Abstract
Nucleotides within the airway surface liquid (ASL) regulate airway epithelial ion transport rates by Ca2+- and protein kinase C-dependent mechanisms via activation of specific P2Y receptors. Extracellular adenine nucleotides also serve as precursors for adenosine, which promotes cyclic AMP-mediated activation of the cystic fibrosis transmembrane regulator chloride channel via A2b adenosine receptors. A biological role for extracellular ATP in ASL volume homeostasis has been suggested by the demonstration of regulated ATP release from airway epithelia. However, nucleotide hydrolysis at the airway surface makes it difficult to assess the magnitude of ATP release and the relative abundance of adenyl purines and, hence, to define their biological functions. We have combined ASL microsampling and high performance liquid chromatography analysis of fluorescent 1,N6-ethenoadenine derivatives to measure adenyl purines in ASL. We found that adenosine, AMP, and ADP accumulated in high concentrations relative to ATP within the ASL covering polarized primary human normal or cystic fibrosis airway epithelial cells. By using immortalized epithelial cell monolayers that endogenously express a luminal A2b adenosine receptor, we found that basal as well as forskolin-promoted cyclic AMP production was reduced by exogenous adenosine deaminase, suggesting that A2b receptors sense endogenous adenosine within the ASL. The physiological role of adenosine was further established by illustrating that adenosine removal or inhibition of adenosine receptors in primary cultures impaired ASL volume regulation. Our data reveal a complex pattern of nucleotides/nucleosides in ASL under resting conditions and suggest that adenosine may play a key role in regulating ASL volume homeostasis.
Airways continuously remove noxious materials through a mucus clearance process that requires a complex regulation of airway surface liquid (ASL)1 volume by active ion transport and coordinated ciliary beating. In normal airways, the cystic fibrosis transmembrane conductance regulator (CFTR) protein mediates liquid balance on airway surfaces by regulating Na+ absorption and by acting as a cyclic AMP-regulated Cl− channel. Proper regulation of salt and water movement across epithelial cell surfaces is required in both ASL compartments: (i) sufficient volume in the periciliary liquid ensures efficient cilia movement; and (ii) sufficient volume optimizes the viscoelastic properties of the mucus layer for transport (1). Defective CFTR activity causes the syndrome of cystic fibrosis (CF), which is characterized by accelerated Na+-dependent volume absorption and failure to secrete Cl−, leading to ASL volume depletion (2). An alternative Ca2+-regulated Cl− channel that is functional in CF airway epithelial cells is also present on the mucosal surface but is relatively inactive in nonstimulated cells (3).
The expression of purinergic receptors in airway epithelia that couple via different mechanisms to salt (and water) transport suggests a role of purinergic agonists in the autocrine regulation of ASL volume and mucociliary clearance (MCC). Indeed, the accumulation of physiologically active concentrations of ATP in human airway secretions in vivo (4) and the recognition that airway epithelial cells release ATP constitutively in vitro (4–6) suggest that release and metabolism of nucleotides may control the basal activity of ion transport and, hence, ASL volume homeostasis. Functional data suggest that ATP mediates acute responses via P2Y2 receptor stimulation (7, 8), and that ATP metabolism provides a source of adenosine for stimulation of the A2b adenosine receptor (9). However, because of the continuous metabolic interconversion and removal of purinergic molecules, it has not been possible to predict the relative concentrations of agonists for the various purinergic receptors in airway epithelia and hence predict the relative contribution of the A2b and P2Y receptors to the control of ASL volume and MCC.
By adopting the chloroacetaldehyde derivatization technique for the ethenylation of adenyl purines (10), we sought to measure the concentration of adenine nucleotides and adenosine in ASL. We utilized two cell models with complementary features to address the issues of polarity of ATP release and feedback of purine nucleotides in cell signaling. By using well differentiated primary human airway epithelia as a model of the multilayered epithelia in vivo, we asked whether ASL volume regulation is controlled by ASL adenosine concentration in an autocrine fashion and whether the levels of adenyl purines in ASL were dependent on CFTR expression. By using Calu-3 cells as a model of a polarized epithelial monolayer, we asked whether cell surface domains are specialized for purine nucleotide release, whether ASL adenosine levels autocrinely control cellular cyclic AMP levels, and whether cyclic AMP production, and hence CFTR activation, promotes purine nucleotide release.
EXPERIMENTAL PROCEDURES
Cell Cultures and Incubations
Primary human nasal epithelial (HNE) and human bronchial epithelial (HBE) cultures from CF or non-CF subjects (provided by the University of North Carolina Cystic Fibrosis Center Tissue Culture Core Lab) were grown on 12-mm Transwell-Col (Costar, Cambridge, MA) supports under air-liquid conditions and studied (at 3–5 weeks) as fully differentiated stratified cultures, as described previously (11). Human Calu-3 airway epithelial cells were grown as polarized monolayers, as described previously (9). Cultures were washed three times with serum-free Dulbecco’s modified Eagle’s medium, followed by the addition to the luminal surface of either 300 or 25 µl of Hanks’ balanced salt solution (HBSS) for standard sampling and microsampling procedures (see below), respectively. At the indicated times, aliquots were removed, boiled for 2 min, and either processed immediately for nucleotide analysis or stored at −20 °C. All incubations were at 37 °C in a humidified incubator supplemented with 5% CO2. For intracellular determinations, the cells were rinsed and subsequently lysed with 0.3 ml of ice-cold 5% trichloroacetic acid, and trichloroacetic acid was extracted three times with 2 ml of ethyl ether.
Microsampling
A microscope-mounted micromanipulator (Southern Micro Instruments, Fostec Inc., Auburn, NY) was used to apply the tip of a 1.2-mm capillary glass tube (World Precision Instruments Inc., Sarasota, FL) to the meniscus of the ~20-µm depth of luminal surface liquid, as described previously (12). A single aliquot of 5–10 µl was removed from each culture and processed as detailed below.
Luciferin-Luciferase Assay
This assay has been described previously in detail (5). Typically, a 5–10-µl sample was added to a test tube, and the volume was adjusted to 300 µl with HPLC-grade H2O. The luciferin-luciferase reaction mix (100 µl) was added to tubes with a built-in injector into the light-protected chamber of an Auto-Lumat LB953 luminometer. Luminescence was subsequently recorded over 10 s and compared against an ATP standard curve performed in parallel. Luminescence was linear (slope = 1) between 0.1 and 1000 nm ATP.
Derivatization of Adenosine and Adenine Nucleotides
We have adopted and slightly modified the derivatization protocol originally described by Levitt et al. (10), according to conditions delineated experimentally as described under “Results.” In a typical derivatization assay, aliquots of either 150 µl from standard incubations or 20–40 µl from pooled microsamples were incubated for 30 min at 72 °C in the presence of 1.0 m chloroacetaldehyde and 25 mm Na2HPO4 (pH 4.0) in a final volume of 200 µl. Samples were transferred to ice, alkalinized with 50 µl of 0.5 m NH4HCO3, and analyzed by HPLC within 24 h. Up to 40 samples were processed in a single assay.
