Abstract
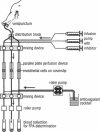
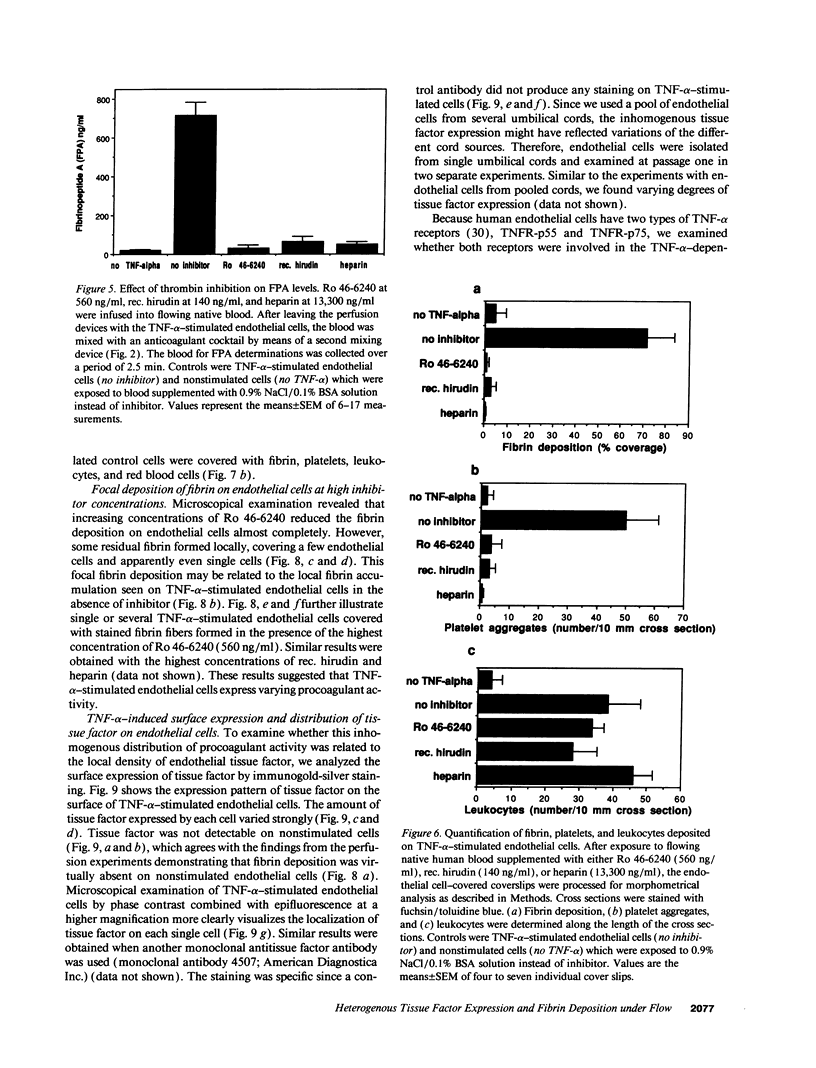
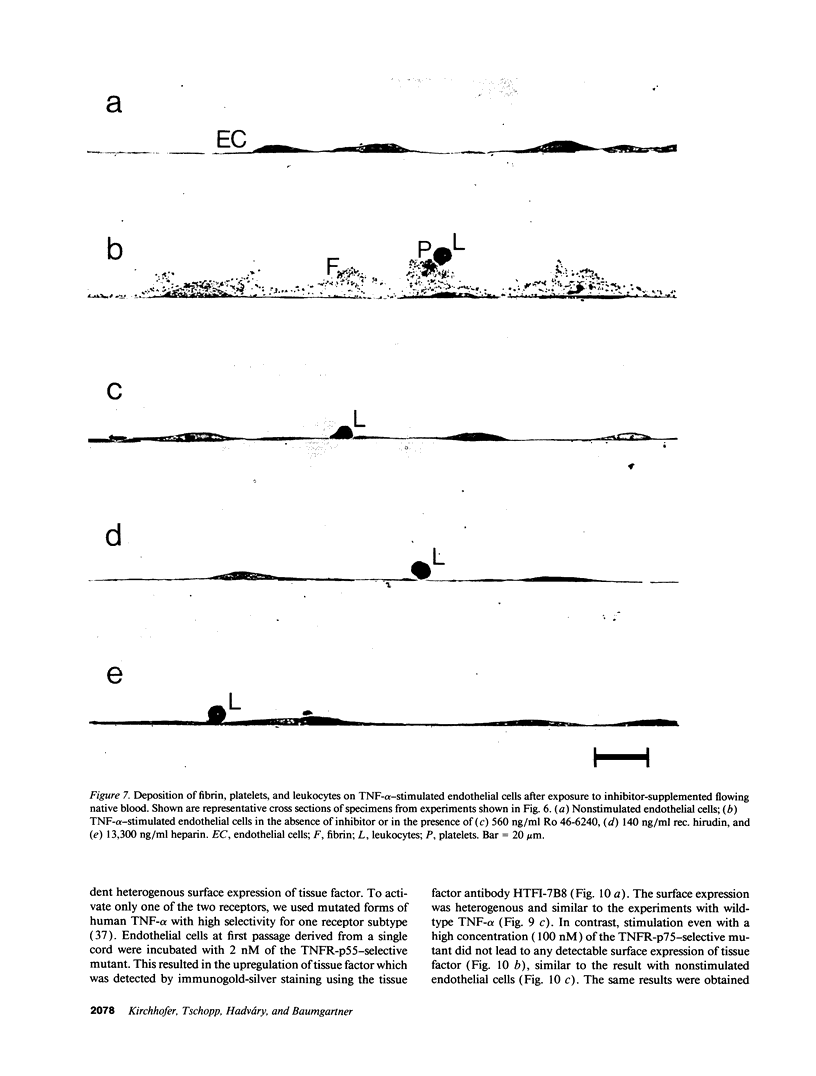
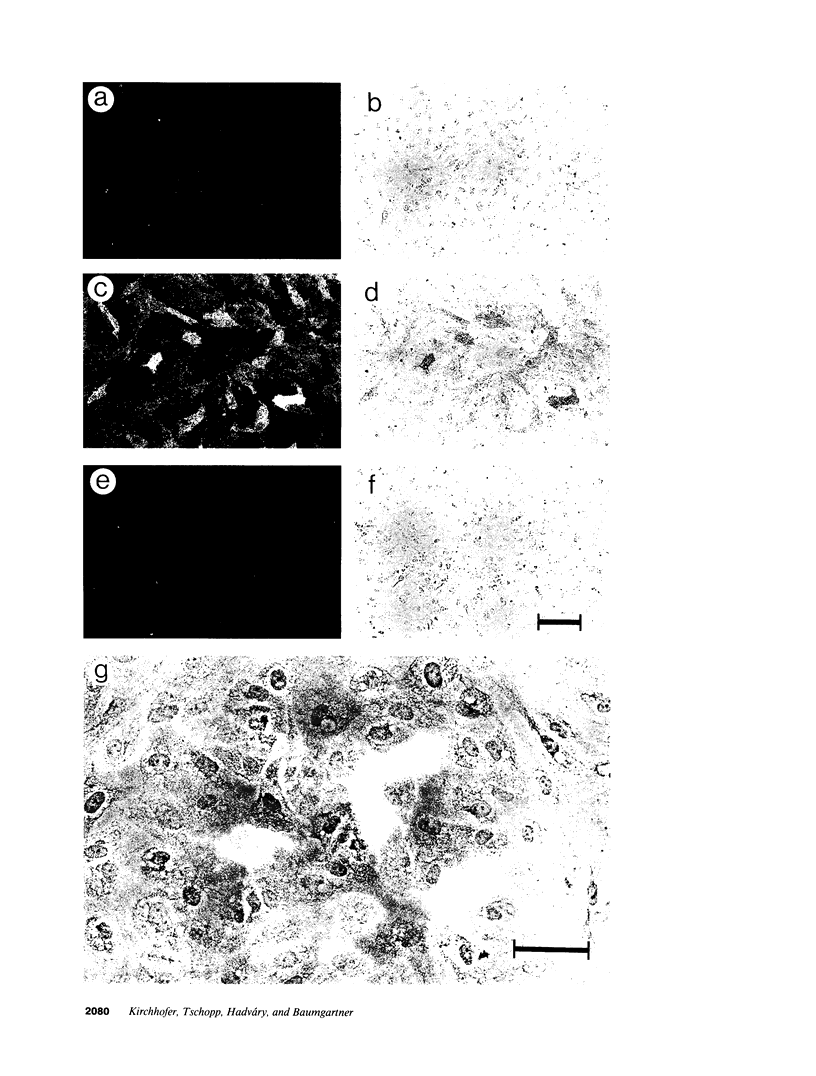
TNF-alpha induces changes in endothelial cell functions, such as upregulation of tissue factor, resulting in endothelial procoagulant activity which may play a role in disseminated intravascular coagulation. The procoagulant activity of TNF-alpha-stimulated endothelial cell monolayers was studied in a human ex vivo native (nonanticoagulated) blood flow system using the three thrombin inhibitors recombinant hirudin, Ro 46-6240, and heparin. Under venous blood flow conditions (shear rate 65 s-1) recombinant hirudin, Ro 46-6240, and heparin inhibited fibrin deposition on the endothelial cells by 50% at concentrations of 14, 28, and 412 ng/ml, respectively. The highest tested concentrations of the thrombin inhibitors reduced the postchamber fibrinopeptide A levels from 713 +/- 69 to < 70 ng/ml. Surprisingly, even at relatively high inhibitor concentrations, some local fibrin deposits were found on TNF-alpha-stimulated cells, suggesting that some endothelial cells possess higher procoagulant activity than others. Therefore, the surface expression pattern of tissue factor, the primary initiator of coagulation in this system, was examined by immunogold-silver staining. The results showed that the tissue factor density on the cell surface varied strongly among TNF-alpha-stimulated endothelial cells. Using TNF receptor-selective agonistic mutants of TNF-alpha, it was demonstrated further that the heterogenous surface expression of tissue factor was mediated entirely by the 55-kD TNF receptor and did not involve the 75-kD TNF receptor. We conclude that in this system TNF-alpha induces heterogenous tissue factor expression which may lead to a high local thrombin concentration, such that even in the presence of thrombin inhibitors focal fibrin deposition occurs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh K., Pettersen K. S., Filion-Myklebust C., Prydz H. Observations on the cell biology of tissue factor in endothelial cells. Thromb Haemost. 1990 Apr 12;63(2):298–302. [PubMed] [Google Scholar]
