Abstract
Background
Treatment for lexical retrieval impairment has been shown to yield positive outcomes in individuals with aphasia due to focal lesions, but there has been little research regarding the treatment of such impairments in individuals with progressive aphasia.
Aims
The purpose of this study was to examine the therapeutic effects of a semantic treatment for anomia in progressive aphasia relative to the outcome in an individual with stroke-induced aphasia.
Methods & Procedures
Two individuals with progressive aphasia and one with aphasia resulting from stroke participated in the study. Each participant presented with fluent, anomic aphasia; however, one of the patients with progressive aphasia demonstrated characteristics indicating a likely progression towards non-fluency. Each participant received a brief, intensive treatment intended to improve lexical retrieval in the context of generative naming for selected semantic categories. Treatment tasks included guided lexical retrieval prompted by the identification and elaboration of items within semantic subcategories, as well as other semantic tasks. Treatment outcomes were quantified using standard effects sizes as well as nonparametric tests comparing pre- versus post-treatment performance.
Outcomes & Results
One of the individuals with progressive aphasia showed large treatment effects for lexical retrieval of items from targeted semantic categories. The other progressive aphasia patient showed very small effects overall for treated categories. The individual with the focal lesion due to stroke showed medium-sized effects for trained categories as well as significant improvement on a standardised measure of naming.
Conclusions
Findings indicate that intensive, semantically based treatment for lexical retrieval can result in positive outcomes in individuals with progressive as well as stroke-induced aphasia. Examination of individual differences suggests that the status of semantic and episodic memory may provide predictive information regarding responsiveness to treatment.
Keywords: Progressive aphasia, Treatment, Rehabilitation, Anomia, Lexical retrieval
Primary progressive aphasia (PPA) is an acquired impairment of language, with relative sparing of other aspects of cognition, which results from degenerative neurological disease. Progressive aphasia is often compared with stroke-induced aphasia in its behavioural manifestations, with published case reports comprising a range of language profiles. These are often characterised using traditional aphasia types, including anomic, Broca’s, conduction, Wernicke’s, and transcortical aphasias. It has been suggested, however, that these are imperfect descriptors for the language deficits observed in progressive aphasia because the damage to the brain in PPA is degenerative and less likely to be purely focal (Clark, Charuvastra, Miller, Shapira, & Mendez, 2005; Mesulam, 2001). An alternative view is that, aside from the degenerative nature of PPA, aphasia represents the same fundamental disorder in its progressive and static forms (McNeil & Duffy, 2001). Whether the same treatment approaches can be applied successfully in cases of focal and progressive damage is an area as yet largely unexplored.
Lexical retrieval impairment, or anomia, is the most pervasive of the language deficits associated with aphasia. This is the case for both stroke-induced and progressive forms of the disorder. Models of lexical retrieval posit that two distinct central processing levels, one semantic and the other phonological, are critically involved in spoken word production (for a review, see Raymer & Gonzales-Rothi, 2001). Lexical retrieval impairment may result from damage to either the semantic store itself, to lexical phonological representations, or to the connections between the two levels. It follows that treatments for anomia fall into two categories: those that are phonological and those that are semantic in nature. Many treatments, however, contain elements of both (Nickels, 2002).
Semantically based anomia treatment has proven effective in patients with aphasia resulting from focal damage, including those with and without damage to the semantic system (e.g., Boyle, 2004; Drew & Thompson, 1999; Hillis, 1991; Lowell, Beeson, & Holland, 1995; Nickels & Best, 1996). Some semantic treatments have attempted to improve naming performance through remediation of a damaged semantic system. In these cases, improved naming is thought to result from rebuilding of underspecified or degraded representations. For example, Hillis (1991) implemented a treatment wherein a patient with an underlying semantic impairment was provided with semantic information in the event of written naming errors. When the patient produced a semantic error, semantic features of the target item were contrasted with those of the patient’s semantic error. This treatment resulted in improved performance for treated items across a variety of lexical tasks. Other treatments have utilised semantic tasks to improve lexical retrieval by increasing semantic activation when there is no frank semantic impairment, but rather a post-semantic deficit affecting retrieval of items in the phonological output lexicon. For example, semantic feature matrix training, in which individuals are encouraged to engage in systematic retrieval of semantic attributes of items, has proven beneficial in individuals with impaired retrieval of phonological word forms (e.g., Boyle, 2004; Lowell et al., 1995).
