Abstract
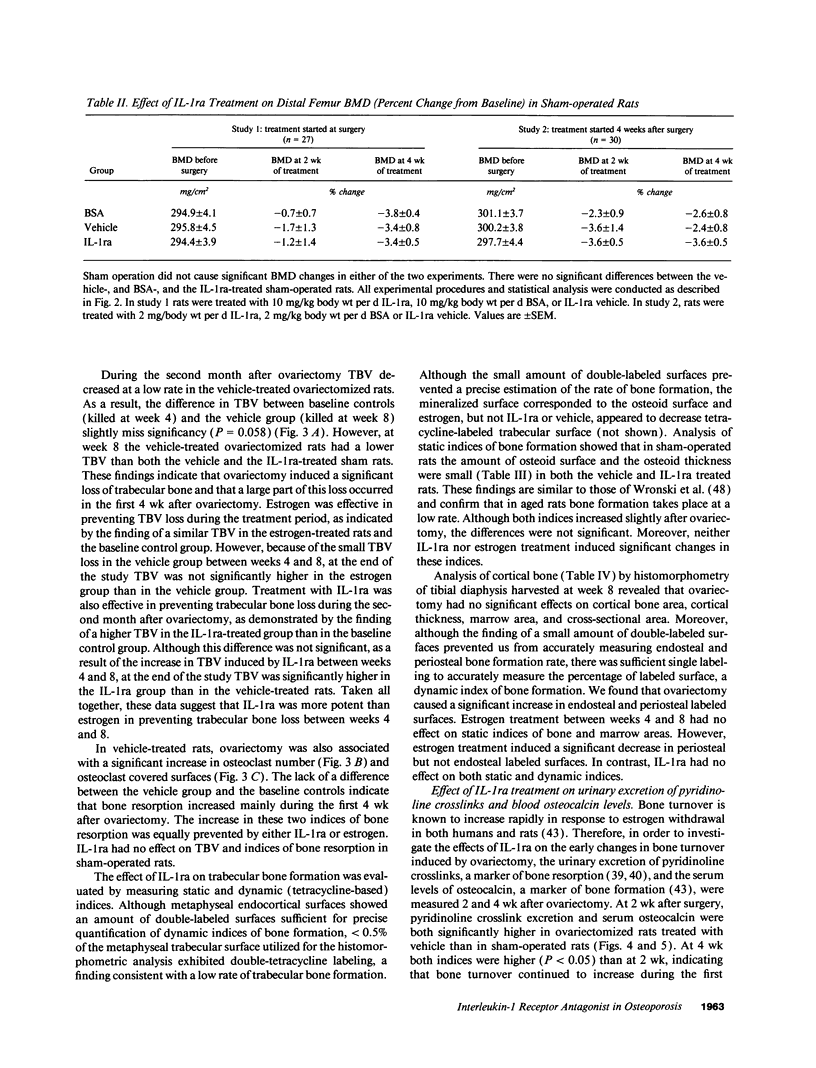
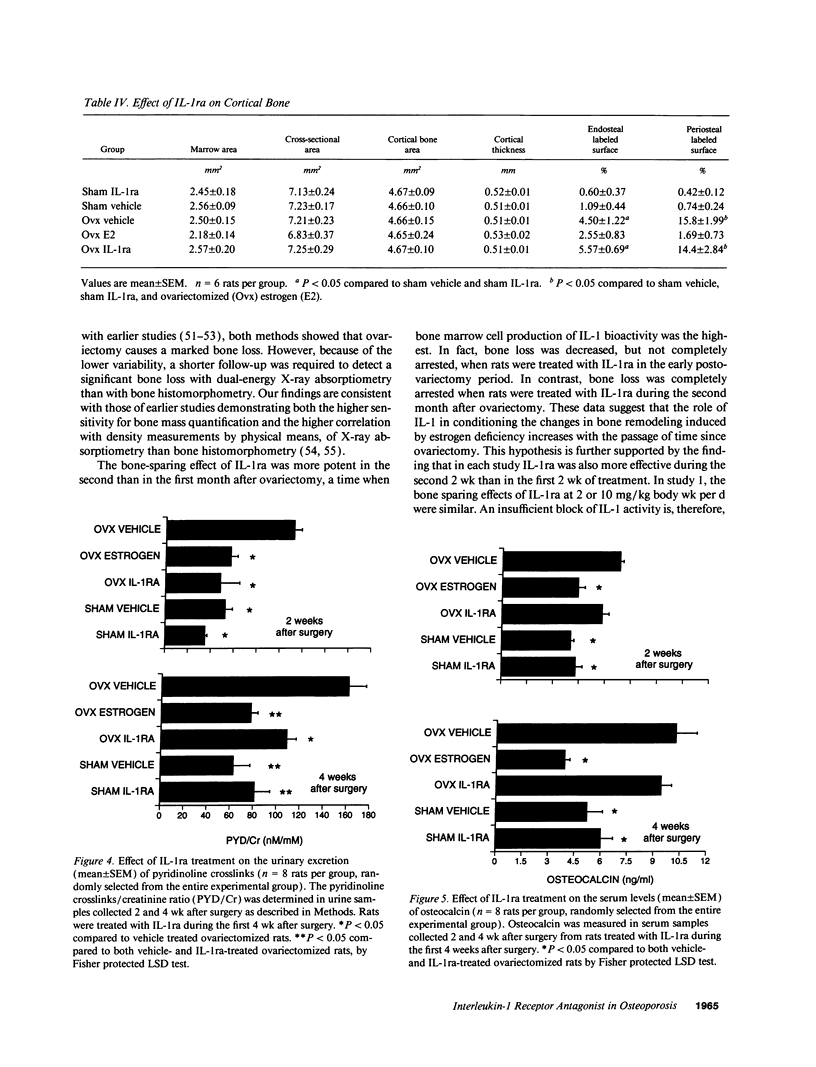
Interleukin-1 (IL-1), a cytokine produced by bone marrow cells and bone cells, has been implicated in the pathogenesis of postmenopausal osteoporosis because of its potent stimulatory effects on bone resorption in vitro and in vivo. To investigate whether IL-1 plays a direct causal role in post ovariectomy bone loss, 6-mo-old ovariectomized rats were treated with subcutaneous infusions of IL-1 receptor antagonist (IL-1ra), a specific competitor of IL-1, for 4 wk, beginning either at the time of surgery or 4 wk after ovariectomy. The bone density of the distal femur was measured non invasively by dual-energy X-ray absorptiometry. Bone turnover was assessed by bone histomorphometry and by measuring serum osteocalcin, a marker of bone formation, and the urinary excretion of pyridinoline cross-links, a marker of bone resorption. Ovariectomy caused a rapid increase in bone turnover and a marked decrease in bone density which were blocked by treatment with 17 beta estradiol. Ovariectomy also increased the production of IL-1 from cultured bone marrow cells. Ovariectomy induced-bone loss was significantly decreased by IL-1ra treatment started at the time of ovariectomy and completely blocked by IL-1ra treatment begun 4 wk after ovariectomy. In both studies IL-1ra also decreased bone resorption in a manner similar to estrogen, while it had no effect on bone formation. In contrast, treatment with IL-1ra had no effect on the bone density and the bone turnover of sham-operated rats, indicating that IL-1ra specifically blocked estrogen-dependent bone loss. In conclusion, these data indicate that IL-1, or mediators induced by IL-1, play an important causal role in the mechanism by which ovariectomy induces bone loss in rats, especially following the immediate post ovariectomy period.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991 Nov;88(5):1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Thompson R. C., Hannum C. H. An IL-1 inhibitor from human monocytes. Production and characterization of biologic properties. J Immunol. 1989 Sep 15;143(6):1851–1858. [PubMed] [Google Scholar]
- Arend W. P., Welgus H. G., Thompson R. C., Eisenberg S. P. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990 May;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé R. M., Dantzer R., Kelley K. W. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992 Feb 28;573(2):318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Aufdemorte T. B., Garrett I. R., Yates A. J., Mundy G. R. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989 Sep;125(3):1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- Carter D. B., Deibel M. R., Jr, Dunn C. J., Tomich C. S., Laborde A. L., Slightom J. L., Berger A. E., Bienkowski M. J., Sun F. F., McEwan R. N. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990 Apr 12;344(6267):633–638. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- Delmas P. D., Gineyts E., Bertholin A., Garnero P., Marchand F. Immunoassay of pyridinoline crosslink excretion in normal adults and in Paget's disease. J Bone Miner Res. 1993 May;8(5):643–648. doi: 10.1002/jbmr.5650080516. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Thompson R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991 Nov;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Felix R., Fleisch H., Elford P. R. Bone-resorbing cytokines enhance release of macrophage colony-stimulating activity by the osteoblastic cell MC3T3-E1. Calcif Tissue Int. 1989 May;44(5):356–360. doi: 10.1007/BF02556317. [DOI] [PubMed] [Google Scholar]
- Finkelman R. D., Linkhart T. A., Mohan S., Lau K. H., Baylink D. J., Bell N. H. Vitamin D deficiency causes a selective reduction in deposition of transforming growth factor beta in rat bone: possible mechanism for impaired osteoinduction. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3657–3660. doi: 10.1073/pnas.88.9.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Chaplin D. D., Kiely J. M., Unanue E. R. Regulation of interleukin 1 gene expression by adherence and lipopolysaccharide. J Immunol. 1987 Jun 1;138(11):3799–3802. [PubMed] [Google Scholar]
- Geusens P., Dequeker J., Nijs J., Bramm E. Effect of ovariectomy and prednisolone on bone mineral content in rats: evaluation by single photon absorptiometry and radiogrammetry. Calcif Tissue Int. 1990 Oct;47(4):243–250. doi: 10.1007/BF02555926. [DOI] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M., Mundy G. R. Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol. 1986 Apr 1;136(7):2478–2482. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Granowitz E. V., Porat R., Mier J. W., Pribble J. P., Stiles D. M., Bloedow D. C., Catalano M. A., Wolff S. M., Dinarello C. A. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992 Sep;4(5):353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- Griffin M. G., Kimble R., Hopfer W., Pacifici R. Dual-energy x-ray absorptiometry of the rat: accuracy, precision, and measurement of bone loss. J Bone Miner Res. 1993 Jul;8(7):795–800. doi: 10.1002/jbmr.5650080704. [DOI] [PubMed] [Google Scholar]
- Hannum C. H., Wilcox C. J., Arend W. P., Joslin F. G., Dripps D. J., Heimdal P. L., Armes L. G., Sommer A., Eisenberg S. P., Thompson R. C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990 Jan 25;343(6256):336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Kalu D. N., Liu C. C., Salerno E., Hollis B., Echon R., Ray M. Skeletal response of ovariectomized rats to low and high doses of 17 beta-estradiol. Bone Miner. 1991 Sep;14(3):175–187. doi: 10.1016/0169-6009(91)90021-q. [DOI] [PubMed] [Google Scholar]