- Baumgartner H. R., Sakariassen K. S. Factors controlling thrombus formation on arterial lesions. Ann N Y Acad Sci. 1985;454:162–177. doi: 10.1111/j.1749-6632.1985.tb11855.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Turitto V., Weiss H. J. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. II. Relationships among platelet adhesion, thrombus dimensions, and fibrin formation. J Lab Clin Med. 1980 Feb;95(2):208–221. [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson S. D., Ross S. E., Bach R., Guha A. An inhibitory monoclonal antibody against human tissue factor. Blood. 1987 Aug;70(2):490–493. [PubMed] [Google Scholar]
- Clozel M., Kuhn H., Baumgartner H. R. Procoagulant activity of endotoxin-treated human endothelial cells exposed to native human flowing blood. Blood. 1989 Feb 15;73(3):729–733. [PubMed] [Google Scholar]
- Colucci M., Balconi G., Lorenzet R., Pietra A., Locati D., Donati M. B., Semeraro N. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983 Jun;71(6):1893–1896. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Cheng J., Chang A., Taylor F. B., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993 May;142(5):1458–1470. [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Gemmell C. H., Nemerson Y., Turitto V. The effects of shear rate on the enzymatic activity of the tissue factor-factor VIIa complex. Microvasc Res. 1990 Nov;40(3):327–340. doi: 10.1016/0026-2862(90)90031-l. [DOI] [PubMed] [Google Scholar]
- Grabowski E. F., Zuckerman D. B., Nemerson Y. The functional expression of tissue factor by fibroblasts and endothelial cells under flow conditions. Blood. 1993 Jun 15;81(12):3265–3270. [PubMed] [Google Scholar]