Only a handful of studies have examined the rehabilitation of anomia in cases of progressive language impairment (Frattali, 2004; Graham, Patterson, Pratt, & Hodges, 1999, 2001; Jokel, Cupit, Rochon, & Graham, 2007; Jokel, Rochon, & Leonard, 2002, 2006; McNeil, Small, Masterson, & Fossett, 1995). One study examined the use of a semantic and phonological cueing hierarchy for training predicate adjectives in an individual with PPA whose naming impairment was described as an “accessing deficit” (McNeil et al., 1995). A positive treatment outcome was observed for trained items, with generalisation to untargeted words and word classes.
Several more studies have examined the effects of treatment for anomia in individuals with semantic dementia (SD), a type of progressive language impairment wherein the gradual loss of semantic knowledge results in anomia, comprehension impairment and, in some cases, face and object recognition deficits (Hodges, Patterson, Oxbury, & Funnell, 1992; Neary et al., 1998). Graham and colleagues explored the effect of repeated rehearsal of names paired with pictures and/or descriptions of targets in an individual with SD (Graham et al., 1999, 2001). The participant demonstrated significant improvement for trained items and categories; however, constant exposure to trained items was necessary to prevent a decline in performance. Further, the authors suggested that improved word naming in target categories was more likely attributable to episodic than semantic memory. In other words, improvement was considered to be a result of rote memorisation, mediated by medial temporal lobe structures, rather than enhanced semantic representations for targets.
Snowden and Neary (2002), intrigued by the ability of some of their SD patients to learn new, personally relevant information, despite the loss of common concepts, examined word learning in two SD patients. They found that the ability to relearn lexical targets via training with pictures paired with written/spoken words was dependent on the degree of residual semantic knowledge for those items. They also observed that the inclusion of personally relevant cues linking targets with a participant’s own experience was beneficial, resulting in improvements in naming that were maintained to some degree for 6 months. Finally, the authors found that improved naming performance was mediated by contextual information from training sessions, such that performance on probes was enhanced when the test was formatted (order of items as well as colour of paper) in the same manner as the practice materials. The authors concluded that spared conceptual knowledge pertaining to a particular target, as well as spatial and temporal contextual information, could mediate word learning in SD.
Jokel et al. (2002, 2006) implemented a homework-based treatment for lexical retrieval in a patient with SD, which involved rehearsal of picture names with cues consisting of personally relevant semantic information. Trained items included those that the patient could name and comprehend, could not name but could comprehend, and those that could neither be named nor comprehended. Significant improvement was observed for both sets of items that the participant could not name prior to treatment, with greater and longer-lasting effects for items that were comprehended prior to the initiation of treatment. Results also suggested that the practice regimen slowed the progression of naming impairment for targeted items that the participant was able to name prior to treatment.
While not directly comparing outcomes in patients with focal versus progressive brain damage, these studies, which include elements of both semantic and phonological approaches, indicate that similar treatment techniques may benefit both types of patients. The studies involving SD patients suggest that individuals with semantic deficits may, however, require special consideration, given that erosion of the semantic store may make relearning of lexical items particularly difficult.
The purpose of the present study was to directly compare a single treatment approach in patients with progressive disease relative to an individual with focal damage. To do so, we examined the therapeutic effects of an intensive semantic treatment for lexical retrieval in three individuals with aphasia—two with progressive aphasia and one with aphasia resulting from stroke.
METHOD
Two individuals with progressive language impairment (PA 1 and PA 2) and one with aphasia resulting from left hemisphere stroke (LH stroke) participated in this study (see Table 1). PA 1 began experiencing a decline in language approximately 5 years prior and PA 2 approximately 6 years prior to the start of the study. LH stroke was 6 years, 11 months post onset of aphasia. All individuals presented with anomic aphasia according to the Western Aphasia Battery (WAB; Kertesz, 1982). Of the two participants with progressive aphasia, one (PA 1) demonstrated fluent language with some characteristics indicating a progression towards non-fluency including mild agrammatism, phonemic paraphasias, and a mild apraxia of speech (Neary et al., 1998). The other individual with progressive language impairment (PA 2) presented with fluent, somewhat empty spontaneous speech, mild impairment on a nonverbal measure of semantic relations (The Pyramids and Palm Trees Test, PPT; Howard & Patterson, 1992), as well as surface dysgraphia. Nonverbal semantic processing was spared in PA 1 and the focal lesion patient, as evidenced by normal performance on the PPT test. The status of episodic memory was assessed using the Warrington Recognition Memory Test, which examines recognition memory for both faces and words in a two-alternative forced choice format (Warrington, 1984). Of the three participants, only PA 2 showed evidence of impairment on this measure. His performance suggested some degree of impairment to episodic memory, with a slightly greater deficit in verbal recognition memory. This pattern is consistent with the presence of primarily left hemisphere atrophy.
TABLE 1.