HPLC Analysis
Identification and quantification of ethenylated species were performed with an automated Waters HPLC apparatus equipped with a fluorescence detector. Derivatized samples were transferred to 0.7-ml plastic vials (Sun-SRi, Duluth, GA) and kept at 4 °C in the sample injector rack. A 100-µl sample aliquot was injected into a 250-mm, 10-µm Hamilton PRP-X100 anion exchange column. The mobile phase (2 ml/min, 30% methanol) developed linearly from 0.250 to 0.275 m NH4HCO3 (pH 8.5) during the first 8 min, remaining isocratic at 0.275 m NH4HCO3 for an additional 4 min. The column was subsequently rinsed for 3 min with 0.425 m NH4HCO3 in 30% methanol and re-equilibrated to the initial conditions for 15 min. Typical elution times (in minutes) of authentic etheno-standards are as follows: ε-ADO, 3.2; ε-AMP, 5.9; ε-ADP, 6.8; and ε-ATP, 8.4. For intracellular cyclic AMP measurements, separation of ε-cyclic AMP from ε-ATP was optimized by using a slightly modified mobile phase (30% methanol, 1.5 ml/min) developed from 0.20 to 0.25 m NH4HCO3 for 10 min and from 0.25 to 0.30 m NH4HCO3 over an additional 4 min. ε-ATP and ε-cyclic AMP eluted at 5.2 and 10.1 min, respectively. In experiments using radio-chemicals, samples were separated and analyzed with a Shimadzu HPLC coupled on-line to a Packard Flo-One detector. ε-[3H]ATP was separated and analyzed with the ion exchange column under mobile phase conditions similar to those indicated above. Separation of [3H]adenosine from [3H]inosine and [γ-32P]ATP from 32Pi and 32PPi were accomplished through the C18 column and mobile phase described previously (13).
Confocal Microscopy Measurement of ASL
Texas Red dextran (10 kDa, 2 mg/ml, Molecular Probes) was added in 20 µl of phosphate-buffered saline (± drugs) to the mucosal surface (1.13 cm2) of rinsed cultures. The excess volume was aspirated, and the cultures were kept undisturbed at 37 °C in a humidified incubator until microscopy analysis. At the times indicated (see Fig. 7), cultures were transferred to the stage of the confocal microscope over a serosal reservoir (80 µl of TES-buffered Ringer) (14, 15). Perfluorocarbon was added mucosally to prevent evaporation of the ASL during measurements, and cultures were subsequently X-Z scanned as described previously (14, 15).
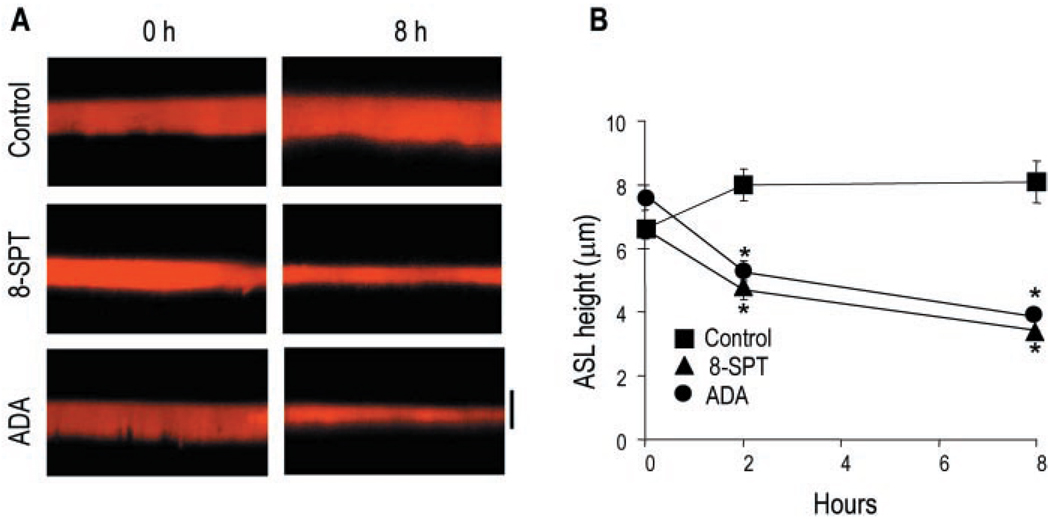
Fig. 7. Regulation ASL height by endogenous adenosine.
A, X-Z confocal images of ASL at 0 and 8 h after mucosal addition of Texas Red dextran in the absence (control) or in the presence of 100 µm 8-SPT or 5 units/ml adenosine deaminase (ADA) to primary HBE cell cultures. The bar indicates 7 µm. B, the data represent the mean ± S.E. (n = 5). *, significantly different from control.
Reagents
[3H]Adenosine (20 Ci/mmol), [3H]ATP (41 Ci/mmol), [γ-32P]ATP (3000 Ci/mmol), and molecular biology grade ATP were purchased from Amersham Biosciences. ADP, AMP, adenosine, cyclic AMP, and adenosine deaminase were obtained from Roche Applied Sciences. 8-(Sulfophenyl)theophylline (8-SPT), chloroacetaldehyde, and ε-adenyl standards were obtained from Sigma. Firefly luciferase and luciferin were purchased from Pharmingen. All other chemicals were of the highest purity available.
Data Analysis
HPLC fluorescence was integrated and analyzed by using Millennium software (Waters). Data from test samples were compared against known concentrations of ATP, ADP, AMP, and adenosine, which were derivatized and analyzed in parallel. These controls were injected at the beginning and again at the end of each HPLC injection set to determine within-run precision (<3%). Flow-One Analysis software (Packard Instrument Co.) was used for analyzing the radioactive tracings. Differences between means were determined by unpaired Student’s t test and were considered significant at p <0.05.
