Abstract
The ompA gene of Escherichia coli codes for a major protein of the outer membrane. When this gene was moved between various unrelated strains (E. coli K-12 and two clinical isolates of E. coli) by transduction, the gene was expressed very poorly. Recombinants carrying “foreign” genes produced no OmpA protein which could be detected on polyacrylamide gels and became resistant to bacteriophage K3, which uses this protein as receptor. The recombinants were sensitive to host-range mutants of K3, indicating a very low level of OmpA protein was produced. When an E. coli K-12 recombinant carrying an unexpressed foreign ompA allele was subjected to two cycles of selection for an OmpA+ phenotype, a mutant strain was obtained which was sensitive to K3 and which expressed nearly normal levels of OmpA protein in the outer membrane. This strain carried mutations in the foreign ompA gene, as indicated both by genetic mapping and the alteration of a peptide in the mutant OmpA protein. The ability of the OmpA protein to bind to lipopolysaccharide (LPS) showed similar strain specificity, and the mutant OmpA protein which was expressed in an unrelated host showed enhanced ability to bind LPS from its new host. Thus, cell surface expression of the ompA gene appears to depend upon the ability of the gene product to bind LPS, suggesting that an interaction between the protein and LPS plays an essential role in biosynthesis of this outer membrane protein.
Full text
PDF




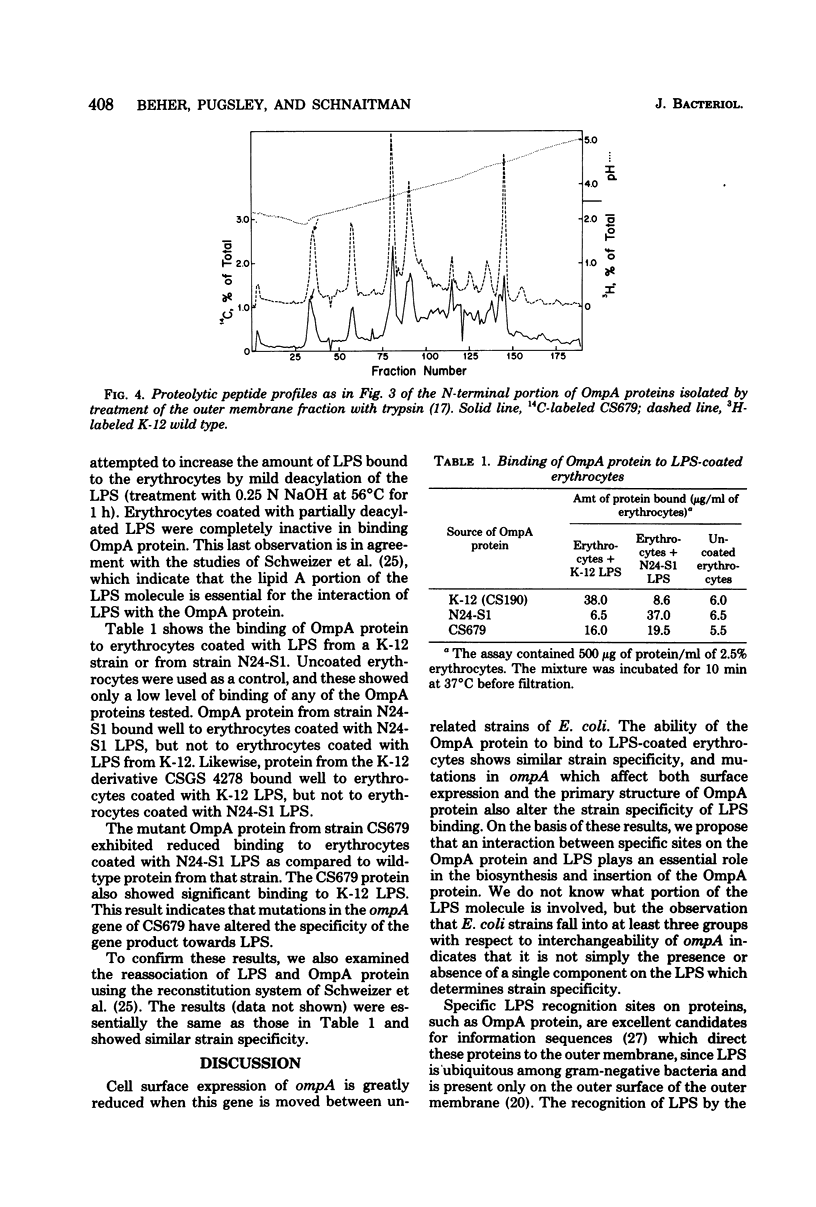


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Krämer C., Henning U. Diploidy for a structural gene specifying a major protein of the outer cell envelope membrane from Escherichia coli K-12. J Bacteriol. 1976 Dec;128(3):834–841. doi: 10.1128/jb.128.3.834-841.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Summers A. O., Schnaitman C. A. Outer membrane proteins of Escherichia coli. V. Evidence that protein 1 and bacteriophage-directed protein 2 are different polypeptides. J Bacteriol. 1977 Aug;131(2):598–607. doi: 10.1128/jb.131.2.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Hindennach I., Haller I. The major proteins of the Escherichia coli outer cell envelope membrane: evidence for the structural gene of protein II. FEBS Lett. 1976 Jan 1;61(1):46–48. doi: 10.1016/0014-5793(76)80168-8. [DOI] [PubMed] [Google Scholar]
- Henning U., Royer H. D., Teather R. M., Hindennach I., Hollenberg C. P. Cloning of the structural gene (ompA) for an integral outer membrane protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4360–4364. doi: 10.1073/pnas.76.9.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. R., Schnaitman C. A., Pugsley A. P. Chemical heterogeneity of major outer membrane pore proteins of Escherichia coli. J Bacteriol. 1979 Jun;138(3):861–870. doi: 10.1128/jb.138.3.861-870.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Pugsley A. P., Reeves P. Defective growth functions in mutants of Escherichia coli K12 lacking a major outer membrane protein. J Mol Biol. 1977 Oct 25;116(2):285–300. doi: 10.1016/0022-2836(77)90217-0. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Puspurs A., Reeves P. Outer membrane of Escherichia coli K-12: isolation of mutants with altered protein 3A by using host range mutants of bacteriophage K3. J Bacteriol. 1976 Sep;127(3):1080–1084. doi: 10.1128/jb.127.3.1080-1084.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Sekizawa J., Inouye S., Halegoua S., Inouye M. Precursors of major outer membrane proteins of Escherichia coli. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1126–1133. doi: 10.1016/s0006-291x(77)80095-8. [DOI] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Insertion of newly synthesized proteins into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1978 Sep 22;512(2):365–376. doi: 10.1016/0005-2736(78)90260-2. [DOI] [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Nature of the regions involved in the insertion of newly synthesized protein into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1979 May 17;553(2):224–234. doi: 10.1016/0005-2736(79)90227-x. [DOI] [PubMed] [Google Scholar]
- van Alphen W., Lugtenberg B., Berendsen W. Heptose-deficient mutants of Escherichia coli K12 deficient in up to three major outer membrane proteins. Mol Gen Genet. 1976 Sep 23;147(3):263–269. doi: 10.1007/BF00582877. [DOI] [PubMed] [Google Scholar]