Demographic characteristics and standardised test scores for participants
| Age | Sex | WAB AQ | BNT | PPT | Memory:faces | Memory:words | |
|---|---|---|---|---|---|---|---|
| PA 1 | 71 | F | 88.8* | 37* | 51 | 46 | 43 |
| PA 2 | 78 | M | 88.0* | 31* | 46* | 37* | 31* |
| LH stroke | 79 | M | 93.4* | 24* | 49 | 43 | 43 |
Clinically significant impairment.
WAB AQ: Western Aphasia Battery Aphasia Quotient; max. score = 100.
BNT: Boston Naming Test; max. score = 60.
PPT: Pyramids and Palm Trees Test; max. score = 52.
Memory: faces: Warrington Recognition Memory Test, faces subtest; max. score = 50.
Memory: words: Warrington Recognition Memory Test, words subtest; max. score = 50.
Each individual received a regimen of intensive treatment, conducted over a brief span of time, which was intended to improve lexical retrieval in the context of generative naming for selected semantic categories. Participants received treatment once daily for 90 minutes, for a total of 12 treatment sessions over 16 days. Each of three categories of living things and three of non-living things was trained for two sessions, at which point treatment for a new category was begun. Each category to be trained was matched in terms of difficulty (based on the performance of a group of 13 age-matched normal controls) with an untrained category. Treated and untreated categories were held constant across individuals, but were randomised for order of treatment (see Table 2 for a listing of paired trained and untrained categories).
TABLE 2.
Verbal fluency probe data for each participant for trained (in bold) and matched untrained categories
| Pre1 | Pre2 | Pre3/Tr1 | Tr2 | Post1 | Post2 | Post3 | Post4 | Post5 | Post6 | FU1 | FU2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA 1 | Tools | 6 | 5 | 6 | 10 | 19 | 17 | 15 | 18 | 16 | 17 | 19 | 9 |
| Furniture | 10 | 12 | 11 | 11 | 12 | 12 | 14 | 12 | 15 | 15 | 14 | 11 | |
| Musical instruments | 9 | 8 | 7 | 11 | 14 | 15 | 13 | 12 | 16 | 13 | 12 | ||
| clothing | 18 | 15 | 14 | 17 | 20 | 15 | 20 | 15 | 16 | 17 | |||
| Vegetables | 14 | 14 | 13 | 11 | 22 | 19 | 24 | 21 | 19 | ||||
| Fruits | 12 | 13 | 13 | 12 | 14 | 14 | 15 | 14 | 13 | ||||
| Dogs | 6 | 5 | 6 | 15 | 14 | 16 | 15 | 14 | 11 | ||||
| Flowers | 7 | 11 | 8 | 10 | 9 | 9 | 9 | 11 | 6 | ||||
| Kitchen utensils | 9 | 11 | 6 | 19 | 18 | 15 | 15 | 9 | |||||
| Modes of transportation | 10 | 12 | 13 | 13 | 14 | 10 | 8 | ||||||
| Birds | 13 | 14 | 15 | 16 | 20 | 18 | 13 | ||||||
| Sea animals | 6 | 7 | 8 | 10 | 9 | 9 | 8 |
| Pre1 | Pre2 | Pre3/Tr1 | Tr2 | Post1 | Post2 | Post3 | Post4 | Post5 | FU | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PA 2 | Tools | 7 | 5 | 9 | 7 | 8 | 8 | 6 | 8 | 9 | 9 |
| Furniture | 3 | 3 | 5 | 3 | 5 | 8 | 5 | 5 | 5 | 5 | |
| Vegetables | 3 | 6 | 7 | 6 | 12 | 8 | 2 | 6 | 4 | ||
| Fruits | 5 | 4 | 4 | 4 | 2 | 3 | 2 | 3 | 2 | ||
| Musical instruments | 5 | 4 | 5 | 5 | 7 | 5 | 6 | 4 | |||
| clothing | 5 | 7 | 5 | 8 | 9 | 8 | 7 | 6 | |||
| Birds | 3 | 4 | 6 | 6 | 10 | 7 | 7 | ||||
| Sea animals | 4 | 3 | 2 | 2 | 4 | 2 | 1 | ||||
| Kitchen utensils | 4 | 6 | 4 | 4 | 6 | 6 | 3 | ||||
| Modes of transportation | 7 | 5 | 6 | 5 | 6 | 5 | |||||
| Dogs | 2 | 3 | 4 | 3 | 10 | 5 | |||||
| Flowers | 3 | 5 | 3 | 3 | 3 | 4 |
| Pre1 | Pre2 | Pre3/Tr1 | Tr2 | Post1 | Post2 | Post3 | Post4 | Post5 | Post6 | FU1 | FU2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LH stroke | Tools | 4 | 3 | 4 | 7 | 7 | 10 | 7 | 11 | 11 | 10 | 8 | 9 |
