Abstract
Twenty-five panic disorder (PD) patients, 19 social phobics (SP), and 20 healthy controls (HC) sat quietly for 15 minutes, rating their anxiety and dyspnea every 30 seconds while respiratory, cardiovascular, and electrodermal responses were recorded. No panic attacks were reported. For self-reported anxiety and dyspnea, within-subject variability over time was higher in PD than in SP or HC. In PD within-subject correlations across 30-second epochs were significant for (a) self-reported anxiety versus dyspnea, end-tidal pCO2, minute volume, duty cycle, skin conductance level, and interbeat interval, and for (b) dyspnea versus end-tidal pCO2, minute volume, tidal volume, and inspiratory flow rate. Several positive or negative correlations were greater in PD than in other groups. Thus in PD, experienced anxiety and dyspnea are temporally unstable but are correlated with each other and with fluctuations in respiratory and autonomic variables, even in the absence of panic attacks.
Keywords: anxiety disorder, respiration, psychophysiology, physiological instability, autonomic nervous system, emotional coherence
Introduction
The hallmark of panic disorder (PD) are panic attacks, which patients describe as a rapidly increasing feeling of anxiety accompanied by perceptions of alarming bodily changes. This description implies some kind of instability in anxiety mechanisms at both the cognitive or experiential level and the physiological level. The relationship between experience and physiology in PD has been a subject of discussion among anxiety researchers and clinicians (e.g., Wilhelm & Roth, 2001). One idea is that small but perceived physiological fluctuations are falsely interpreted as threats of impending psychological or medical catastrophe, creating a positive feedback loop that escalates to panic (Clark, 1986; Goldstein & Chambless, 1978). Patients prone to panic may have greater physiological fluctuations or may tend to interpret any perceived fluctuations more catastrophically. Fluctuations in dyspnea and respiration are of particular interest because of the suffocation false alarm hypothesis that sees the biological root of PD in an unstable mechanism that generates a feeling of dyspnea (Klein, 1993; Preter & Klein, 2008; for a review see Meuret & Ritz, in press) and because of evidence that hyperventilation causes anxiety (Ley, 1985). Regardless of the specific mechanism, these considerations suggest that PD patients at times will have greater fluctuations than patients with other anxiety disorders or than non-anxious controls in several kinds of variables: self-reported anxiety and dyspnea, and respiratory and autonomic measures reactive to emotion such as heart rate, heart rate variability, and skin conductance. Whether greater fluctuations would be restricted to periods before attacks, or whether they would be present during most waking hours or even sleep is uncertain.
Evidence points to greater variability of physiological measures of respiration in PD. Several laboratory studies report increased tidal volume variability, higher respiratory rate, and higher sigh frequency during resting conditions (e.g. Abelson, Weg, Nesse, & Curtis, 2001; Caldirola, Bellodi, Cammino, & Perna, 2004; Wilhelm, Trabert, & Roth, 2001a,b; Wilhelm, Gerlach, & Roth, 2001). These irregularities persist even after cognitive intervention and thus may be a stable trait of PD patients (Abelson, et al., 2001). In a recent review on breathing in PD, Nardi, Freire and Zin (2009) hypothesize that respiratory irregularities observed in laboratory panic provocations are trait markers of panic vulnerability and expressions of an imbalanced adaptation mechanism in a hypersensitive fear network. Roth (2005) speculated that respiratory instability could result from emotional thoughts interfering with breathing and disturbing its natural rhythmicity. In a recent ambulatory study, compared to duration-matched control periods the hour prior to the onset of a reported panic attack was marked by significant instability in cardiorespiratory function. Most intriguingly, levels of end-tidal pCO2 were elevated (relative to initial levels) significantly just prior to the onset of the panic attacks (Rosenfield et al., 2010).
Autonomic measures reactive to emotion often distinguish PD from comparison groups. Greater electrodermal variability in PD on a second-by-second time scale is manifested in a higher rate of skin conductance (SC) fluctuations in relaxation or baseline laboratory paradigms (Roth, Wilhelm & Trabert, 1998). Also common is a higher SC level, which declines more slowly in PD patients than in non-anxious controls (Roth, Ehlers, Taylor, Margraf, & Agras, 1990; Roth, Wilhelm, & Trabert, 1998). These findings, however, are not always observed (Blechert, Michael, Grossman, Lajtman, & Wilhelm, 2007). Cardiovascular variability on a second-by-second time scale is often less in PD, but this should not be interpreted as greater emotional stability. Respiratory sinus arrhythmia (RSA), the rhythmic fluctuation of HR with breathing related to vagal efferent activity to the heart, is often attenuated in PD, while heart rate is elevated (Blechert, et al., 2007; Friedman, et al., 1993; Wilhelm & Roth, 1998).
Presumably fluctuations between emotional systems, i.e., cognitive, behavioral, and physiological, and within the physiological subsystems responsive to emotional activation would intercorrelate. However, the temporal correlation (or “coherence”) of these systems is sometimes meager. Coherence in emotion theory is defined as “integrated, concurrent, emotion-specific changes in different emotion response domains” (Rosenberg & Ekman, 2005, p. 63). Since higher emotional arousal results in higher response coherence (Bonanno & Keltner, 2004), anxiety disorder patients might be expected to show higher coherence at least when they are anxious, but few studies have examined this. Mauss, Wilhelm, and Gross (2004) found low correlations between anxiety experience and physiological activation in both high and low trait social anxiety subjects giving an impromptu speech. However, in a study inducing moods with films, correlations between anxiety and respiratory rate were higher in general anxiety disorder (GAD) patients than in controls (Hubert & de Jong-Meyer, 1991). A third study reported a significant correlation between heart rate and anxiety in a patient with PD and agoraphobia who took 30 15-minute trips in a chairlift (Lewis & Drewett, 2006). Whether this correlation was higher than that of non-anxious controls was not tested.
