Abstract
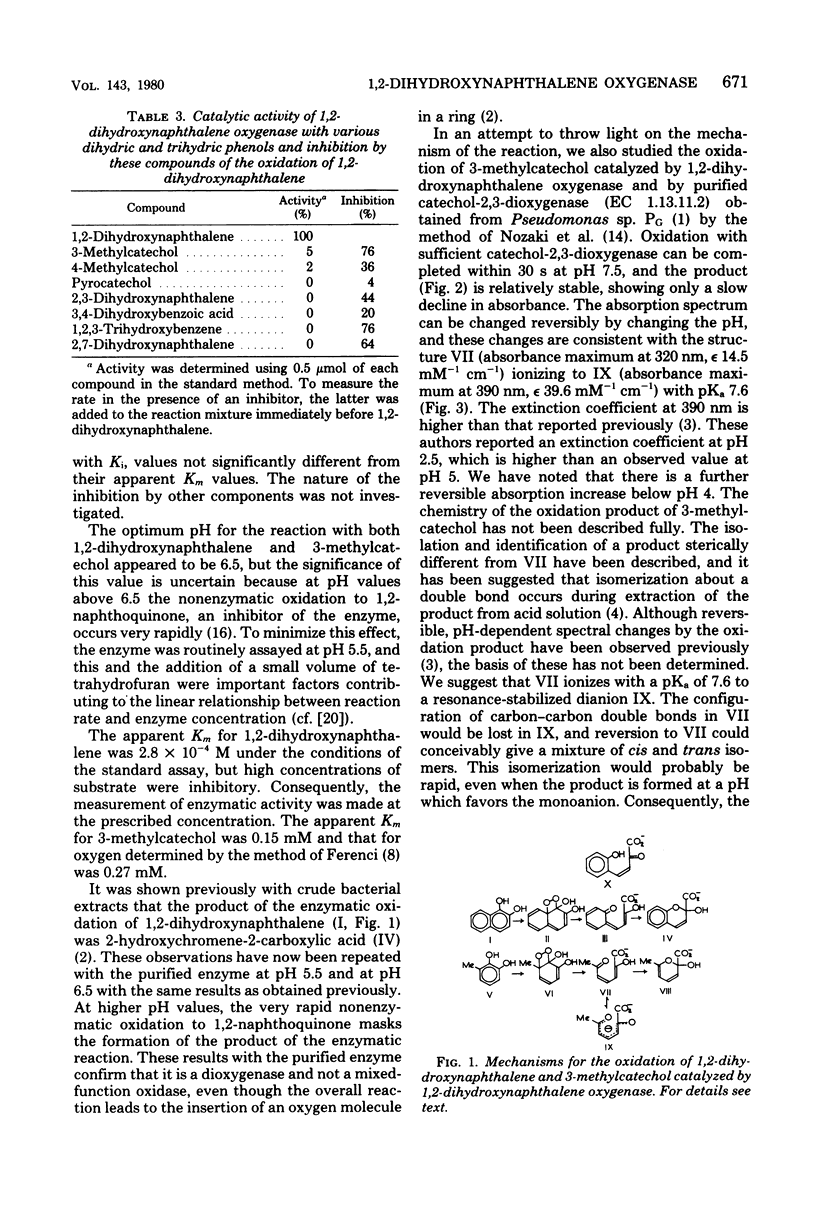
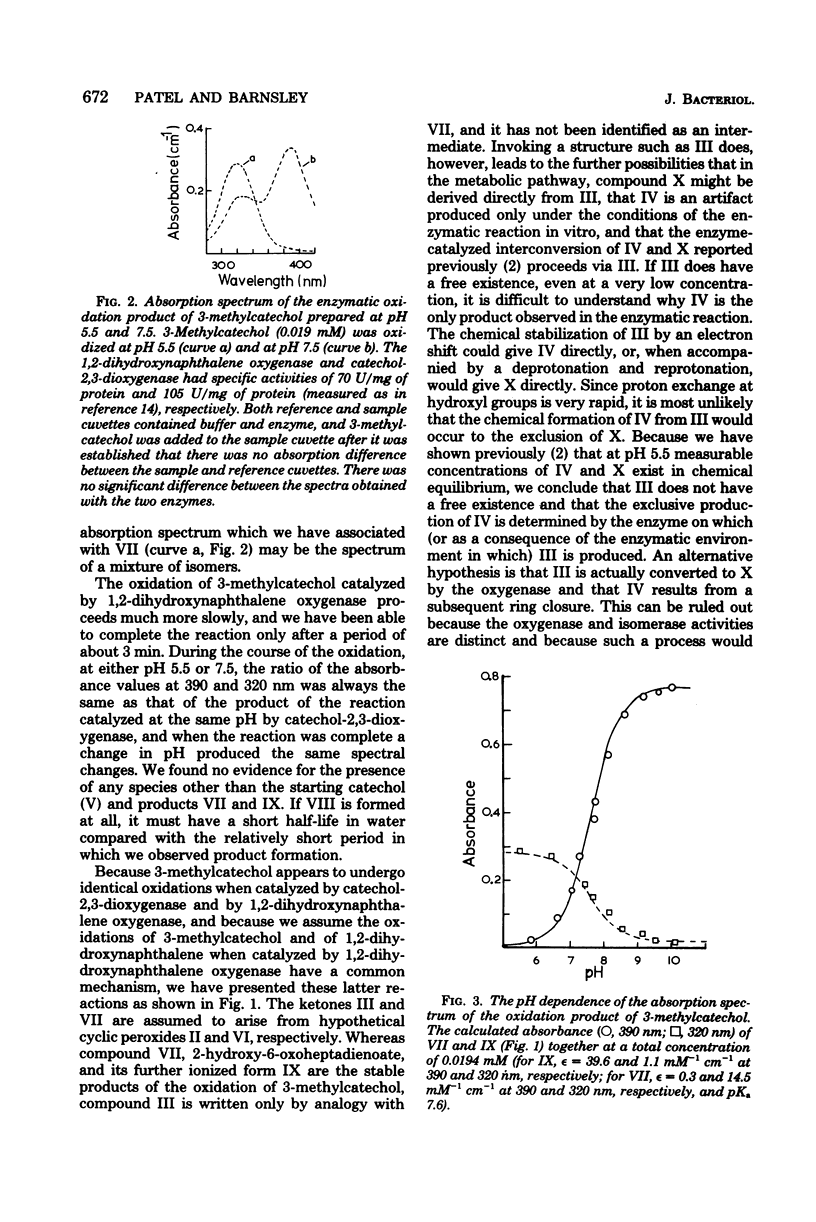
1,2-Dihydroxynaphthalene oxygenase was purified from Pseudomonas putida NCIB 9816 grown on naphthalene as the sole source of carbon and energy. The enzyme had a subunit molecular weight of 19,000 and in a medium containing phosphate buffer, 1 mM mercaptoethanol, and 10% (vol/vol) ethanol had a native molecular weight greater than 275,000. The enzyme required Fe2+ for activity. It was inactivated slowly on standing, and inactivation was accelerated by dilution with aerated buffers and by H2O2. Bathophenanthroline sulfonate, o-phenanthroline, 8-hydroxyquinoline, and 2,2'-dipyridyl also inhibited the enzyme. The inactive enzyme was reactivated by anaerobic incubation with ferrous sulfate and ferrous ammonium sulfate. Thiol reagents and acetone, ethanol, or glycerol decreased the rate of loss of activity. The enzyme was most active with 1,2-dihydroxynaphthalene, for which the Km was 2.8 X 10(-4) M. 3-Methyl- and 4-methylcatechols were oxidized at 3 and 1.5%, respectively, of the rate of 1,2-dihydroxynaphthalene, and the Km for 3-methylcatechol was 1.5 X 10(-4) M. Purified 1,2-dihydroxynaphthalene oxygenase catalyzed the oxidation of 1,2-dihydroxynaphthalene, leading to the appearance of 2-hydroxychromene-2-carboxylic acid, but 3-methylcatechol was oxidized by this enzyme to 2-hydroxy-6-oxoheptadienoic acid. Thus, a product structurally analogous to 2-hydroxychromene-2-carboxylic acid was not observed when 3-methylcatechol was oxidized. This may indicate that 2-hydroxychromene-2-carboxylic acid results from cyclization of a ring fission product before release from the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnsley E. A. Naphthalene metabolism by pseudomonads: the oxidation of 1,2-dihydroxynaphthalene to 2-hydroxychromene-2-carboxylic acid and the formation of 2'-hydroxybenzalpyruvate. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1116–1121. doi: 10.1016/s0006-291x(76)80247-1. [DOI] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNER E. D., YOUNG L. Biochemical studies of toxic agents. VII. The metabolism of naphthalene in animals of different species. Biochem J. 1954 Dec;58(4):647–655. doi: 10.1042/bj0580647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Fiecchi A., Galli E. Formation of 2-hydroxy-6-oxo-2, trans-4, trans-heptad-ienoic acid from 3-methylcatechol by a Pseudomonas. Experientia. 1968 Feb 15;24(2):113–113. doi: 10.1007/BF02146927. [DOI] [PubMed] [Google Scholar]
- Catterall F. A., Williams P. A. A comparison of some properties of the 1,2-dihydroxynaphthalene oxygenase and catechol 2,3-oxygenase activities in naphthalene-grown pseudomonas sp. NCIB 9816. Biochem J. 1972 Jul;128(3):88P–89P. doi: 10.1042/bj1280088pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. I., Evans W. C. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem J. 1964 May;91(2):251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T. Oxygen metabolism in Pseudomonas methanica. Arch Microbiol. 1976 Jun;108(2):217–219. doi: 10.1007/BF00428954. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Uyeda M., Kojima Y., Nozaki M., Hayaishi O. Protocatechuate 3,4-dioxygenase. II. Electron spin resonance and spectral studies on interaction of substrates and enzyme. J Biol Chem. 1972 Jul 10;247(13):4414–4421. [PubMed] [Google Scholar]