| Furniture | 5 | 6 | 7 | 7 | 8 | 8 | 6 | 7 | 12 | 12 | 7 | 8 | |
| Birds | 5 | 4 | 4 | 8 | 9 | 7 | 8 | 10 | 8 | 11 | 7 | 9 | |
| Sea animals | 1 | 1 | 3 | 4 | 0 | 1 | 1 | 2 | 2 | 5 | 2 | 3 | |
| Kitchen utensils | 8 | 7 | 7 | 8 | 10 | 13 | 9 | 11 | 10 | 8 | |||
| Modes of transportation | 8 | 10 | 7 | 10 | 13 | 13 | 10 | 10 | 9 | 13 | |||
| Vegetables | 6 | 5 | 7 | 8 | 9 | 13 | 12 | 16 | 11 | 7 | |||
| Fruits | 4 | 1 | 9 | 4 | 3 | 4 | 3 | 6 | 3 | 6 | |||
| Musical instruments | 7 | 8 | 6 | 8 | 9 | 10 | 8 | 10 | 7 | 8 | |||
| clothing | 8 | 13 | 17 | 15 | 11 | 14 | 14 | 16 | 14 | 12 | |||
| Dogs | 5 | 3 | 2 | 3 | 3 | 7 | 4 | 5 | |||||
| Flowers | 1 | 2 | 2 | 3 | 2 | 3 | 4 | 2 |
Pre = pre-treatment probes; Pre/Tr = Day1 of treatment, when last pre-treatment probe was taken; Tr = probes taken on treatment days; Post = probes taken after treatment for that category was completed; FU = follow-up probes.
Treatment tasks involved guided retrieval prompted by identification and elaboration of items within subcategories, as well as other semantic tasks. These included sorting pictures and words by subcategory (e.g., for the category “tools” → things used for cutting, things used for pounding, types of screwdrivers), identifying semantic attributes of exemplars, comparing and contrasting between exemplars, and picture naming using a picture dictionary organised thematically by category. The treatment tasks were designed to encourage production of category exemplars by providing a boost to semantic representations within a given category and/or by facilitating improved transmission of information between intact semantic representations and specific lexical items. There was also a strategic element to the training. For example, participants were encouraged to produce items within categories by thinking of logical sub-groupings of items and exhausting one subcategory before switching to another. In this way, the treatment and homework tasks were intended to promote strategies for generation of novel exemplars, rather than purely item-specific learning. Spoken production of exemplars was required during training, and therefore an element of phonological rehearsal was included in addition to the semantic stimulation. Daily homework involved tasks similar to those completed in treatment sessions.
Performance probes, consisting of attempts to name items within trained and untrained categories for 1 minute, were collected three times pre-treatment and at the start of each treatment session. Additional verbal fluency trials were conducted during treatment sessions, as part of the therapy protocol. Once treatment for a given category was complete, post-treatment probes were collected over subsequent sessions. Follow-up probes were administered at 3 weeks and 4 months follow-up for two participants (PA 1 and LH stroke). PA 2 was examined 1 week post-treatment; however, further assessment was not possible. Feedback regarding performance was given following fluency tasks during the training sessions, but feedback was not provided for the pre- and post-treatment probes. When probes were analysed for number of items produced within 1 minute, each unique lexical item that was produced in an intelligible manner (minor literal paraphasias or apraxic errors were considered acceptable) was counted. If a superordinate term was given, in addition to specific exemplars (e.g., spoon, teaspoon, soup spoon), each was counted as correct.