In the present study experienced anxiety and dyspnea as well as physiological measures of emotional arousal were assessed every 30 s during a 15-minute period of quiet sitting in the laboratory. This allowed us to evaluate the temporal variability of each measure and the intercorrelations between measures. Three groups were tested: PD patients, social phobia (SP) patients, and healthy controls. No fear-inducing stimuli or tasks were given, although being tested in a laboratory should be considered a mild stressor. We expected to find more psychophysiological variability in PD patients than in the other groups, since the central complaint in PD is temporal instability of fear and its physical symptoms. Our previous study on baseline activation in PD (Wilhelm, et al., 2001a) had found particularly elevated physiological variability in PD patients in the respiratory system. We also expected that psychological and physiological fluctuations would be more closely coupled in PD patients than in SP patients or in healthy controls, since perception of bodily changes and reactions are a central feature in self-descriptions of PD anxiety.
Methods and Materials
Participants
Twenty-five PD patients and 19 SP patients were recruited by newspaper advertisements offering evaluation and treatment. Twenty healthy controls (HC) selected to sex- and age-match the patients were recruited by advertisements offering payment for participation. Exclusion criteria for all subjects were a lifetime history of bipolar disorder, psychosis, mental disability, drug dependence or abuse, or medical conditions that might affect the physiological systems being tested (e.g., asthma, myocardial infarction, thyroid dysfunction). All participants were interviewed by trained clinical psychologists using the Structured Clinical Interview for DSM-IV, SCID (First, Spitzer, Gibbon, & Williams, 1996). The following comorbid disorders were found among the anxiety disorder patients: generalized anxiety disorder (2 in the PD group / 3 in the SP group), major depression (1/2), and specific phobia (3/0). Controls were selected never to have had a psychiatric diagnosis.
Procedure
Before laboratory testing, all participants gave informed consent. We tried to create a benign setting without fear-inducing stimuli or tasks. Subjects sat upright in a comfortable chair in a quiet, temperature-controlled room. They were instructed to sit quietly and to move as little as possible to avoid disturbances in the physiological recording. They were to keep their eyes open and their mouth tightly closed, breathing only through their nose where nasal prongs sampled the air they breathed in and out. A microphone was installed in the room for communication with the experimenter outside the room via intercom. The quiet sitting lasted 15 minutes. In our previous study, 10-min time segments of a 30-minute session had been long enough to demonstrate temporal instability of relevant variables (Wilhelm, et al., 2001a).
Self-Report Measures
In the week before testing, participants filled out questionnaires that had been mailed to them: the Anxiety Sensitivity Index (ASI) (Reiss, Peterson, Gursky, & McNally, 1986), the Mobility Inventory (Chambless, Caputo, Jasin, Gracely, & Williams, 1985), the Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), and the Suffocation Fear Scale (SFS) (Rachman & Taylor, 1994), which comprises 15 items about claustrophobic situations relevant to suffocation fears (e.g., being “at the furthest point from an exit on a tour of an underground mine shaft”). Subjects indicated their degree of endorsement on 5-point scales ranging from 0 (“not at all anxious”) to 4 (“extremely anxious”).
Before the testing, participants rated their level of anxiety they typically experience in an average day, their level of anxiety right before they went to bed the day before the testing, and their anxiety level on the testing day after waking up. An 11-point scale from 0 (“not at all”) to 10 (“extremely”) was used.
While quietly sitting and being physiologically recorded, participants rated their feelings of anxiety and dyspnea every 30 seconds in response to a recorded signal on an 11-point scale from 0 (“not at all”) to 10 (“extremely”), which is the most valid method of assessing emotions and emotional response coherence (Bonanno & Keltner, 2004; Robinson & Clore, 2002).
Immediately afterwards they marked on the rating sheet if during the sitting they had had a panic attack, defined as a discrete period of intense fear or discomfort accompanied by at least four DSM-IV (American Psychiatric Association, 1994) symptoms. They then filled out a symptom questionnaire asking about experiences during the quiet sitting period. The questionnaire contained the anxiety-related items “anxious”, “worried” and “relaxed” (negatively coded) on a 0 (“not at all”) to 10 (“extremely”) scale, which was averaged to give a composite anxiety score (Cronbach's Alpha=.83). As a measure of awareness of bodily sensations, the questionnaire asked about 7 of the 13 DSM-IV bodily panic symptoms (American Psychiatric Association, 1994), which were rated on a 0 (“not at all”) to 4 (“extremely”) scale: (1) heart pounding or racing; (2) chest pain or discomfort; (3) shortness of breath or smothering; (4) dizziness, unsteadiness, lightheadedness, or faintness; (5) feelings of unreality or detachedness (6) numbness or tingling sensations; and (7) trembling or shakiness. These items were averaged for a composite panic symptom score (Cronbach's Alpha=.66). Participants also rated each symptom on how anxious it made them (“How much did this scare you?” 0-4), yielding a second composite score, the symptom sensitivity score (Cronbach's Alpha=.59). Awareness and control of breathing was assessed by two items (“Were you aware of your breathing?” and “Did you feel you had to consciously control your breathing?”) on a 0 (“not at all”) to 4 (“extremely”) scale.