- Heimark R. L., Schwartz S. M. Binding of coagulation factors IX and X to the endothelial cell surface. Biochem Biophys Res Commun. 1983 Mar 16;111(2):723–731. doi: 10.1016/0006-291x(83)90365-0. [DOI] [PubMed] [Google Scholar]
- Inauen W., Baumgartner H. R., Bombeli T., Haeberli A., Straub P. W. Dose- and shear rate-dependent effects of heparin on thrombogenesis induced by rabbit aorta subendothelium exposed to flowing human blood. Arteriosclerosis. 1990 Jul-Aug;10(4):607–615. doi: 10.1161/01.atv.10.4.607. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
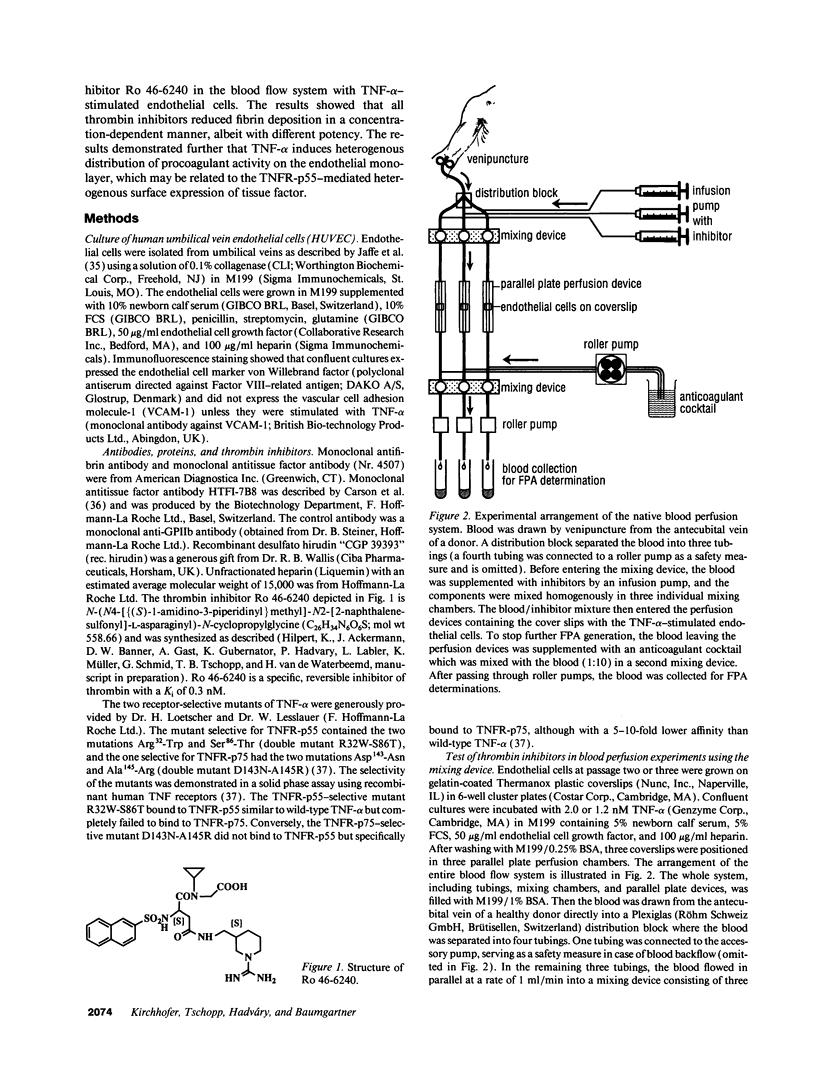
- Kirchhofer D., Sakariassen K. S., Clozel M., Tschopp T. B., Hadváry P., Nemerson Y., Baumgartner H. R. Relationship between tissue factor expression and deposition of fibrin, platelets, and leukocytes on cultured endothelial cells under venous blood flow conditions. Blood. 1993 Apr 15;81(8):2050–2058. [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Stueber D., Banner D., Mackay F., Lesslauer W. Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J Biol Chem. 1993 Dec 15;268(35):26350–26357. [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Loetscher H., Stueber D., Gehr G., Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993 May 1;177(5):1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., Rosenberg R. D. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986 Jun 5;261(16):7507–7517. [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry. 1984 Apr 10;23(8):1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Selectins: novel receptors that mediate leukocyte adhesion during inflammation. Thromb Haemost. 1991 Mar 4;65(3):223–228. [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerson Y., Turitto V. T. The effect of flow on hemostasis and thrombosis. Thromb Haemost. 1991 Sep 2;66(3):272–276. [PubMed] [Google Scholar]