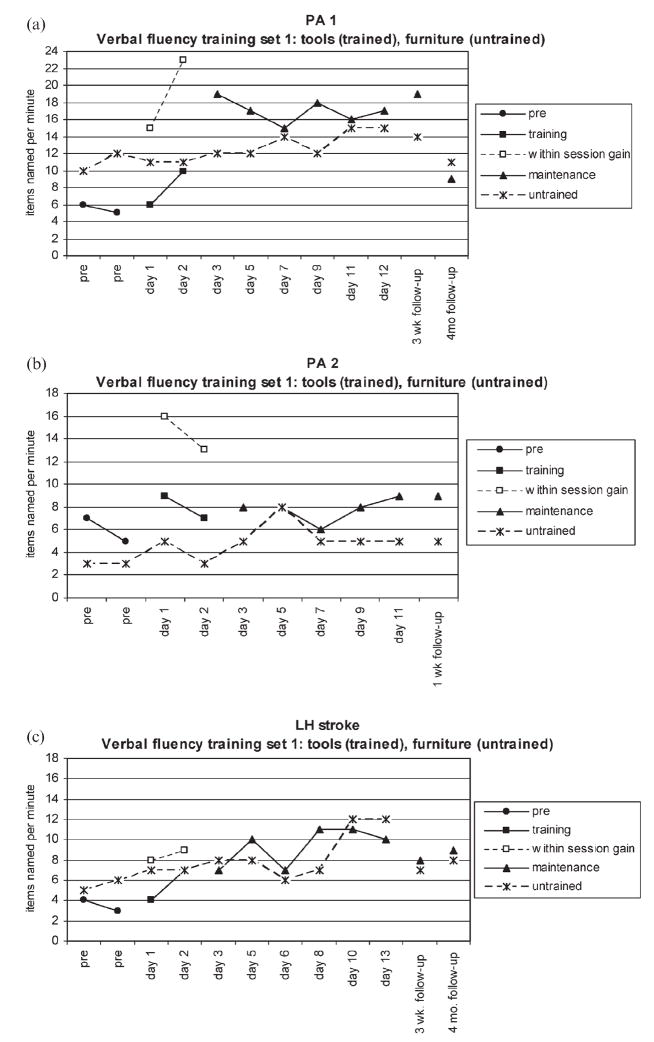
Single-subject multiple baseline data were collected for each participant. In order to illustrate the treatment design, Figure 1 presents performance data for the first set of trained and untrained categories. Treatment outcomes were quantified using effect sizes, as suggested by Beeson and Robey (2006). The effect sizes were calculated using Busk and Serlin’s d, wherein the mean baseline performance is subtracted from the mean performance for the post-treatment period (i.e., the period following treatment for a given category, in which items were probed but not treated). The resulting value is divided by the standard deviation during the baseline phase. For each d statistic comparing pre- to post-treatment performance in a given category, three baseline data points (Pre1, Pre2, and Pre3 in Table 2) were collected and compared with all probes taken after training for that category stopped (Post1–6 in Table 2), with the exception of follow-up probes. The d statistic was also calculated for maintenance effects by comparing follow-up performance (FU1 and FU2 in Table 2) to baseline performance. Weighted mean effect sizes were calculated for each participant by multiplying the effect size for each category by the number of observations for that category and dividing by the total number of observations.
Figure 1.
(a) Performance for PA 1 on first training set. (b) Performance for PA 2 on first training set. (c) Performance for focal lesion patient (LH stroke) on first training set (pre = pre-treatment probes; training = probes taken at the start of each training session; within-session gain = best performance achieved during training sessions; maintenance = probes taken post-treatment; untrained = performance on untrained category matched for difficulty based on normal data).
Effect sizes were evaluated relative to benchmarks derived by Robey and colleagues from single-subject studies examining treatments for lexical retrieval impairment in aphasia (small: d = 4.0, medium: d = 7.0, and large: d = 10.1; Beeson & Robey, 2006; Robey, Shultz, Crawford, & Sinner, 1999). Wilcoxon signed-rank tests were also conducted to compare pre-treatment performance with post-treatment and follow-up performance. McNemar tests were used to compare pre-versus post-treatment scores on the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 2001) and the Pyramids and Palm Trees Test.
RESULTS
All three participants demonstrated improved lexical retrieval on the generative naming task for the trained categories, as indicated in Table 2, but only PA1 and LH stroke maintained improved performance on the follow-up probes. Each participant’s response to treatment is exemplified in Figures 1a, 1b, and 1c, which show the probes during pre-treatment, training, post-treatment, and follow-up phases for the first of the six pairs of trained and untrained categories.
The magnitude of change in performance for each participant is reported as standardised effects sizes in Table 3. PA 1 had a strong, positive response to treatment indicated by large effect sizes (d >10) for the trained categories for the comparison from baseline to post-treatment and at the 3-week follow-up, and a smaller effect size for the maintenance of gains at the 4-month follow-up (d = 4.56). These improvements from baseline to post-treatment and the two follow-up probes were all significant when tested with the Wilcoxon signed-rank test, W = 21, N = 6, p =.025; W = 21, N = 6, p =.025; W = 17, N = 6, p =.05, respectively. Although the effect size for the change on the untrained items was small, the improvement was significant for the post-treatment probes, W = 21, N = 6, p =.025, indicating some degree of generalisation. However, at the 4-month follow-up probe, performance had declined for the untreated items relative to pre-treatment performance (d = −.24), resulting in a notable discrepancy between performance on the treated and untreated items at that time. In contrast to PA 1, PA 2 demonstrated a small (d = 2.0) but significant change in performance for trained categories (W = 21, N = 6, p =.025), and there was no maintenance or generalisation of the learning.