Physiological measures
In parallel with subjective ratings, mean scores for the physiological channels were computed for 30-second segments yielding a total of 30 repeated measurements for the 15-min recording. We mainly focused on respiratory measures, which were based on chest plethysmography and blood gas monitoring. Two channels of respiration were recorded with Respibands (Respitrace Corporation, Ardsley, NY) placed around the chest and abdomen. Calibration against spirometry was made by the least-squares method. Instantaneous respiratory rate, tidal volume, minute volume, mean inspiratory flow rate, and duty cycle were calculated breath-by-breath using customized programs. Expiratory pCO2, which is close to arterial pCO2, was recorded continuously by a calibrated infrared capnograph (N-1000, Nellcor, Hayward, CA) from air samples sucked in from a dual nostril prong. End-tidal pCO2 was determined as the level at which pCO2 stopped rising at the end of expiration (final maximum).
In addition we measured electrodermal and cardiovascular variables. As a sympathetic electrodermal measure, skin conductance level was recorded from the palmar surfaces of the middle phalanx of the left index and middle fingers. Instantaneous interbeat intervals (IBIs) were derived from an electrocardiogram lead. Respiratory sinus arrhythmia (RSA), which quantifies cardiac vagal control, was calculated using complex demodulation of IBI time series for the frequency band of 0.15-0.4 Hz. This method is less vulnerable to nonstationarity than spectral analysis and is particularly sensitive to changes in heart rate variability (Wilhelm, Grossman, & Roth, 2005).
Statistical analyses
One-way analyses of variance (ANOVA) were performed for the entire 15-minute period for each of the measures, followed by post-hoc Tukey HSD tests between groups if significant. To index variability within the 15 minutes, root mean squared successive differences (RMSSDs) were calculated for the measures. Variability, defined as the dispersion of scores from a central tendency, does not consider the temporal order of scores when measured by standard deviations, while RMSSD takes into account both the temporal order of scores and reflects the two other components of instability, amplitude and frequency (Ebner-Priemer, Eid, Kleindienst, Stabenow, & Trull, 2009; Larsen, 1987). Thus, RMSSD is a general and more sensitive index of psychological and physiological instability than its alternatives such as within-subject standard deviation, power spectral density, or autocorrelation. One-way ANOVA was also applied to the RMSSDs to test for group differences. Due to not normally distributed self-report data, we used the nonparametric Kruskal-Wallis test to assess group differences regarding the anxiety and dyspnea, followed post-hoc by Mann-Whitney U-tests.
To assess correspondence among physiological variables and self-reported continuous anxiety and dyspnea measures, overall within-subject correlations for each subject were calculated for the 30 repeated measurements over the 15 minutes. This within-individual approach is more sensitive to temporal correspondence or ‘coherence’ than between-individual or between-group analyses (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005; Mauss & Robinson, 2009; Reisenzein, 2000). We consider effect sizes according to Cohen (1988) as being small at r = .10, moderate at r = .30, and large at r = .50.
Statistical significance of the coherence values was tested by one-sample t-tests, comparing the correlations to 0 for each group separately. Group differences in these correlations were tested with one-way ANOVA followed post-hoc by Tukey HSD tests if significant.
Results
Sociodemographic and psychometric assessment
As seen in Table 1, the three groups did not differ in gender distribution, age, years of education, or body mass index (which is known to influence respiratory function; Pelosi, et al., 1998). PD patients scored higher on the Anxiety Sensitivity Index (Reiss, et al., 1986) and on the Mobility Inventory (Chambless, et al., 1985) than either other group. Both patient groups had higher scores on the Beck Depression Inventory (Beck, et al., 1961) than HC. On the Suffocation Fear Scale (Rachman & Taylor, 1994), both patient groups scored higher than HC, especially PD. Groups did not differ in the number of cigarettes they usually smoke or the amount of alcohol they consumed the 24 hours before the testing.
Table 1.