RESULTS
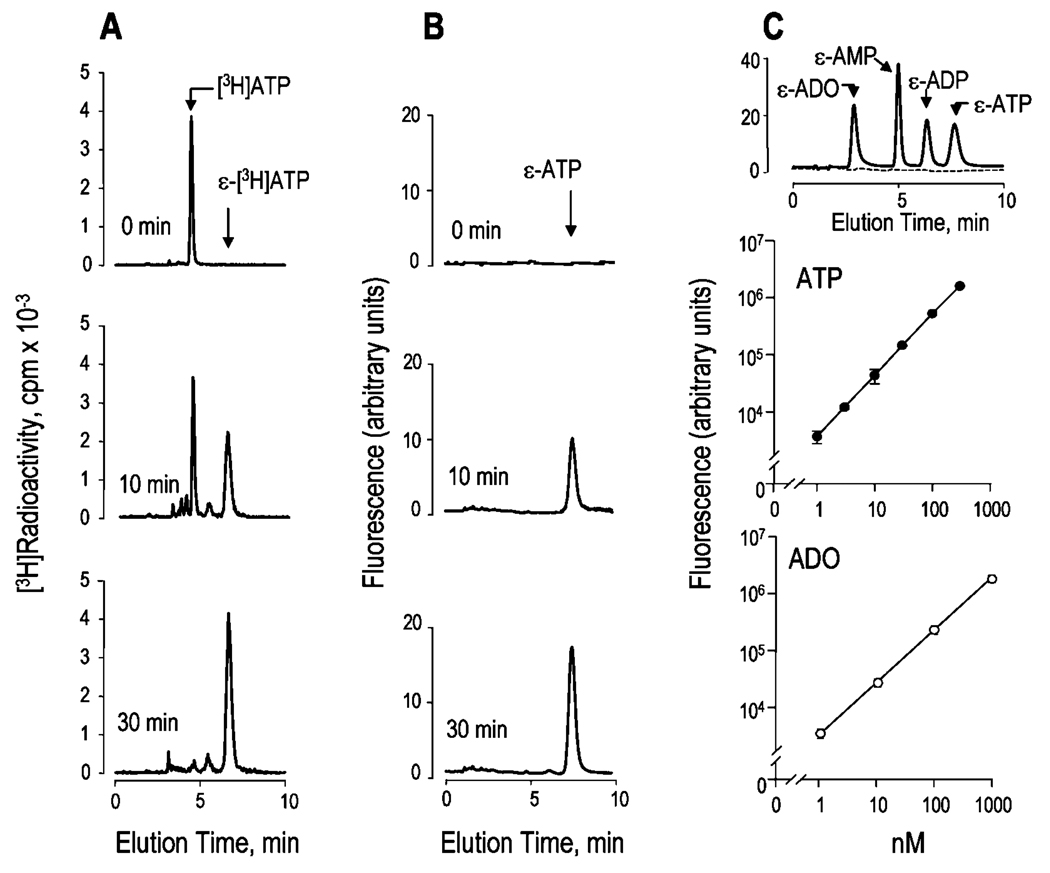
Chloroacetaldehyde derivatization allows for the sensitive quantitation of adenyl purines as fluorescent 1,N6-etheno species (10). To identify and quantitate ATP and other adenyl purines within the ASL, we optimized the ethenylation reaction conditions by monitoring changes in radioactive and fluorescent species during the derivatization of 0.1 µCi of [3H]ATP (100 nm). Ethenylation of ATP was quantitatively completed within 30 min at 72 °C in the presence of 1 m chloroacetaldehyde, as indicated by the shift in elution times of radioactive species (Fig. 1A) that was paralleled by formation of fluorescent ε-ATP (Fig. 1B). Most importantly, conversion of [3H]ATP to ε-[3H]ATP and formation of fluorescent species occurred with negligible loss of radioactive material or ATP breakdown. The reaction either slowed at lower temperatures (~70% completion after 45 min at 60 °C) or resulted in increased fluorescence background at 80 °C (data not shown). Under optimal conditions (72 °C for 30 min, 1 m chloroacetaldehyde), adenine-containing species could be resolved by ion exchange HPLC (see “Experimental Procedures”) and quantified to a sensitivity of 200 fmol (Fig. 1C). An HPLC tracing representative of a derivatization reaction performed in the presence of 100 nm adenosine, AMP, ADP, and ATP is shown in Fig. 1C (top). The linearity of the reaction as a function of ATP concentration is illustrated in Fig. 1C (middle), and similar calibration curves were obtained with adenosine (Fig. 1C, bottom) and with AMP, ADP, and cyclic AMP (data not shown).
Fig. 1. Derivatization of adenyl purines.
A, conversion of 100 nm [3H]ATP to ε-[3H]ATP was monitored by liquid scintillation detector-coupled HPLC. B, fluorescent HPLC tracings corresponding to the derivatization reaction shown in A. C, fluorescent HPLC tracings from a derivatization reaction performed either in the absence (dashed line) or in the presence (solid line) of 100 nm ADO, AMP, ADP, and ATP (top), and calibration curves for ATP (middle), and adenosine (bottom). All reactions were at 72 °C in the presence of 1 m chloroacetaldehyde, and they took place for the times indicated (A and B) or for 30 min (C). The elution times of authentic [3H]ATP and ε-purine standards are indicated with arrows. HPLC tracings are representative of three (A and B) or at least 20 (C) independent experiments. The data in the middle and bottom panels of C indicate the mean value (±S.D.) from one experiment with triplicate samples, and the results were representative of at least five impendent calibration curves performed under similar conditions.
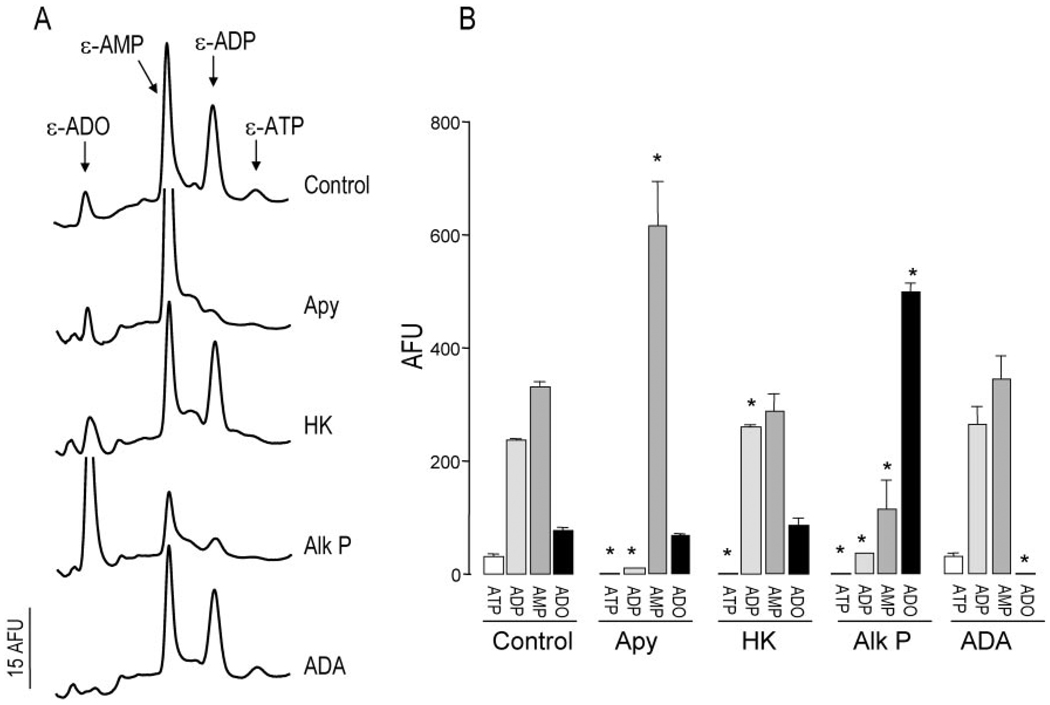
By having defined optimal derivatization conditions, we next examined the fluorescent pattern resulting from derivatization reactions performed with bulk solutions bathing the resting primary airway epithelial cells (“bulk” solutions were defined as those that have typically been used for nucleotide measurements, e.g. 300–500 µl (4, 5), but are in great excess to those volumes that likely occur in vivo). Primary cultures of nasal epithelial cells from normal subjects were incubated for 3 h (after the addition of 300 µl of HBSS to the mucosal surface) to allow the cells to recover from culture manipulations that potentially result in ATP release (17). A 200-µl aliquot was gently removed from the mucosal bath and subjected to derivatization and HPLC analysis. A representative HPLC fluorescence chromatogram is illustrated in Fig. 2A (top tracing). Species co-eluting with authentic ε-adenosine, εAMP, ε-ADP, and ε-ATP were detected. To verify further the identity of the fluorescent species, samples were subjected to enzymatic treatment prior to derivatization. As expected, incubation with apyrase caused a marked depletion of species eluting as ε-ADP and ε-ATP, whereas incubation with hexokinase (18) resulted in selective loss of the peak corresponding to ε-ATP and an increase of ε-ADP. Incubation of the samples with alkaline phosphatase eliminated or markedly attenuated the fluorescence eluting as ε-ATP, ε-ADP, and ε-AMP and increasing that of ε-adenosine. Adenosine deaminase selectively removed species eluting as ε-adenosine. Similar data were obtained with primary cultures of HBE cells (not shown) and with the Calu-3 airway epithelial cell line (9). Quantitative changes in fluorescence species after enzymatic treatment of HNE samples are summarized in Fig. 2B. These results confirm that the post-derivatization species detected in the extracellular medium of resting airway epithelial cells represent endogenous nucleotides and their nucleoside product adenosine.