TABLE 3.
Weighted (by number of observations) mean effect size (d) for six treated and six untreated categories
| Direct effect size (d) |
Follow-up effect size (d) 1† |
Follow-up effect size (d) 2‡ |
||||
|---|---|---|---|---|---|---|
| Treated | Untreated | Treated | Untreated | Treated | Untreated | |
| PA 1 | 11.69 | 1.65 | 10.29 | 1.25 | 4.56 | −.24 |
| PA 2 | 2.00 | .03 | .25 | −.77 | n/a | n/a |
| LH stroke | 6.06 | 1.11 | 3.70 | .94 | 3.59 | 1.16 |
Data collected at 3 weeks post-treatment for PA 1 and LH stroke, and at 1 week post-treatment for PA 2.
Data collected at 4 months post-treatment.
The gains made by LH stroke were intermediate between the two other participants. He showed medium-sized treatment effects for trained categories (d >6.0), with moderate maintenance of improvement on the two follow-up probes (d = 3.70 and 3.59). These changes in performance were significant from baseline to post-treatment (W = 21, N = 6, p =.025), and at the follow-up evaluations (W = 15, N = 5, p =.05; W = 21, N = 6, p =.025). Generalised improvement to the untrained categories was modest (d = 1.11) but significant (W = 17, N = 6, p =.05) at post-treatment, and some degree of maintenance was observed at the 4-month follow-up (d = 1.16; W = 17, N = 6, p =.05).
Data utilised for calculation of effect sizes were collected at the start of treatment sessions and thus do not represent changes in behaviour that took place within a session, which were often substantial. In order to represent the effect of stimulation within a treatment session, the sample data for each participant in Figure 1 also include the best performance obtained for the trained category during the two treatment sessions for that category (labelled “within-session gain”). These data illustrate the effects of priming and facilitation during training; however, they were not included in statistical analyses, as they do not necessarily reflect lasting, meaningful changes in behaviour. Nonetheless, it is worth mention that, in the majority of treatment sessions for each participant, there was an improvement of at least 50% relative to the probe at the beginning of the session.
Pre- to post-treatment scores on the BNT and PPT showed relatively stable performance for the three participants (see Table 4), with the notable exception of the stroke patient’s 14-point improvement on the naming test (McNemar test, p<.001). Improvement on this measure indicates generalisation to an untrained naming task and to untreated items. Importantly, this significant improvement was maintained at 3 weeks (36/60 items correct; McNemar test, p<.001) and 4 months (33/60 items correct; McNemar test, p<.01) post-treatment. There was also a small but non-significant change in performance on the PPT for PA 2, which resulted in a post-treatment score that fell within normal limits.
TABLE 4.
Comparison of pre- and post-treatment scores on standardised language measures
| WAB AQ |
Naming: BNT |
Semantics: PPT |
||||
|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | |
| PA 1 | 88.8 | 88.6 | 37 | 39 | 51 | 52 |
| PA 2 | 88.0 | 84.6 | 31 | 30 | 46 | 50 |
| LH stroke | 93.4 | 95.2 | 24 | 38** | 49 | 50 |
Significant change (p<.001) on McNemar test (BNT and PPT).
WAB AQ: Western Aphasia Battery Aphasia Quotient; max. score = 100.
BNT: Boston Naming Test; max. score = 60.
PPT: Pyramids and Palm Trees Test; max. score =52.
DISCUSSION
In this study, a strong positive response to intensive, semantically based treatment for lexical retrieval was documented in an individual with progressive aphasia (PA 1), similar to that observed in a participant with stroke-induced aphasia. This is contrasted with the limited response to treatment by a second individual with progressive aphasia (PA 2). Although the pre-treatment language profiles of all three participants were similar with regard to aphasia severity and degree of anomia, PA 2 demonstrated impairment of conceptual knowledge, whereas the other two participants showed a relative sparing of semantic processing. Thus, we consider that the differential response to treatment may reflect differences regarding the nature of the underlying cognitive impairment.
The mechanism for improved naming in PA 1 and LH stroke appeared to involve a strengthening of links between intact semantic representations and corresponding phonological word forms, as treatment involved explicit enumeration of semantic attributes combined with spoken naming. Semantically motivated retrieval strategies (e.g., generating items by sub-category based on shared semantic attributes) also proved beneficial, as a means of organising the lexical search and as a guide for production of novel exemplars. Application of semantic strategies for retrieval was apparent during probes as these two participants produced items that were grouped semantically and also in their overt comments (e.g., “Okay, now I’ll think of ones that you use for cutting” during the probe for “tools”).