Sample characteristics and clinical questionnaires: means (SD) and group differences
| Panic Disorder | Social Phobia | Healthy Controls | Statistic | Post-hoc | |
|---|---|---|---|---|---|
| N=25 | N=19 | N=20 | |||
| Women (%) | 62.5 | 52.6 | 65.0 | χ2(2)=0.70, p=.705 | |
| Age (years) | 39.7 (10.7) | 39.5 (10.6) | 38.4 (10.9) | F(2,61)=0.86, p=.917 | |
| Education (years) | 16.5 (1.9) | 15.2 (3.0) | 16.8 (4.0) | F(2,46)=1.34, p=.273 | |
| Body Mass Index (kg/m2) | 25.2 (7.0) | 24.6 (5.4) | 24.2 (3.8) | F(2,44)=0.12, p=.891 | |
| Anxiety Sensitivity Index | 28.6 (12.8) | 21.7 (10.5) | 7.4 (4.9) | F(2,48)=15.20, p<.001 | PD>SP>HC |
| Mobility Inventory | 23.9 (17.4) | 17.2 (16.8) | 1.2 (1.3) | F(2,48)=8.91, p=.001 | PD>SP>HC |
| Suffocation Fear Scale | 22.0 (12.8) | 13.2 (9.9) | 3.2 (5.0) | F(2,48)=12.77, p<.001 | PD>SP>HC |
| Beck Depression Inventory | 12.0 (7.9) | 14.3 (9.0) | 2.0 (2.6) | F(2,48)=10.37, p<.001 | PD=SP>HC |
| Anxiety in an average day | 4.98 (1.82) | 4.63 (2.06) | 1.40 (1.57) | F(2,48)=24.56, p<.001 | PD=SP>HC |
| Anxiety night before testing | 3.92 (2.14) | 4.42 (2.52) | 0.50 (1.00) | F(2,48)=23.12, p<.001 | PD=SP>HC |
| Anxiety wake up testing day | 4.76 (2.31) | 3.47 (2.32) | 0.75 (1.12) | F(2,48)=22.28, p<.001 | PD=SP>HC |
Average anxiety before the testing
The anxiety groups rated their typical anxiety level in an average day, their anxiety level before going to bed the day before the testing and their anxiety level when they woke up on the testing day higher than HC (Table 1).
Anxiety and dyspnea during quiet sitting
Figure 1 shows that while sitting both patient groups reported significantly higher anxiety than did HC (Figure 1) [χ2(2) = 24.98, p < 0.001; PD vs. SP, U = 192.00, p = 0.281; PD vs. HC, U = 42.00, p < 0.001; SP vs. HC, U = 61.50, p < 0.001]. HC reported almost no anxiety or dyspnea. Feelings of dyspnea were higher in PD than in both SP and HC groups, which did not differ in their dyspnea ratings [χ2(2) = 24.32, p < 0.001; PD vs. SP, U = 129.00, p = 0.009; PD vs. HC, U = 45.00, p < 0.001; SP vs. HC, U = 120.00, p = 0.050].
Figure 1.
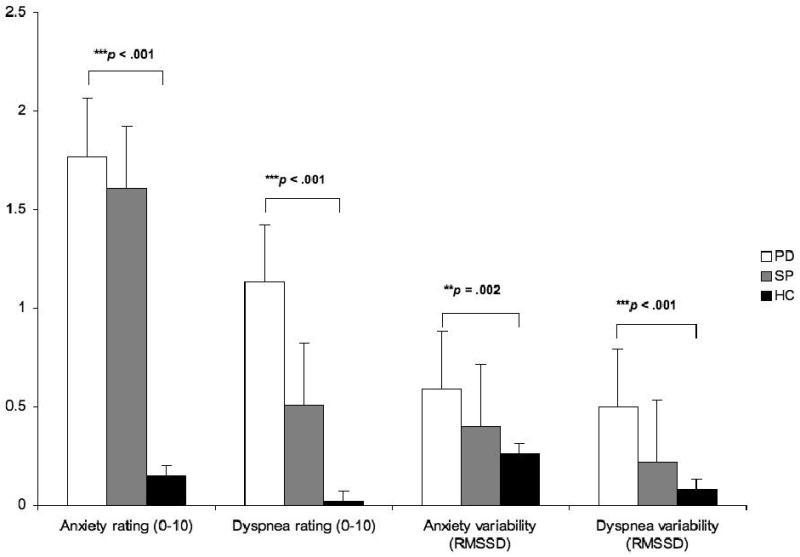
Anxiety and dyspnea: group means and variabilities (root mean squared successive differences, RMSSD) of 30-sec interval ratings
Note: Bars indicate standard errors. ** p < .01; *** p < .001. Post hoc results: Anxiety PD=SP>HC; dyspnea PD>SP=HC; anxiety variability PD>SP=HC; dyspnea variability PD>SP=HC
The variability (RMSSD) of anxiety and dyspnea showed highly significant group differences: PD patients showed the highest values of anxiety variability [χ2(2) = 12.38, p = 0.002; PD vs. SP, U = 148.00, p = 0.033; PD vs. HC, U = 105.50, p = 0.001; SP vs. HC, U = 135.00, p = 0.127]. Variability of dyspnea, too, was higher in PD than in either other group, while SP did not differ from HC in either measure [χ2(2) = 19.39, p < 0.001; PD vs. SP, U = 122.00, p = 0.005; PD vs. HC, U = 79.00, p < 0.001; SP vs. HC, U = 131.50, p=0.101].
Retrospective symptom questionnaire
As presented in Table 2, anxiety on the retrospective symptom questionnaire was higher in both patient groups than in HC. PD scored higher than HC in panic symptoms and in symptom sensitivity, while SP was intermediate, not differing from either group in either score. The clinical anxiety groups differed from each other only in anxiety and not in panic symptoms or symptom sensitivity. No participant reported having experienced a panic attack during the procedure.
Table 2.