Fig. 2. Enzymatic identification of ε-species in ASL.
Samples collected from 300-µl mucosal baths of primary cultures of normal resting HNE cells were preincubated with vehicle or with 2 units/ml of the indicated enzyme for 15 min at 30 °C prior to derivatization. A, HPLC tracings. B, changes in fluorescent species (mean ± S.D., n = 3). * indicates significantly different from control. Apy, apyrase; HK, hexokinase, Alk P, alkaline phosphatase; ADA, adenosine deaminase; AFU, arbitrary fluorescence units.
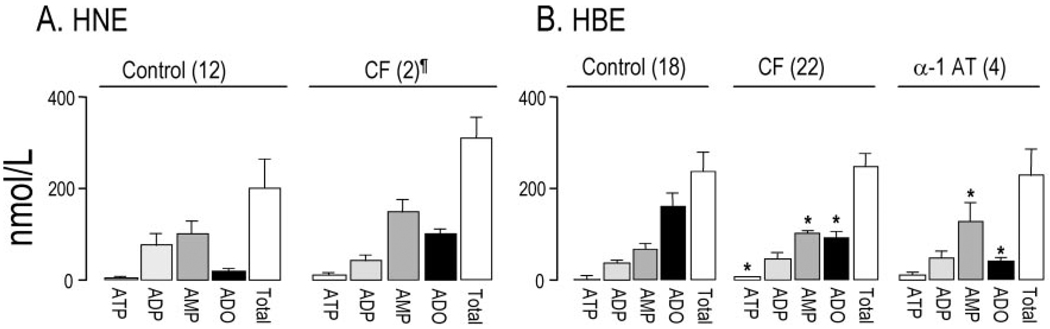
Fig. 3 summarizes measurements obtained with bulk samples from cultures of well differentiated primary nasal and bronchial epithelial cells. ATP concentrations in the 3–4 nm range were observed in the mucosal baths of control HNE and HBE cells, suggesting that these epithelia exhibit similar ratios of ATP release:breakdown on the luminal surface. The observation that ATP was the least abundant species (<2%) in HNE and HBE cells (Fig. 3) suggested that the ATP metabolism is efficiently coupled to ATP release on both cell types. However, and in agreement with recent studies (19, 20) indicating higher rates of hydrolysis of ATP, ADP, and AMP on bronchial cells relative to nasal cells, bronchial and nasal cells exhibited opposite nucleotide:adenosine accumulation ratios (Fig. 3).
Fig. 3. Extracellular accumulation of adenyl purines on airway epithelial cells.
Confluent cultures of HNE (A) and HBE (B) cells were grown in air/liquid interface and incubated for 3 h with 300 µl of HBSS added bilaterally. The bulk content of the adenyl species was determined by etheno derivatization, as indicated under “Experimental Procedures.” The data represent the mean value ± S.E., or ¶ indicates the mean value ± difference between duplicates; sample number is indicated in parentheses. * indicates significantly different from control cells.
Fig. 3 also suggests that luminal purine accumulation is not impaired in CF cells. Results in Fig 3A, although not conclusive because of the small sample number of CF HNE cultures, are consistent with our previous measurements of ATP release rates and nucleotide accumulation on nasal cultures and in nasal secretions from CF patients (4, 21). Moreover, comparative analysis of results from 18 to 22 HBE cultures from normal donors and CF patients also revealed essentially no difference in the total accumulation of mucosal purines (Fig. 3B). In addition, no difference in total accumulation of adenyl species was observed with HBE cells from patients with α-1 antitrypsin deficiency (Fig. 3B), suggesting that release of ATP to the lumen of the airway epithelia is not impaired in chronic airway inflammation.
Variability among patient groups was observed at the level of individual species (Fig. 3B). For example, adenosine levels were lower and AMP levels higher on CF and α-1 antitrypsin cells relative to control cells, suggesting that ecto-5′-nucleotidase activity is expressed more robustly in normal cells. ATP levels were slightly higher in CF relative to control samples.
Intracellular ATP levels were approximately the same between normal (32 ± 6 nmol/well) and CF bronchial cultures (34 ± 3 nmol/well) and represented >85% of the cellular adenyl purine pool, followed by ADP (12–14%) and AMP and adenosine (<2%, together).