In the case of PA 2, impaired semantic processing was evident on the PPT test, but was most striking as he struggled with the treatment tasks themselves. Relative to the other two participants, PA 2’s performance during treatment was quantitatively and qualitatively different on the semantically based treatment tasks. For example, his attempts to sort items by semantic relatedness often reflected idiosyncratic groupings of items, and he had difficulty comparing and contrasting items within a given category. In addition, PA 2’s written homework showed limited variation in the exemplars generated for target categories, giving the impression that he attempted to memorise lists of items, rather than generating items using the semantic strategies implemented during treatment. Thus, it appeared that PA 2 tended to rely more on episodic memory to retrieve items, much like the SD patient described by Graham et al. (2001). Despite these limitations, it was interesting to note that PA 2 made notable improvement on the category “dogs” (d =7.00 for “dogs”; weighted d for other categories combined = 1.36), which can be attributed to both his residual knowledge of and his intrinsic interest in this topic. This differential performance highlights the obvious practical consideration of selecting personally relevant items when conducting this type of treatment in a clinical setting. In fact, evidence from prior studies with SD patients suggests that targeting items of importance to the individual, using personally relevant cues during training, and treating items for which there is some degree of residual semantic knowledge may be critical in successfully treating anomia in these patients (Jokel et al., 2006; Snowden & Neary, 2002).
It is important to note that, despite PA 2’s apparent attempts to memorise items in the trained categories, he did in fact show an impairment of episodic memory. This was documented by his performance on the Warrington Recognition Memory Test (see Table 1) but was also evident during treatment sessions, as he had considerable difficulty recalling the lexical retrieval strategies for specific categories from one session to the next. This was not the case with the other two participants. PA 2 also differed from the others in that he demonstrated some of the obsessive practice behaviours reported by Graham and colleagues (2001), as well as difficulty in switching task set during sessions, which may be indicative of some degree of frontal lobe pathology. Such behaviours may present additional considerations when implementing treatment with individuals with probable frontotemporal lobar degeneration (FTLD). Indeed, it has been shown that patients with the semantic impairment of SD may demonstrate behavioural deficits similar to those observed in patients with the frontal variant of FTLD (Williams, Nestor, & Hodges, 2005). In PA 2, an impairment of frontal lobe function may have contributed to poor performance on verbal fluency probes, which are reliant on both frontal and temporal cortices (Jokel et al., 2006), and may also have been a limiting factor in his ability to apply strategies for word retrieval. In sum, PA 2’s progress appeared to be constrained by a degradation of semantic processing abilities, as well as other cognitive factors, including episodic memory impairment and possibly reduced frontal lobe functioning.
Returning attention to PA 1 and LH stroke, we noted that they appeared to have relatively preserved semantic knowledge. We acknowledge, however, that our testing was not extensive enough to rule out the possibility that a subtle semantic deficit was a contributing factor in their anomia. If so, treatment tasks involving explicit delineation of semantic features within and between categories may have aided in more efficient lexical selection by augmenting degraded semantic representations. However, these patients’ high degree of accuracy (nearly 100%) in semantic sorting tasks with pictures of items that they often could not name suggests that the deficit was, at least in part, one of impaired access to or activation of lexical forms, rather than semantic degradation.
The positive response to treatment documented in PA 1 offers support for the consideration of behavioural treatment to improve language performance in individuals with progressive aphasia. In fact, it was noteworthy that PA 1’s improvement occurred in the context of a gradual decline in performance over time on the untrained items. Specifically, a positive treatment effect was maintained for trained categories at the 4-month follow-up (d>4) relative to a negative change in performance on the untrained categories (d= −.24). These findings suggest that a protective benefit may have been afforded by the treatment of these categories. These treatment effects might have been further enhanced by the implementation of a maintenance programme following treatment.
In sum, this study supports the notion that intensive semantically based treatment may serve to improve lexical retrieval for some individuals with progressive aphasia in a manner similar to individuals with focal left hemisphere damage due to stroke. These findings contribute to the small body of literature regarding treatment for the language impairment in progressive aphasia, and prompt additional work to further clarify the best candidates and therapeutic approaches for treatment with this population.