Retrospective symptom questionnaire: Means (SD) and group differences
| Panic | Social | Healthy | Statistic | Post-hoc | |
|---|---|---|---|---|---|
| Disorder | Phobia | Controls | |||
| Anxiety | 3.05 (1.69) | 2.66 (1.78) | 0.90 (0.68) | χ2(2) =23.61, p<.001 | PD=SP>HC |
| Panic symptoms | 0.43 (0.36) | 0.25 (0.39) | 0.29 (0.75) | χ2(2) =23.64, p<.001 | PD>SP>HC |
| Symptom sensitivity | 0.19 (0.27) | 0.11 (0.18) | 0.00 (0.00) | χ2(2) =11.21, p=.004 | PD>SP=HC |
| Awareness of breathing | 2.08 (1.08) | 1.89 (0.99) | 1.70 (1.38) | χ2(2) =1.41, p=.493 | |
| Control of breathing | 0.84 (0.94) | 1.05 (1.08) | 0.65 (1.04) | χ2(2) =2.15, p=.342 | |
| Panic attack | 0% | 0% | 0% |
Physiological measures during quiet sitting
Groups did not differ in means or RMSSDs of physiological measures.
Correspondence between response systems
Figure 2 gives an example of the time courses of self-reports and end-tidal pCO2 for one PD patient. Anxiety and dyspnea in this patient parallel each other over time, having a high positive correlation over the 30 measurements [ranx/dys (30) = .83, p <.001]. In contrast anxiety and dyspnea show high negative correlations with pCO2 [ranx/pCO2(30) = -.67, p <.001, rdys/pCO2(30) = -.71, p <.001], with opposite time courses.
Figure 2.
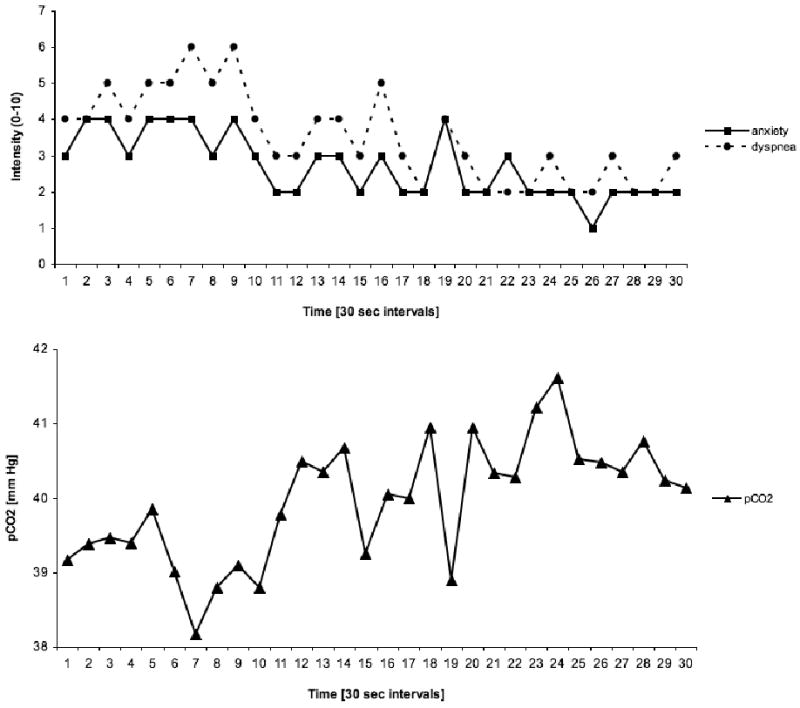
Anxiety, dyspnea, and end-tidal pCO2 of Case No. 25 (PD patient)
Note: Anxiety and dyspnea cohere positively with one another (ranx/dys = .83, p < .001), but negatively with pCO2 (ranx/pCO2 = -.67, p < .01; rdys/pCO2 = -.71, p < .01)
As shown in Table 3, within-subject correlations during quiet sitting were highest in the patient groups between anxiety and dyspnea. Medium effect sizes (Cohen, 1988) were found for PD and SP, but not for HC. Nine HC subjects did not experience any anxiety, and 15 did not report any dyspnea. For them, correlations between the measures were set at 0. Anxiety was temporally associated with respiratory variables only in PD (minute volume, end-tidal pCO2, duty cycle). In addition, the correlation between tidal volume and anxiety was small and positive in PD, but small and negative in the other groups, resulting in a significant group difference. Both autonomic variables (skin conductance level and interbeat interval) were correlated with anxiety in PD. Dyspnea was significantly correlated with respiratory variables only in PD (tidal volume, minute volume, end-tidal pCO2, inspiratory flow rate). The strongest correlation was found with end-tidal pCO2. In addition, there were group differences in correlations for dyspnea and interbeat interval, where the correlation in PD was more negative than in the other groups.
Table 3.