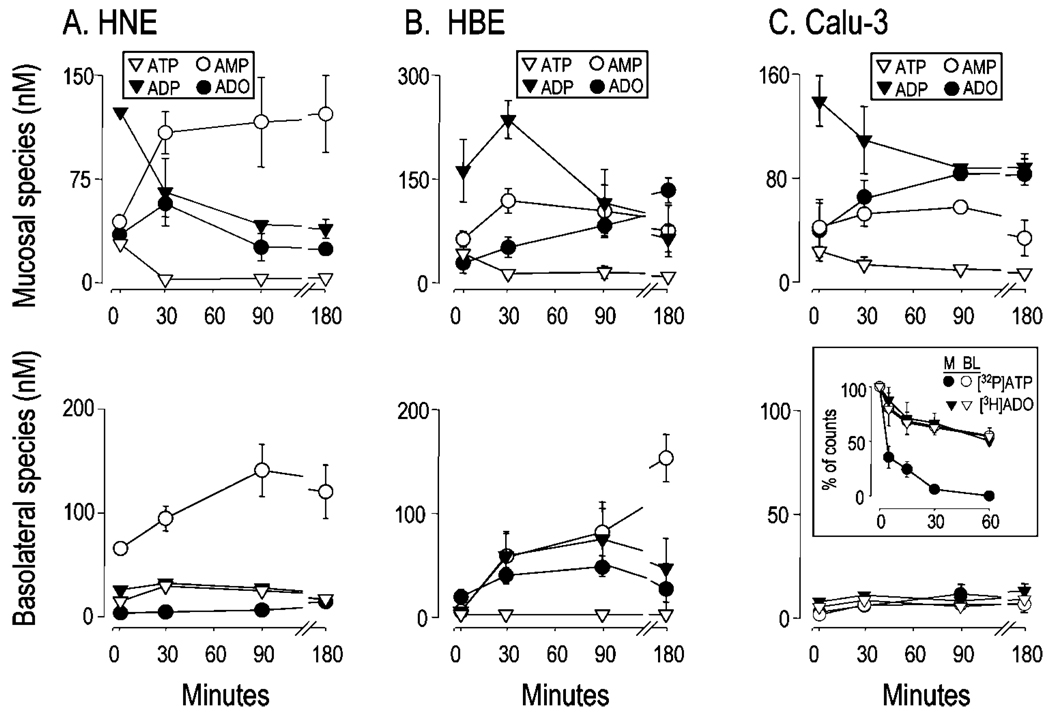
To examine further the kinetics of nucleotide and nucleoside accumulation, purine measurements were performed with samples collected bilaterally at various times from 2 to 180 min following a medium replacement on HNE and HBE cells (Fig. 4, A and B). As a complementary approach to address the sidedness of purine accumulation, measurements also were performed with Calu-3 cells (Fig. 4C), a CFTR-expressing epithelial cell line grown as a polarized monolayer (9, 22, 23). Consistent with the extreme sensitivity of airway epithelia to shear-induced ATP release (4, 6, 21, 24), a gentle medium change resulted in rapid mucosal ATP release by all three cell types. Furthermore, all three cell types achieved a concentration of 25–60 nm ATP within 2 min of challenge, which subsequently decayed within 30 min to lower but detectable (1–5 nm) levels that remained constant over the remainder of the 3-h incubation (Fig. 4, upper panels). The medium change-initiated ATP release was accompanied by robust accumulation of ADP, which was the predominant species (100–200 nm) at 2 min on all cell types. There were, however, features that distinguished these three cell types. In HNE cells, AMP levels reached a maximum after 30 min and remained >100 nm for up to 180 min. Adenosine reached a maximum (63 ± 19 nm) at 30 min but decayed to ~50% of that value after 180 min (Fig. 4A, upper panel). On HBE and Calu-3 cells, adenosine was readily detected at 2 min and substantially increased until 180 min (Fig. 4, B and C, upper panels).
Fig. 4. Bilateral accumulation of adenyl purines.
Well differentiated HNE (A) and HBE cells (B), and Calu-3 cell monolayers (C) were rinsed and incubated in 0.3 ml of bilateral HBSS, and 200 µl were collected at 2, 30, 90, or 180 min and derivatized. Trace amounts (0.1 µCi) of [γ-32P]ATP or [3H]adenosine were added for the indicated times to 0.3 ml of HBSS bathing the mucosal (M) and basolateral (BL) surfaces of Calu-3 cells (C, inset). Fluorescent and radioactive species were analyzed by HPLC, as indicated under “Experimental Procedures.” The data indicate the mean value (±S.D., n = 3) from a single experiment that was representative of two independent experiments performed under similar conditions. Mass measurements at t = 0 (i.e. nominally purine-free HBSS) are not depicted.
With respect to the basolateral compartment, ATP, ADP, and adenosine concentrations in HNE cells during the 3-h incubation period were <30 nm, but AMP levels were robust at all time points (Fig. 4A, lower panel). In HBE cells, ADP, AMP, and adenosine were detected after 30 min, but ADP and adenosine slightly decreased thereafter, whereas AMP levels increased to up to ~150 nm; little or no ATP was observed at all times (Fig. 4B, lower panel). Negligible accumulation of adenyl purines was detected in the basolateral solution of Calu-3 cells during the 3-h incubation period following a medium change (Fig. 4C, lower panel).
Taken together, these results reveal a complex pattern of nucleotide release and accumulation in the bulk mucosal and basolateral solutions of airway epithelial cultures. These data confirm previous ATP measurements consistent with basal ATP release by resting epithelia (4, 5) and enhanced ATP release during mechanical stimulation of epithelia (4, 5, 21, 24). The data also illustrate the efficiency and diversity of the nucleotide hydrolysis enzymes expressed on the epithelial cell surfaces, which converts ATP to AMP and ultimately to adenosine, and the data also suggest striking differences in nucleotide release in the basolateral compartments among the three epithelial cells.
To investigate whether accelerated basolateral metabolism in Calu-3 cells could account for the absence of purines in this compartment, trace quantities of [γ-32P]ATP and [3H]adenosine were added to each surface, and the radioactivity present in the medium at various times was monitored by HPLC. Basolateral [γ-32P]ATP decay (t½ = 56 min) was >10-fold slower than mucosal [γ-32P]ATP decay (t½ = 4 min) (Fig. 4C, inset). [3H]Adenosine decayed at similar rates (t½ ~60 min) on both surfaces of Calu-3 cells (Fig. 4C, inset). These results indicate that the absence of ε-purines in the basolateral bath of resting Calu-3 cell monolayers was not a consequence of faster basolateral clearance but rather reflected little or undetectable nucleotide release through the basolateral membrane.
Bulk measurements may underestimate the nucleotide concentration in the physiologically relevant ASL volume (height). Because the height (~7–50 µm) and hence volume of ASL normally bathing airway epithelial surfaces is small, and because airway epithelial cells are extremely sensitive to luminal stress, a major problem in measuring the concentrations of nucleotides in “small” ASL volumes that mimic those in vivo is to sample the thin airway surface liquid without mechanically triggering ATP release. Consequently, we adopted a two-step strategy to measure resting levels of nucleotides and adenosine in primary cultures bathed with ASL volumes that approximate those in vivo. First, we measured the clearance rates of trace amounts of [γ-32P]ATP and [3H]adenosine. Second, we utilized a micromanipulator to sample small ASL volumes on resting cells for nucleotide/nucleoside mass measurements.