Acknowledgments
This work was supported by grants DC008286 and DC007646 from the National Institute on Deafness and Other Communication Disorders. Support was also provided by the Arizona Alzheimer’s Consortium (Arizona Alzheimer’s Disease Core Center) grant NIA P30AG19610.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Maya L. Henry, University of Arizona, Tucson, AZ, USA
Pélagie M. Beeson, University of Arizona, Tucson, AZ, USA
Steven Z. Rapcsak, University of Arizona, and Southern Arizona VA Health Care System, Tucson, AZ, USA
References
- Beeson PM, Robey RR. Evaluating single-subject treatment research: Lessons learned from the aphasia literature. Neuropsychology Review. 2006;16(4):161–169. doi: 10.1007/s11065-006-9013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. Semantic feature analysis treatment for anomia in two fluent aphasia syndromes. American Journal of Speech-language Pathology. 2004;13(3):236–249. doi: 10.1044/1058-0360(2004/025). [DOI] [PubMed] [Google Scholar]
- Clark DG, Charuvastra A, Miller BL, Shapira JS, Mendez MF. Fluent versus nonfluent primary progressive aphasia: A comparison of clinical and functional neuroimaging features. Brain and Language. 2005;94(1):54–60. doi: 10.1016/j.bandl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Drew RL, Thompson CK. Model-based semantic treatment for naming deficits in aphasia. Journal of Speech, Language, and Hearing Research. 1999;42(4):972. doi: 10.1044/jslhr.4204.972. [DOI] [PubMed] [Google Scholar]
- Frattali C. An errorless learning approach to treating dysnomia in frontotemporal dementia. Journal of Medical Speech-Language Pathology. 2004;12(3):11–24. [Google Scholar]
- Graham KS, Patterson K, Pratt KH, Hodges JR. Relearning and subsequent forgetting of semantic category exemplars in a case of semantic dementia. Neuropsychology. 1999;13(3):359–380. doi: 10.1037//0894-4105.13.3.359. [DOI] [PubMed] [Google Scholar]
- Graham KS, Patterson K, Pratt KH, Hodges JR. Can repeated exposure to “forgotten” vocabulary help alleviate word-finding difficulties in SD? An illustrative case study. Neuropsychological Rehabilitation. 2001;11:429–454. [Google Scholar]
- Hillis AE. Effects of separate treatments for distinct impairments within the naming process. In: Prescott T, editor. Clinical aphasiology. Vol. 19. Austin, TX: Pro-Ed; 1991. pp. 255–265. [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain: a Journal of Neurology. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm Trees: A test of semantic access from pictures and words. Bury St. Edmunds, UK: Thames Valley; 1992. [Google Scholar]
- Jokel R, Cupit J, Rochon E, Graham N. Errorless re-training in semantic dementia using MossTalk words. Brain and Language. 2007;103(1–2):205–206. [Google Scholar]
- Jokel R, Rochon E, Leonard C. Therapy for anomia in semantic dementia. Brain and Cognition. 2002;49(2):241–244. [PubMed] [Google Scholar]
- Jokel R, Rochon E, Leonard C. Treating anomia in semantic dementia: Improvement, maintenance, or both? Neuropsychological Rehabilitation. 2006;16(3):241–256. doi: 10.1080/09602010500176757. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Baltimore: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Kertesz A. The Western Aphasia Battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Lowell S, Beeson PM, Holland AL. The efficacy of a semantic cueing procedure on naming performance of adults with aphasia. American Journal of Speech-Language Pathology. 1995;4(4):109. [Google Scholar]
- McNeil M, Duffy J. Primary progressive aphasia. In: Chapey R, editor. Language intervention strategies in aphasia and related neurogenic communication disorders. 4. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 472–486. [Google Scholar]
- McNeil MR, Small SL, Masterson RJ, Fossett TRD. Behavioural and pharmacological treatment of lexical-semantic deficits in a single patient with primary progressive aphasia. American Journal of Speech-Language Pathology. 1995;4(4):76–87. [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Annals of Neurology. 2001;49(4):425–432. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nickels L. Therapy for naming disorders: Revisiting, revising, and reviewing. Aphasiology. 2002;10/11:935–979. [Google Scholar]
- Nickels L, Best W. Therapy for naming disorders (part II): Specifics, surprises and suggestions. Aphasiology. 1996;10(1):109–136. [Google Scholar]
- Raymer AM, Gonzales-Rothi LJ. Cognitive approaches to impairments of word comprehension and production. In: Chapey R, editor. Language intervention strategies in aphasia and related neurogenic communication disorders. 4. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 524–550. [Google Scholar]
- Robey RR, Schultz MC, Crawford AB, Sinner CA. Review: Single-subject clinical-outcome research: Designs, data, effect sizes, and analyses. Aphasiology. 1999;13(6):445–473. [Google Scholar]
- Snowden JS, Neary D. Relearning of verbal labels in semantic dementia. Neuropsychologia. 2002;40(10):1715–1728. doi: 10.1016/s0028-3932(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Recognition Memory Test. Windsor, UK: NFER-Nelson; 1984. [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. NeuroImage. 2005;24(4):1042–1051. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]