Mean within-subject correlations (coherences), group differences, and post-hoc results
| Anxiety | Dyspnea | ||||||
|---|---|---|---|---|---|---|---|
| Group | Mean r (SD) | Statistic | Post-hoc | Mean r (SD) | Statistic | Post-hoc | |
| Dyspnea | PD | .38*** (.36) | F(2,61)=5.63, p=.006 | PD > HC | |||
| SP | .23** (.33) | ||||||
| HC | .06 (.23) | ||||||
| SC level (μSiemens) | PD | .19* (.36) | F(2,58)=0.71, p=.495 | .08 (.34) | F(2,58)=0.01, p=.995 | ||
| SP | .20* (.36) | .07 (.35) | |||||
| HC | .09 (.23) | .09 (.17) | |||||
| Interbeat interval (ms) | PD | -.17** (.27) | F(2,61)=2.44, p=.096 | -.11 (.29) | F(2,61)=3.84, p=.027 | PD < SP | |
| SP | -.03 (.32) | .05 (.21) | |||||
| HC | -.02 (.15) | .04 (.11) | |||||
| Respiratory sinus arrhythmia | PD | -.05 (.22) | F(2,61)=0.50, p=.611 | .00 (.22) | F(2,61)=0.57, p=.569 | ||
| SP | -.03 (.28) | .03 (.19) | |||||
| HC | .02 (.20) | -.03 (.10) | |||||
| Respiratory rate (breaths/min) | PD | .02 (.22) | F(2,61)=1.19, p=.312 | -.05 (.18) | F(2,61)=2.04, p=.139 | ||
| SP | .13 (.29) | -.06 (.26) | |||||
| HC | .07 (.20) | .06 (.17) | |||||
| Tidal volume (mL) | PD | .10 (.27) | F(2,61)=3.96, p=.024 | PD > SP | .11** (.20) | F(2,61)=1.22, p=.301 | |
| SP | -.08 (.21) | .06 (.16) | |||||
| HC | -.02 (.16) | .03 (.18) | |||||
| Minute volume (L/min) | PD | .13* (.28) | F(2,61)=1.92, p=.156 | .12* (.25) | F(2,61)=2.45, p=.095 | ||
| SP | .04 (.14) | .00 (.17) | |||||
| HC | .01 (.18) | .03 (.11) | |||||
| End-tidal pCO2 (mm Hg) | PD | -.17* (.36) | F(2,60)=2.76, p=.072 | -.20** (.32) | F(2,60)=5.08, p=.009 | PD < HC | |
| SP | -.09 (.33) | -.07 (.29) | |||||
| HC | .04 (.13) | .05 (.14) | |||||
| Duty cycle (ratio) | PD | .17** (.23) | F(2,61)=4.73, p=.012 | PD > HC | .06 (.25) | F(2,61)=0.67, p=.517 | |
| SP | .03 (.29) | .00 (.19) | |||||
| HC | -.05 (.21) | .05 (.14) | |||||
| Inspiratory flow rate (mL/sec) | PD | .07 (.26) | F(2,61)=0.83, p=.441 | .11* (.22) | F(2,61)=2.22, p=.118 | ||
| SP | -.02 (.24) | .00 (.15) | |||||
| HC | .03 (.22) | .03 (.19) | |||||
Note. PD, panic disorder (n= 25); SD, standard deviation; SP, social phobia (n=19); HC, healthy controls (n= 20); SC, skin conductance. Significance tests for r were one-sample t-tests comparing values to 0;
p < .05;
p < .01;
p < .001
Discussion
For self-reported anxiety and dyspnea, within-subject variability over 30-second time periods was higher in PD than in SP or HC. Reported anxiety during testing was higher in both PD and SP than in HC. This suggests that the higher variability is not a general feature of higher anxiety levels but is somewhat specific to PD. Puzzling, however, is that variability of the individual physiological measures was not greater in PD, since these patients commonly report fluctuating somatic symptoms. Furthermore, sighing and other physiological signs of respiration and arousal are often more variable in PD studies (e.g., Abelson et al., 2001; Roth, et al., 1998; Wilhelm, et al., 2001a). While recent laboratory (Blechert et al., 2007) and ambulatory (Pfaltz, Michael, Grossman, Blechert, & Wilhelm, 2009; Pfaltz, Grossman, Michael, Margraf, & Wilhelm, 2010) studies of respiration in PD did not find convincing evidence of greater respiratory variability, respiratory deviations were observed in the hour preceding naturally occurring panic attacks (Rosenfield et al., 2010). The observed differences are likely due to both setting and emotional states (i.e., laboratory versus ambulatory setting; proximity to panic versus non-panic states).
Since in PD variability of self-report was greater but not variability of physiological measures, it is somewhat surprising that correlations between self-report and physiological variables were higher in PD than in other groups. If measure A is more variable in a certain group, and measure B correlates with measure A, one might expect measure B to be more variable in this group too. To a certain extent this inconsistency can be laid to sizes of the correlations, which, although often different from 0, are small. Apparently, sources of variance in physiological variables unrelated to fluctuations in emotional state, mask statistical differences due to emotional state, which nevertheless are detectable as significant correlations with self-report variables.
Greater correlations in PD can be interpreted as greater emotional coherence, which can be viewed as a sign of healthy emotional regulation. In the case of PD, however, the greater coherence may be a product of a positive feedback loop between anxiety and somatic manifestations of anxiety, which in turn generates more anxiety, spiralling upwards in a vicious circle. Alternatively, an unstable biological mechanism may be causing parallel fluctuations in experienced fear and bodily signs of fear.
The directions of the correlations of anxiety with respiratory and autonomic variables were consistently in the expected direction, which increases the likelihood that these findings are not due to chance. In PD, anxiety during sitting was positively correlated with dyspnea and SC level and minute volume and negatively correlated with IBI (which is inversely related to HR) and end-tidal pCO2. This is what would be expected for anxiety activating the sympathetic branch of the autonomic nervous system, and causing mild hyperventilation. However, it is hazardous to draw causal conclusions from correlations as evidence for one theory of panic over another (Roth, Wilhelm, & Pettit, 2005). Whether respiratory variability was caused by fluctuating thoughts of threat, whether these thoughts arose from interoception of respiratory changes, or whether hypothalamic or amygdala instability drove simultaneous changes in experience, thinking, respiration, and autonomic activation cannot be inferred from correlations between simultaneous time series.