- Repke D., Gemmell C. H., Guha A., Turitto V. T., Broze G. J., Jr, Nemerson Y. Hemophilia as a defect of the tissue factor pathway of blood coagulation: effect of factors VIII and IX on factor X activation in a continuous-flow reactor. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7623–7627. doi: 10.1073/pnas.87.19.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers G. M., Shuman M. A. Characterization of the interaction between factor Xa and bovine aortic endothelial cells. Biochim Biophys Acta. 1985 Mar 21;844(3):320–329. doi: 10.1016/0167-4889(85)90133-8. [DOI] [PubMed] [Google Scholar]
- Ryan J., Brett J., Tijburg P., Bach R. R., Kisiel W., Stern D. Tumor necrosis factor-induced endothelial tissue factor is associated with subendothelial matrix vesicles but is not expressed on the apical surface. Blood. 1992 Aug 15;80(4):966–974. [PubMed] [Google Scholar]
- Sakariassen K. S., Joss R., Muggli R., Kuhn H., Tschopp T. B., Sage H., Baumgartner H. R. Collagen type III induced ex vivo thrombogenesis in humans. Role of platelets and leukocytes in deposition of fibrin. Arteriosclerosis. 1990 Mar-Apr;10(2):276–284. doi: 10.1161/01.atv.10.2.276. [DOI] [PubMed] [Google Scholar]
- Sakariassen K. S., Kuhn H., Muggli R., Baumgartner H. R. Growth and stability of thrombi in flowing citrated blood: assessment of platelet-surface interactions with computer-assisted morphometry. Thromb Haemost. 1988 Dec 22;60(3):392–398. [PubMed] [Google Scholar]
- Scarpati E. M., Sadler J. E. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989 Dec 5;264(34):20705–20713. [PubMed] [Google Scholar]
- Semeraro N., Triggiani R., Montemurro P., Cavallo L. G., Colucci M. Enhanced endothelial tissue factor but normal thrombomodulin in endotoxin-treated rabbits. Thromb Res. 1993 Sep 15;71(6):479–486. doi: 10.1016/0049-3848(93)90121-4. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stern D. M., Drillings M., Nossel H. L., Hurlet-Jensen A., LaGamma K. S., Owen J. Binding of factors IX and IXa to cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4119–4123. doi: 10.1073/pnas.80.13.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Kaiser E., Nawroth P. P. Regulation of the coagulation system by vascular endothelial cells. Haemostasis. 1988;18(4-6):202–214. doi: 10.1159/000215808. [DOI] [PubMed] [Google Scholar]