- Kita H. Crystallization and some properties of 3,4-dihydroxyphenylacetate 2,3-oxygenase from Pseudomonas ovalis. J Biochem. 1965 Aug;58(2):116–122. doi: 10.1093/oxfordjournals.jbchem.a128172. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Fujisawa H., Nakazawa A., Nakazawa T., Kanetsuna F., Taniuchi H., Nozaki M., Hayaishi O. Studies on pyrocatechase. I. Purification and spectral properties. J Biol Chem. 1967 Jul 25;242(14):3270–3278. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- NOZAKI M., KAGAMIYAMA H., HAYAISHI O. METAPYROCATECHASE. I. PURIFICATION, CRYSTALLIZATION AND SOME PROPERTIES. Biochem Z. 1963;338:582–590. [PubMed] [Google Scholar]
- Nozaki M., Ono K., Nakazawa T., Kotani S., Hayaishi O. Metapyrocatechase. II. The role of iron and sulfhydryl groups. J Biol Chem. 1968 May 25;243(10):2682–2690. [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsuzzaman K. M., Barnsley E. A. The regulation of naphthalene metabolism in pseudomonads. Biochem Biophys Res Commun. 1974 Sep 23;60(2):582–589. doi: 10.1016/0006-291x(74)90280-0. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman K. M., Barnsley E. A. The regulation of naphthalene oxygenase in pseudomonads. J Gen Microbiol. 1974 Jul;83(0):165–170. doi: 10.1099/00221287-83-1-165. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams P. A., Catterall F. A., Murray K. Metabolism of naphthalene, 2-methylnaphthalene, salicylate, and benzoate by Pseudomonas PG: regulation of tangential pathways. J Bacteriol. 1975 Nov;124(2):679–685. doi: 10.1128/jb.124.2.679-685.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