One limitation of a short-term laboratory study compared to a longer-term ambulatory one is the length of the observation period (Wilhelm & Grossman, 2010). Are the greater fluctuations and coherence we observe in PD patients for certain variables in the laboratory also present outside the laboratory, or does the unfamiliar and restraining laboratory environment induce a less representative mental state? A recent ambulatory study indicated that PD patients are characterized by prominent variability of symptomatic experience in daily life, albeit this was investigated only on a time scale of hours, not minutes (Pfaltz, Michael, Meyer, Grossman, Margraf, & Wilhelm, in press). Although the test room in the present study was large, subjects were tethered to a stationary apparatus by electrical leads, which could elicit claustrophobic fears in PD patients. Furthermore, the time resolution of our testing procedure was 30 seconds, a sampling rate that cannot resolve fluctuations with a period less than one minute. A higher temporal resolution of ratings might have increased effect sizes, but would be more likely to have disturbed the natural flow of emotions. Mauss et al. (2005) circumvented this problem by a post-hoc cued recall rating procedure following the psychophysiological measurements, with participants' facial videos and emotional film stimuli serving as cues. It is unclear if our quiet sitting context without external stimulation would have resulted in sufficiently distinct facial expressions to be detected by such a procedure. In any case, a possible extension of our study design would be to include the analysis of facial expression, which showed higher correlations with self-report than with the psychophysiological measures in Mauss et al. (2005).
In conclusion, we have documented that even in the absence of panic attacks, PD is characterized by instability of self-reported feelings of anxiety and dyspnea on a time scale of minutes. Furthermore, these feelings correlate with bodily changes in respiration and autonomic activation. Although SP patients reported anxiety levels similar to those of the PD patients, SP patients did not show fluctuations or correlations to the same degree, indicating that the observed phenomena are specific to the PD diagnosis rather than a reflection of state anxiety. The results of this study strengthen the case for respiration-centered biofeedback treatments for PD with their focus on teaching patients to recognize and regularize breathing sensations and breathing behavior (Meuret, Wilhelm, Ritz, & Roth, 2003; Meuret, Wilhelm, & Roth, 2004, 2008). An interesting question for future studies is to what degree symptomatic and physiological variability can be normalized by cognitive-behavioral (including biofeedback) or pharmacological treatments, and if a reduction of psychophysiological coherence in PD patients is related to better health.
Acknowledgments
This work was supported by the Department of Veterans Affairs (WTR), NIH Grant MH56094 (WTR, FHW), and the National Centre of Competence in Research (NCCR) Swiss Etiological Study of Adjustment and Mental Health (sesam, SCAB, FHW). The Swiss National Science Foundation (SNF) (project no. 51A240-104890), the University of Basel, the F. Hoffmann-La Roche Corp., and the Freie Akademische Gesellschaft (Basel Scientific Society) provided core support for the NCCR sesam.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biological Psychiatry. 2001;49(7):588–595. doi: 10.1016/s0006-3223(00)01078-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock JE, Erbaugh JK. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosomatic Medicine. 2007;69(9):935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Keltner D. Brief report: The coherence of emotion systems: Comparing “on-line” measures of appraisal and facial expressions, and self-report. Cognition & Emotion. 2004;18(3):431–444. [Google Scholar]
- Caldirola D, Bellodi L, Cammino S, Perna G. Smoking and respiratory irregularity in panic disorder. Biological Psychiatry. 2004;56(6):393–398. doi: 10.1016/j.biopsych.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Jasin SE, Gracely EJ, Williams C. The Mobility Inventory for Agoraphobia. Behaviour Research and Therapy. 1985;23(1):35–44. doi: 10.1016/0005-7967(85)90140-8. [DOI] [PubMed] [Google Scholar]
- Clark DM. A cognitive approach to panic. Behaviour Research and Therapy. 1986;24(4):461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Ebner-Priemer UW, Eid M, Kleindienst N, Stabenow S, Trull TJ. Analytic strategies for understanding affective (in)stability and other dynamic processes in psychopathology. Journal of Abnormal Psychology. 2009;118(1):195–202. doi: 10.1037/a0014868. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- Friedman BH, Thayer JF, Borkovec TD, Tyrrell RA, Johnson BH, Columbo R. Autonomic characteristics of nonclinical panic and blood phobia. Biological Psychiatry. 1993;34(5):298–310. doi: 10.1016/0006-3223(93)90087-t. [DOI] [PubMed] [Google Scholar]
- Goldstein AJ, Chambless DL. A reanalysis of agoraphobia. Behavior Therapy. 1978;9(1):47–59. [Google Scholar]
- Hubert W, de Jong-Meyer R. Psychophysiological response patterns to positive and negative film stimuli. Biological Psychology. 1991;31(1):73–93. doi: 10.1016/0301-0511(90)90079-c. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Archives of General Psychiatry. 1993;50(4):306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Larsen RJ. The stability of mood variability: A spectral analytic approach to daily mood assessments. Journal of Personality and Social Psychology. 1987;52(6):1195–1204. [Google Scholar]