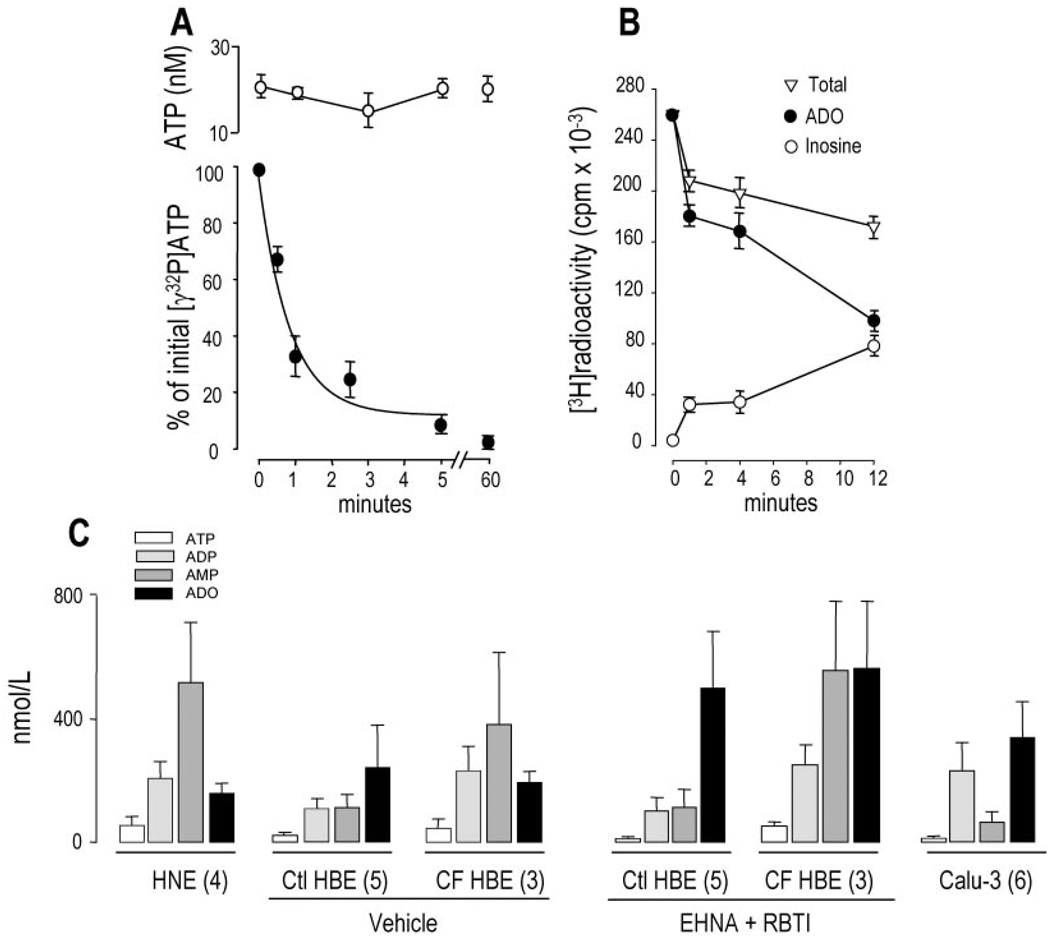
Primary cultures of HBE cells were preincubated with 25 µl of luminal minimum Eagle’s medium for 1 h, followed by the addition of 2 µl of [γ-32P]ATP for the times indicated. Under no circumstances during the time course of the incubation was radioactivity recovered in the contralateral medium. In parallel cultures, 5–10-µl aliquots were removed at various times to assess ATP concentrations by the luciferin-luciferase assay. Despite rapid breakdown of the radiotracer (t½ = 50 s), the mass of ATP in the small volume solutions remained essentially constant in the 15–25 nm range over 60 min (Fig. 5A). These results imply that extracellular ATP hydrolysis was balanced by cellular ATP release. Thus, the rate of basal ATP release (5) was calculated as 417 ± 105 fmol/min per culture (or 369 ± 92 fmol/cm2), similar to previous measurements of mucosal ATP release rates performed with bulk solutions covering airway epithelial cells (4).
Fig. 5. Basal accumulation of adenyl purines in small volume airway surface liquid.
The mucosal surfaces of well differentiated primary HNE and HBE cells and Calu-3 cells were rinsed and preincubated for either 1 (A and B) or 3 h (C) with 25 µl of HBSS. For radiotracer measurements (A and B), 0.1–0.5 µCi of the radiolabeled species were added to the lumen of HBE cultures, and ASL radioactivity was recovered at the times indicated by rapidly rinsing the mucosal cell surface with an excess volume of ice-cold HBSS containing 100 µm of the corresponding nonradioactive purine. [γ-32P]ATP (A, filled circles) and [3H]adenosine (B) were quantified by HPLC as described under “Experimental Procedures.” For ATP measurements by the luciferin-luciferase assay (A, open circles), and for derivatization reactions (C), a micromanipulator was used to collect 5–10 µl of ASL, as described under “Experimental Procedures.” The data in A and B represent the mean value (± S.D.) from at least four independent determinations. C, up to five samples from parallel incubations were pooled and derivatized, and the resulting etheno-adenyl purines were quantified by HPLC. The results are expressed as mean ± S.E.; the number of derivatization reactions is indicated in parentheses. Vehicle or 50 µm erythro-9-(2-hydroxy-3-nonyl)-adenine (EHNA) and 1 µm S-(p-nitrobenzyl)-6-thioinosine (RBTI) were added into the 25 µl of ASL at the beginning of the 3-h incubation period. Ctl, control.
[3H]Adenosine also decayed relatively rapidly on the mucosal HBE cell surface (Fig. 5B), although ASL [3H]adenosine decayed with an apparent t½ = 8 min that was considerable longer than [32P]ATP decay. Accumulation of [3H]inosine within the ASL bathing HBE cells suggested the presence of ecto-adenosine deaminase on the mucosal surface of these cells. Approximately one-third of the radioactivity added to the lumen of HBE cells could not be recovered in the extracellular space after extensive washing (Fig. 5B), suggesting that mucosal nucleoside uptake has occurred.
By having verified that mucosal ATP and adenosine were quickly metabolized under small volume-to-surface ratio incubation conditions, we asked whether endogenous adenyl purines in addition to ATP could be detected in this more physiological ASL volume covering resting cells. Primary nasal and bronchial epithelial cells and Calu-3 cells were preincubated for 3 h in 25 µl of mucosal HBSS, and 5–10 µl were sampled from independent cultures, pooled, and derivatized. Fig. 5C illustrates that, despite incubations that were >20-fold longer than the half-lives of radioactive ATP and adenosine measured above,2 accumulation of purines in small volume ASL exhibited a pattern similar (although not identical) to that in bulk samples. ATP was again the minor species (<6%) in all cell types. AMP:adenosine ratios were >1 in HNE ASL and <1 in control HBE ASL. Differences in total purine accumulation between control and CF HBE cells were not significant. In Calu-3 cells, the relative distribution of purine species in small ASL volume also was consistent with that observed in bulk solutions. However, 2–4-fold higher levels of purines were detected in small volumes of ASL relative to bulk accumulations (compare Fig. 5C with Figs. 3 and 4), suggesting that nucleotide/nucleoside measurements in small volume ASL more closely reflect purine levels in the physiological unstirred thin film. Addition of the adenosine deaminase inhibitor erithro-9-(2-hydroxy-3-nonyl)-adenine and the nucleoside transporter inhibitor S-(p-nitrobenzyl)-6-thioinosine resulted in 2- and 3.4-fold increases in adenosine accumulation on control and CF HBE cells, respectively (Fig. 5C), illustrating high nucleoside turnover on the mucosal surface of these cells.
These results suggest that accumulation of mucosal purines reflects a dynamic steady state in which the rates of hydrolysis are counter-balanced by similar rates of release/formation. Measurements of nucleotides/nucleosides in bulk volumes of ASL underestimate the actual nucleotide/nucleoside levels in ASL under more physiologically small volumes and likely underestimate nucleotide/nucleoside concentrations at the cell surface. Finally, luminal adenosine concentrations on resting cells under small volume ASL conditions may be in a range to activate adenosine receptors.
Given the potential role of A2b adenosine receptors in the control of CFTR-mediated Cl− secretion and ASL volume regulation, the important goals of our study were to determine the following: 1) whether endogenous luminal adenosine was capable of stimulating its cognate receptor to promote changes in second messenger levels, i.e. cyclic AMP; and 2) whether endogenous adenosine conferred A2b receptor-mediated ASL volume regulation.