- Thorp K. M., Southern C., Bird I. N., Matthews N. Tumour necrosis factor induction of ELAM-1 and ICAM-1 on human umbilical vein endothelial cells--analysis of tumour necrosis factor-receptor interactions. Cytokine. 1992 Jul;4(4):313–319. doi: 10.1016/1043-4666(92)90072-y. [DOI] [PubMed] [Google Scholar]
- Tijburg P. N., Ijsseldijk M. J., Sixma J. J., de Groot P. G. Quantification of fibrin deposition in flowing blood with peroxidase-labeled fibrinogen. High shear rates induce decreased fibrin deposition and appearance of fibrin monomers. Arterioscler Thromb. 1991 Mar-Apr;11(2):211–220. doi: 10.1161/01.atv.11.2.211. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T., Baumgartner H. R. Role of shear rate and platelets in promoting fibrin formation on rabbit subendothelium. Studies utilizing patients with quantitative and qualitative platelet defects. J Clin Invest. 1986 Oct;78(4):1072–1082. doi: 10.1172/JCI112663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaginga J. J., Sixma J. J., de Groot P. G. Activation of endothelial cells induces platelet thrombus formation on their matrix. Studies of new in vitro thrombosis model with low molecular weight heparin as anticoagulant. Arteriosclerosis. 1990 Jan-Feb;10(1):49–61. doi: 10.1161/01.atv.10.1.49. [DOI] [PubMed] [Google Scholar]
- Zwaginga J. J., de Boer H. C., IJsseldijk M. J., Kerkhof A., Muller-Berghaus G., Gruhlichhenn J., Sixma J. J., de Groot P. G. Thrombogenicity of vascular cells. Comparison between endothelial cells isolated from different sources and smooth muscle cells and fibroblasts. Arteriosclerosis. 1990 May-Jun;10(3):437–448. doi: 10.1161/01.atv.10.3.437. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Büller H. R., ten Cate J. W., Aarden L. A., Hack C. E., Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990 Dec 15;76(12):2520–2526. [PubMed] [Google Scholar]