- Lewis LE, Drewett RF. Psychophysiological correlates of anxiety: a single-case study. Journal of Anxiety Disorders. 2006;20(6):829–835. doi: 10.1016/j.janxdis.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Ley R. Agoraphobia, the panic attack and the hyperventilation syndrome. Behaviour Research and Therapy. 1985;23(1):79–81. doi: 10.1016/0005-7967(85)90145-7. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Robinson MD. Measures of emotion: A review. Cognition & Emotion. 2009;23(3):209–237. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Wilhelm FH, Gross JJ. Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition & Emotion. 2004;18(5):631–662. [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory feedback for treating panic disorder. Journal of Clinical Psychology/In Session. 2004;60:197–207. doi: 10.1002/jclp.10245. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research. 2008;42(7):560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training in panic disorder treatment: useful intervention or impediment? Behavior Modification. 2003;27(5):731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: Empirical evidence and clinical strategies. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2010.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi AE, Freire RC, Zin WA. Panic disorder and control of breathing. Respiratory Physiology & Neurobiology. 2009;167(1):133–143. doi: 10.1016/j.resp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesthesia & Analgesia. 1998;87(3):654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- Pfaltz MC, Michael T, Grossman P, Blechert J, Wilhelm FH. Respiratory pathophysiology of panic disorder: an ambulatory monitoring study. Psychosomatic Medicine. 2009;71(8):869–876. doi: 10.1097/PSY.0b013e3181b492ff. [DOI] [PubMed] [Google Scholar]
- Pfaltz MC, Michael T, Meyer A, Grossman P, Margraf J, Wilhelm FH. Variability of symptoms in daily life of patients with panic disorder and patients with posttraumatic stress disorder. Journal of Anxiety Disorders. doi: 10.1016/j.janxdis.2010.06.001. in press. [DOI] [PubMed] [Google Scholar]
- Pfaltz MC, Grossman P, Michael T, Margraf J, Wilhelm FH. Physical activity and respiratory behavior in daily life of patients with panic disorder and healthy controls. International Journal for Psychophysiology. 2010 doi: 10.1016/j.ijpsycho.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Preter M, Klein DF. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Progress in Neuropsychopharmacology and Biological Psychiatry. 2008;32(3):603–612. doi: 10.1016/j.pnpbp.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S, Taylor S. Suffocation Fear Scale. Departments of Psychology and Psychiatry, University of British Columbia; 1994. Unpublished questionnaire. [Google Scholar]
- Reisenzein R. Exploring the strength of association between the components of emotion syndromes: The case of surprise. Cognition & Emotion. 2000;14:1–38. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128(6):934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Rosenberg EL, Ekman P. Coherence Between Expressive and Experiential Systems of Emotion. In: Ekman P, Rosenberg EL, editors. What the face reveals: basic and applied studies of spontaneous expression using the facial action coding system (FACS) Oxford: Oxford University Press; 2005. pp. 63–85. [Google Scholar]
- Rosenfield D, Zhou E, Wilhelm FH, Conrad A, Roth WT, Meuret AE. Change point analysis for longitudinal physiological data: Detection of cardiorespiratory changes preceding panic attacks. Biological Psychology. 2010;84:112–120. doi: 10.1016/j.biopsycho.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT. Physiological markers for anxiety: panic disorder and phobias. International Journal of Psychophysiology. 2005;58(2-3):190–198. doi: 10.1016/j.ijpsycho.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Roth WT, Ehlers A, Taylor CB, Margraf J, Agras WS. Skin conductance habituation in panic disorder patients. Biological Psychiatry. 1990;27(11):1231–1243. doi: 10.1016/0006-3223(90)90421-w. [DOI] [PubMed] [Google Scholar]
- Roth WT, Wilhelm FH, Pettit D. Are current theories of panic falsifiable? Psychological Bulletin. 2005;131(2):171–192. doi: 10.1037/0033-2909.131.2.171. [DOI] [PubMed] [Google Scholar]
- Roth WT, Wilhelm FH, Trabert W. Autonomic instability during relaxation in panic disorder. Psychiatry Research. 1998;80(2):155–164. doi: 10.1016/s0165-1781(98)00066-3. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT. The somatic symptom paradox in DSM-IV anxiety disorders: suggestions for a clinical focus in psychophysiology. Biological Psychology. 2001;57:105–40. doi: 10.1016/s0301-0511(01)00091-6. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gerlach AL, Roth WT. Slow recovery from voluntary hyperventilation in panic disorder. Psychosomatic Medicine. 2001;63(4):638–649. doi: 10.1097/00006842-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P. Emotions beyond the laboratory: Theoretical fundaments, study design, and analytic strategies for advanced ambulatory assessment. Biological Psychology. 2010;84:552–569. doi: 10.1016/j.biopsycho.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, Roth WT. Assessment of heart rate variability during alterations in stress: complex demodulation vs. spectral analysis. Biomedical Sciences Instrumentation. 2005;41:346–351. [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT. Taking the laboratory to the skies: ambulatory assessment of self-report, autonomic, and respiratory responses in flying phobia. Psychophysiology. 1998;35(5):596–606. doi: 10.1017/s0048577298970196. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT. Physiologic instability in panic disorder and generalized anxiety disorder. Biological Psychiatry. 2001a;49(7):596–605. doi: 10.1016/s0006-3223(00)01000-3. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT. Characteristics of sighing in panic disorder. Biological Psychiatry. 2001b;49(7):606–614. doi: 10.1016/s0006-3223(00)01014-3. [DOI] [PubMed] [Google Scholar]