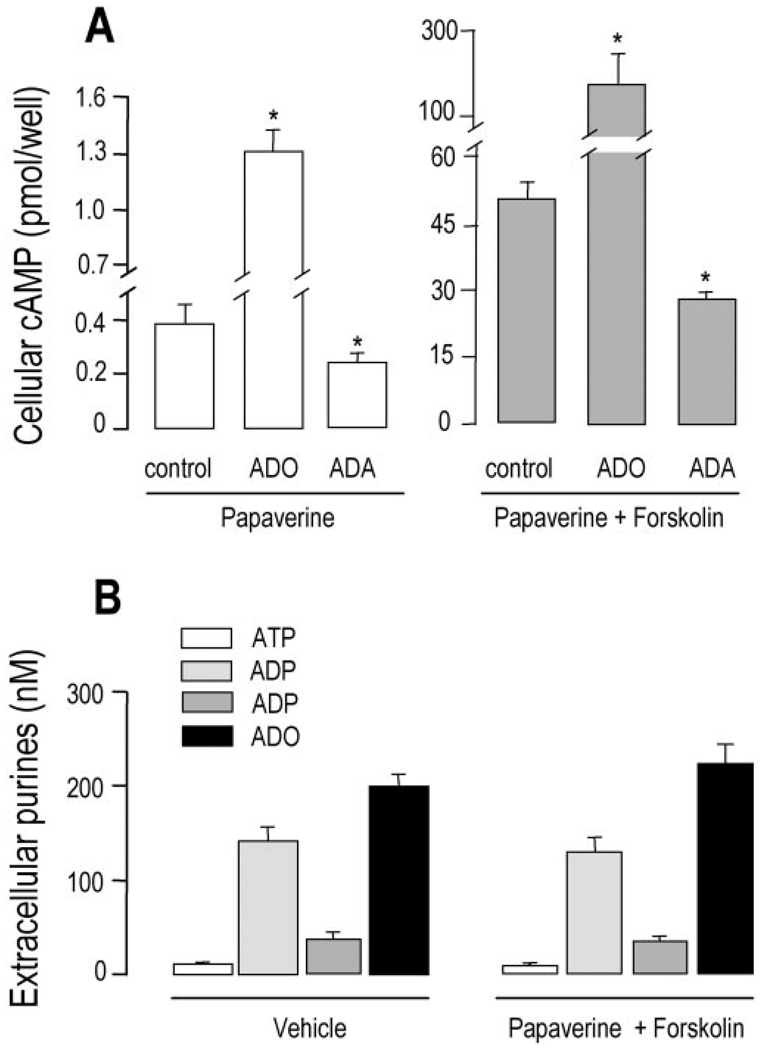
We first used Calu-3 airway epithelial cell monolayers, which express a well characterized luminal A2b receptor functionally coupled to Gs and adenylyl cyclase (9, 26), to quantitate the levels of cellular cyclic AMP mass as a function of endogenous luminal adenosine. Previously, we have illustrated that exogenous adenosine barely increased cyclic AMP detection in untreated Calu-3 and other airway epithelial cells (9, 27) and that no formation of cyclic AMP could be detected in resting airway epithelial cells in the absence of cyclic AMP-phosphodiesterase inhibitors (27). Consistent with these previous observations, there was a negligible accumulation of ε-cyclic AMP in untreated Calu-3 cells (not shown). However, addition of the phosphodiesterase inhibitor papaverine to resting Calu-3 cells resulted in accumulation of 370 ± 120 fmol of cyclic AMP per million cells (Fig. 6A, control). Adenosine (100 µm) promoted an ~3-fold increase in cyclic AMP formation (Fig. 6A). As described previously for this and other Gs-coupled receptors (28, 29), the effect of adenosine was dramatically potentiated by forskolin (Fig. 6A).
Fig. 6. Cyclic AMP accumulation and nucleotide release in Calu-3 cells.
A, polarized cells (0.9 million cells/Transwell) were rinsed and preincubated for 90 min with 300 µl of mucosal HBSS and 500 µl of basolateral Dulbecco’s modified Eagle’s medium. A, 200 µm papaverine was added in the absence or presence of 5 units/ml adenosine deaminase (ADA). After 10 min, 100 µm ADO and either vehicle (empty bars, left) or 30 µm forskolin (filled bars, right) was added for an additional 10 min as indicated. B, the cells were either incubated for 20 min with vehicle or they were preincubated for 10 min with 200 µm papaverine followed by the addition of 30 µm forskolin and preincubation for 10 min. Extracellular purine and cellular cyclic AMP measurements were performed as described under “Experimental Procedures.” With the exception of papaverine, which was added bilaterally, all other additions were to the mucosal bath. The data indicate the mean value (±S.D.) from triplicate samples, and the results are representative of two experiments performed in identical conditions. *, significantly different from control.
We next investigated whether both basal and forskolin-stimulated levels of cyclic AMP in Calu-3 cells potentially reflected subtle activation of the A2b receptor by endogenous adenosine contained within the ASL. In papaverine-treated cells, adenosine deaminase caused a partial but significant reduction of basal cyclic AMP levels (Fig. 6A), suggesting that endogenous adenosine has conferred tonic activation to the Gs-coupled A2b receptor (adenosine deaminase had no effect on cyclic AMP levels in the absence of papaverine, not shown). Adenosine deaminase also reduced cyclic AMP formation in response to forskolin, further suggesting that the effect of forskolin on the adenylyl cyclase partially reflected synergistic interactions with the autocrinally activated A2b receptor. It is worth noting that forskolin caused no substantial changes in the levels of luminal adenosine or adenosine nucleotides (Fig. 6B). These data strongly suggest that extracellular adenosine concentrations are not regulated by cyclic AMP-mediated processes but rather reflect a constitutive process in resting cells upstream from cyclic AMP production.
Cyclic AMP-dependent CFTR Cl− channel activity plays a central role in controlling epithelial electrolyte transport and hence ASL volume. By having determined that endogenous adenosine controls basal levels of cellular cyclic AMP in Calu-3 cell monolayers, we next used well differentiated multilayered HBE cells from normal subjects to examine whether ASL adenosine controls CFTR-regulated ASL volume. Well differentiated airway epithelia regulate the ASL height to just above ~7 µm, the height of extended cilia (2, 15). Accordingly, we designed an assay to test whether the adenosine is a key regulator in maintaining physiological “normal” ASL volume. For this assay, 20 µl of phosphate-buffered saline/Texas Red dextran, containing either vehicle, adenosine deaminase, or the adenosine receptor blocker 8-SPT, was added to the mucosal surface. The liquid excess was aspirated to leave an ~7 µm depth (15) and subsequent changes in ASL height measured by X-Z confocal microscopy. Fig. 7 shows that ASL remained 7–8 µm in height in control cells but decreased to ~4 µm in cells incubated in the presence of 8-SPT or adenosine deaminase. These results suggest that endogenous adenosine mediates physiological ASL volume regulation, in accordance with the notion that cyclic AMP-activated CFTR chloride channels regulate periciliary liquid volume by promoting Cl− ion (and water) secretion and inhibiting Na+-dependent volume absorption (2).
