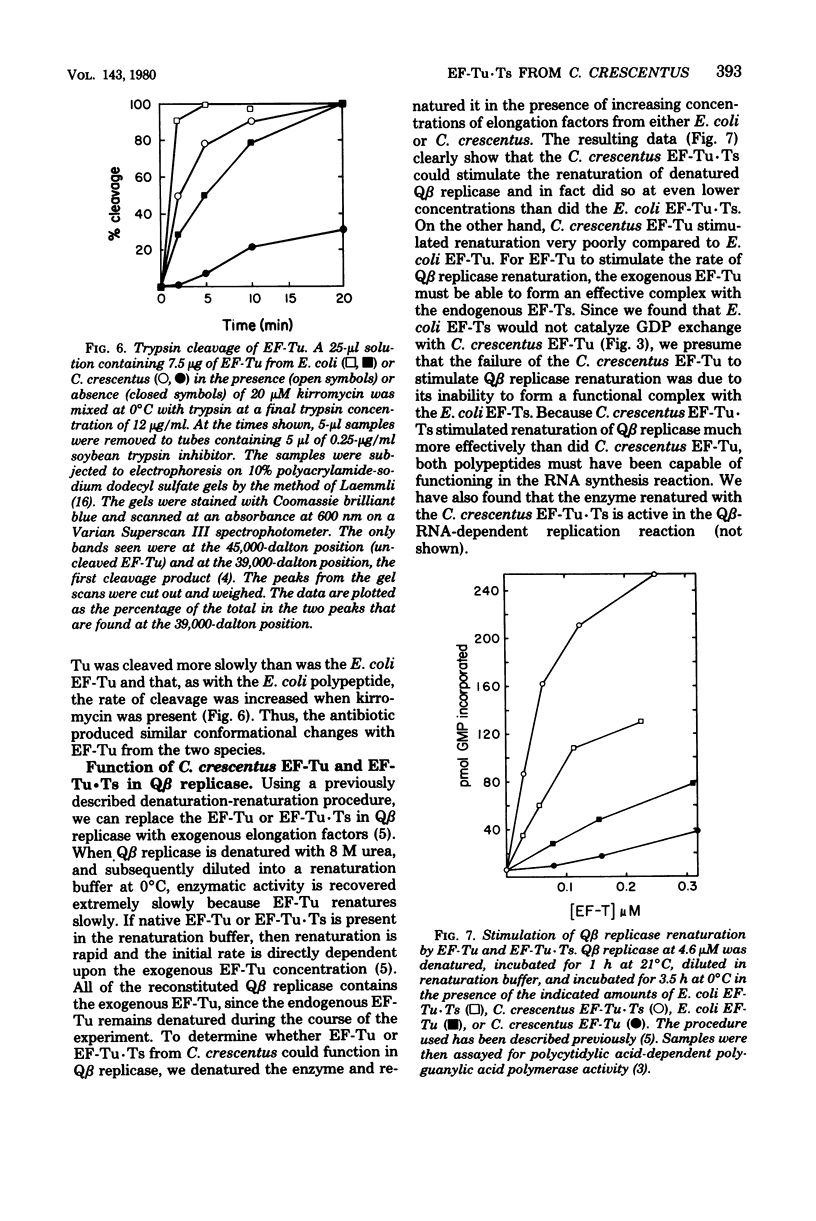
Abstract
The protein synthesis elongation factors Tu and Ts are responsible for binding aminoacyl-transfer ribonucleic acid (RNA) to the ribosome. In addition, they perform an undefined function, as the EF-Tu.Ts complex, in the RNA phage RNA replicases. In an effort to obtain insight into these two apparently unrelated roles, we purified the elongation factors from Caulobacter crescentus and compared them to the analogous Escherichia coli polypeptides. Although most physical and functional characteristics were found to be similar, significant differences were found in the molecular weight of EF-Ts and relative affinities of guanine nucleotides, sensitivity to trypsin cleavage, and rate of heat denaturation of EF-Tu. The antibiotic kirromycin was active with EF-Tu from both bacterial species. When C. crescentus EF-Tu.Ts was substituted for the E. coli elongation factors in Q beta phage RNA replicase, an enzyme capable of apparently normal RNA synthetic activity was formed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Beck B. D. Polymerization of the bacterial elongation factor for protein synthesis, EF-Tu. Eur J Biochem. 1979 Jul;97(2):495–502. doi: 10.1111/j.1432-1033.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A. Renaturation of a multisubunit multiactivity enzyme complex: recovery of phage Qbeta RNA replicase, EF-Tu, and EF-Ts activities after denaturation in urea. Biochemistry. 1976 Jan 27;15(2):422–425. doi: 10.1021/bi00647a028. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T. Qbeta RNA replicase and protein synthesis elongation factors EF-Tu and EF-Ts. Methods Enzymol. 1979;60:628–638. doi: 10.1016/s0076-6879(79)60059-9. [DOI] [PubMed] [Google Scholar]
- Brown S., Blumenthal T. Function and structure in ribonucleic acid phage Qbeta ribonucleic acid replicase. Effect of inhibitors of EF-Tu on ribonucleic acid synthesis and renaturation of active enzyme. J Biol Chem. 1976 May 10;251(9):2749–2753. [PubMed] [Google Scholar]
- Brown S., Blumenthal T. Reconstitution of Qbeta RNA replicase from a covalently bonded elongation factor Tu-Ts complex. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1131–1135. doi: 10.1073/pnas.73.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinali G., Wolf H., Parmeggiani A. Effect of kirromycin on elongation factor Tu. Location of the catalytic center for ribosome-elongation-factor-Tu GTPase activity on the elongation factor. Eur J Biochem. 1977 May 2;75(1):55–65. doi: 10.1111/j.1432-1033.1977.tb11503.x. [DOI] [PubMed] [Google Scholar]