- König A., Mühlbauer R. C., Fleisch H. Tumor necrosis factor alpha and interleukin-1 stimulate bone resorption in vivo as measured by urinary [3H]tetracycline excretion from prelabeled mice. J Bone Miner Res. 1988 Dec;3(6):621–627. doi: 10.1002/jbmr.5650030607. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Forrest C., Baird C. Prevention of spinal osteoporosis in oophorectomised women. Lancet. 1980 Nov 29;2(8205):1151–1154. doi: 10.1016/s0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Sousa S. L., Alander C., Raisz L. G., Dinarello C. A. Comparison of the bone-resorbing activity in the supernatants from phytohemagglutinin-stimulated human peripheral blood mononuclear cells with that of cytokines through the use of an antiserum to interleukin 1. Endocrinology. 1987 Sep;121(3):1164–1170. doi: 10.1210/endo-121-3-1164. [DOI] [PubMed] [Google Scholar]
- Malyak M., Joslin F. G., Verderber E. L., Eisenberg S. P., Arend W. P. IL-1ra ELISA: reduction and alkylation of synovial fluid eliminates interference by IgM rheumatoid factors. J Immunol Methods. 1991 Jul 5;140(2):281–288. doi: 10.1016/0022-1759(91)90381-o. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Matsui K., Shimakoshi Y., Aida Y., Hukuda S. 1-Hydroxyethylidene-1,1-bisphosphonate decreases the postovariectomy enhanced interleukin 1 secretion from peritoneal macrophages in adult rats. Calcif Tissue Int. 1991 Dec;49(6):403–406. doi: 10.1007/BF02555851. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Dewhirst F. E., Hauschka P. V., Stashenko P. Interleukin-1 beta stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res. 1991 Apr;10(1-2):15–21. [PubMed] [Google Scholar]
- Pacifici R., Brown C., Puscheck E., Friedrich E., Slatopolsky E., Maggio D., McCracken R., Avioli L. V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., McCracken R., Vered I., McMurtry C., Avioli L. V., Peck W. A. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2398–2402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Chenu C., Bird A., Mundy G. R., Roodman G. D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989 Feb;4(1):113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- Pioli G., Basini G., Pedrazzoni M., Musetti G., Ulietti V., Bresciani D., Villa P., Bacchi A., Hughes D., Russell G. Spontaneous release of interleukin-I and interleukin-6 by peripheral blood mononuclear cells after oophorectomy. Clin Sci (Lond) 1992 Oct;83(4):503–507. doi: 10.1042/cs0830503. [DOI] [PubMed] [Google Scholar]
- Pødenphant J., Gotfredsen A., Nilas L., Nørgaard H., Braendstrup O. Iliac crest biopsy: representativity for the amount of mineralized bone. Bone. 1986;7(6):427–430. doi: 10.1016/8756-3282(86)90002-5. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med. 1988 Mar 31;318(13):818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- Ralston S. H., Russell R. G., Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990 Sep;5(9):983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Melton L. J., 3rd Involutional osteoporosis. N Engl J Med. 1986 Jun 26;314(26):1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Sabatini M., Boyce B., Aufdemorte T., Bonewald L., Mundy G. R. Infusions of recombinant human interleukins 1 alpha and 1 beta cause hypercalcemia in normal mice. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5235–5239. doi: 10.1073/pnas.85.14.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckinger P., Klein-Nulend J., Alander C., Thompson R. C., Dayer J. M., Raisz L. G. Natural and recombinant human IL-1 receptor antagonists block the effects of IL-1 on bone resorption and prostaglandin production. J Immunol. 1990 Dec 15;145(12):4181–4184. [PubMed] [Google Scholar]
- Seyedin S. M., Kung V. T., Daniloff Y. N., Hesley R. P., Gomez B., Nielsen L. A., Rosen H. N., Zuk R. F. Immunoassay for urinary pyridinoline: the new marker of bone resorption. J Bone Miner Res. 1993 May;8(5):635–641. doi: 10.1002/jbmr.5650080515. [DOI] [PubMed] [Google Scholar]
- Slemenda C., Hui S. L., Longcope C., Johnston C. C. Sex steroids and bone mass. A study of changes about the time of menopause. J Clin Invest. 1987 Nov;80(5):1261–1269. doi: 10.1172/JCI113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Rooney M. L., Desjardins L. A., Heeley J. D. Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 1987 Dec;2(6):559–565. doi: 10.1002/jbmr.5650020612. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Vandersteenhoven J. J., Bell N. H. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987 Apr;2(2):115–122. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- Uebelhart D., Gineyts E., Chapuy M. C., Delmas P. D. Urinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone disease. Bone Miner. 1990 Jan;8(1):87–96. doi: 10.1016/0169-6009(91)90143-n. [DOI] [PubMed] [Google Scholar]
- Uebelhart D., Schlemmer A., Johansen J. S., Gineyts E., Christiansen C., Delmas P. D. Effect of menopause and hormone replacement therapy on the urinary excretion of pyridinium cross-links. J Clin Endocrinol Metab. 1991 Feb;72(2):367–373. doi: 10.1210/jcem-72-2-367. [DOI] [PubMed] [Google Scholar]
- Wronski T. J., Cintrón M., Dann L. M. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988 Sep;43(3):179–183. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- Wronski T. J., Cintrón M., Doherty A. L., Dann L. M. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988 Aug;123(2):681–686. doi: 10.1210/endo-123-2-681. [DOI] [PubMed] [Google Scholar]
- Wronski T. J., Walsh C. C., Ignaszewski L. A. Histologic evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone. 1986;7(2):119–123. doi: 10.1016/8756-3282(86)90683-6. [DOI] [PubMed] [Google Scholar]
- Zamir O., Hasselgren P. O., O'Brien W., Thompson R. C., Fischer J. E. Muscle protein breakdown during endotoxemia in rats and after treatment with interleukin-1 receptor antagonist (IL-1ra). Ann Surg. 1992 Sep;216(3):381–387. doi: 10.1097/00000658-199209000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]