- Douglass J., Blumenthal T. Conformational transition of protein synthesis elongation factor Tu induced by guanine nucleotides. Modulation by kirromycin and elongation factor Ts. J Biol Chem. 1979 Jun 25;254(12):5383–5387. [PubMed] [Google Scholar]
- Filer D., Furano A. V. Portions of the gene encoding elongation factor Tu are highly conserved in prokaryotes. J Biol Chem. 1980 Jan 25;255(2):728–734. [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. The subcellular distribution and state of the elongation factor Tu in extracts of Escherichia coli B. Eur J Biochem. 1976 May 1;64(2):597–606. doi: 10.1111/j.1432-1033.1976.tb10339.x. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. Abundance and membrane association of elongation factor Tu in E. coli. Nature. 1976 May 6;261(5555):23–26. doi: 10.1038/261023a0. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nisen P., Medford R., Mansour J., Purucker M., Skalka A., Shapiro L. Cell-cycle-associated rearrangement of inverted repeat DNA sequences. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6240–6244. doi: 10.1073/pnas.76.12.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. RNA polymerase specificity and the control of growth. Nature. 1976 Oct 21;263(5579):641–646. doi: 10.1038/263641a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weissbach H., Miller D. L., Hachmann J. Studies on the role of factor Ts in polypeptide synthesis. Arch Biochem Biophys. 1970 Mar;137(1):262–269. doi: 10.1016/0003-9861(70)90433-9. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Leberman R. Elongation factor T from Bacillus stearothermophilus and Escherichia coli. Purification and some properties of EF-Tu and EF-Ts from Bacillus stearothermophilus. Eur J Biochem. 1976 Feb 16;62(2):373–382. doi: 10.1111/j.1432-1033.1976.tb10169.x. [DOI] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Kirromycin, an inhibitor of protein biosynthesis that acts on elongation factor Tu. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4910–4914. doi: 10.1073/pnas.71.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Mechanism of the inhibition of protein synthesis by kirromycin. Role of elongation factor Tu and ribosomes. Eur J Biochem. 1977 May 2;75(1):67–75. doi: 10.1111/j.1432-1033.1977.tb11504.x. [DOI] [PubMed] [Google Scholar]
- Wurtz M., Jacobson R. J., Steven A. C., Rosenbusch J. P. Paracrystalline arrays of protein-synthesis elongation factor Tu. Comparison with polymerized actin. Eur J Biochem. 1978 Aug 1;88(2):593–597. doi: 10.1111/j.1432-1033.1978.tb12485.x. [DOI] [PubMed] [Google Scholar]