DISCUSSION
By combining micro-sampling of airway epithelial cultures covered with physiologically “thin” films of ASL and etheno derivatization measurement techniques, we have demonstrated that physiologically relevant amounts of nucleotides and adenosine accumulate within the ASL of well differentiated primary and immortalized cultures of airway epithelial cells. Our results provide evidence that ATP and adenosine concentrations in ASL reflect a dynamic state. Airway epithelia exhibit a continuous release of ATP that offsets ATP hydrolysis, which in turn provides a pathway for generation of adenosine. Indeed, the results in Fig. 5 illustrate that basal ATP and adenosine concentrations in ASL reach levels that would maximally activate purinoceptors if metabolism had not taken place. However, the released ATP is rapidly hydrolyzed and therefore represents a minor species among the adenylyl purines that accumulates within the ASL covering resting airway epithelial cells. Although not formally demonstrated in the current study, the inverse correlation between half-life and concentration of adenosine and ATP on primary airway and Calu-3 cells suggests that released ATP is the primary source of extracellular adenosine. This conclusion is supported by functional and molecular studies indicating that ecto-5′-nucleotidase, but not cytosolic AMP-specific 5′-nucleotidase, is expressed on airway epithelial cells (20) and by electrophysiological studies with Calu-3 cells illustrating that mucosally added ecto-5′-nucleotidase inhibitors markedly reduced ion transport activity, i.e. that of CFTR, known to be under the control of the A2b receptor (9).
One implication of the above observations is that measurements of ATP concentrations alone may result in considerable underestimation of the actual magnitude of ATP release. This conclusion is supported by recent observations by Dubyak and co-workers (30, 31), who measured local ATP concentrations by anchoring luciferase to the cell surface by using a chimeric protein made of the IgG-binding domain of Staphylococcus aureus protein A fused with the luciferase enzyme. With this approach, ATP concentrations at the surface of stimulated cells were found to be at least 20-fold higher than ATP concentrations determined in bulk medium (30, 31). ATP detection in their studies was dramatically potentiated by inhibiting ecto-ATPases, indicating that ATP release and hydrolysis sites may co-localize.
The concentrations of ATP and adenosine generated within the ASL have physiological implications for airway defense. Proper coordination of cyclic AMP- and Ca2+-regulated epithelial functions, e.g. ion and water transport and cilia beat frequency, are essential for efficient mucus clearance. Both cyclic AMP-coupled A2b adenosine receptors and Ca2+-mobilizing P2Y2 receptors (activated by ATP) are expressed throughout the respiratory tract. Because the human airway is poorly innervated and subject to little endocrine regulation, we hypothesize that MCC rates are under autocrine control by extracellular nucleotides. Indeed, airway epithelial cells release nucleotides both constitutively and in response to mechanical stimulation (4, 5, 7, 24, 32). Basal levels of nucleotides, measured in vitro (present study) and in ex vivo nasal lavages (4), suggest that ATP concentrations on resting epithelia approach threshold concentrations for stimulating the P2Y2 receptor. This conclusion is reinforced by the observation that the small but steady basal formation of inositol phosphates in polarized cultures of resting 16HBE14o− human bronchial epithelial cells was abolished when apyrase was present in the luminal bath (6).
The significance of the basal P2Y2 receptor-triggered phospholipase C-mediated ion transport responses in resting airways may be in achieving a balance of ion transport. For example, P2Y2 receptors regulate Na+ absorption rates via inhibition of the epithelial Na+ channel epithelial sodium channel (33) and may influence Cl− transport via protein kinase C potentiation of CFTR activation (34). Because airway epithelial cells are particularly sensitive to shear stress, the P2Y2 receptor likely plays an important role controlling calcium- and protein kinase C-dependent MCC functions in stressed airways, e.g. during coughing and exercise, as suggested by the substantially enhanced rate of ATP release and P2Y2 receptor-mediated ion transport responses in airway cultures subjected to controlled shear stress (8). ATP accumulation in CF airway epithelia potentially may be an important compensation for the lack of cyclic AMP-stimulated CFTR activity via P2Y2 receptor-mediated Ca2+-regulated Cl− channel activation.
Our data suggest that ASL adenosine may be capable of tonically stimulating the epithelial A2b receptor on resting cells. Our microsampling measurements (see Fig. 5) revealed that generation and removal of adenosine in physiologically relevant small ASL volumes are dynamically balanced, resulting in steady state levels of adenosine at 180–350 nm, which approach EC50 values for activating the A2b receptor. Rates of adenosine formation and removal may be airway region-specific, as suggested by differences in ADO:AMP ratios between nasal and bronchial epithelial cells (Figs. 3–5).
The importance of mucosal adenosine accumulation was initially raised by electrophysiological studies indicating that the basal CFTR Cl− channel activity of Calu-3 cells was sensitive to A2b receptor antagonists (9). This observation is complemented by our present results with Calu-3 cells depleted of endogenous adenosine in ASL, which displayed decreased basal and forskolin-promoted intracellular cyclic AMP accumulation (Fig. 6). It is worth noting that because Calu-3 cells were grown as polarized monolayers, they were well suited for examining the contribution of ASL adenosine to cellular cyclic AMP levels, a task difficult to pursue with multilayered primary epithelia. Most importantly, the changes in CFTR-regulated ASL volume in primary airway epithelia indicate that endogenous adenosine accumulates in physiologically relevant concentrations in ASL. The ASL adenosine concentration provides the tonic regulation of the ion transport processes, e.g. CFTR-mediated Cl− secretion, which controls ASL volume, a key component of the innate defense mechanisms in the lung.
If, as indicated above, there is a continuous activation of the A2b receptor by endogenous adenosine, then do these receptors desensitize in resting airway epithelial cells? The basal levels of ASL adenosine do not come close to saturating and likely may not be sufficient to down-regulate the entire A2b receptor population. However, previous results from our laboratory indicated that 5-N-ethyl-carboxamidoadenosine (NECA)-promoted cyclic AMP accumulation in airway epithelial cells was enhanced in the presence of adenosine deaminase (27), suggesting that the A2b receptor may indeed desensitize, although partially, by endogenous adenosine.
An interesting contrast revealed by the present study was that well differentiated primary epithelial cells accumulated comparable quantities of nucleotides on both surfaces, whereas only luminal nucleotide accumulation was detected in Calu-3 cell cultures. Differences in the mucosal versus basolateral Calu-3 nucleotide/nucleoside levels could not be explained by a higher rate of metabolism of extracellular purines at the basolateral surface of Calu-3 cells, because the half-life of basolateral [32P]ATP was greater than that of mucosal [32P]ATP, and [3H]adenosine decayed at apparently similar rates on both cell surfaces. However, structural differences between well differentiated primary epithelia and immortalized Calu-3 cells could account for this apparent discrepancy. For example, primary cultures of bronchial epithelial cells differentiated into a multilayer epithelium containing lumen-facing columnar cells resting on two or more layers of nonpolarized basal cells (11). In contrast, Calu-3 cell cultures exhibited the morphology of true monolayers (9). Therefore, it is possible that the nucleotide content in the basolateral compartment of primary cultures reflected nucleotide release by nonpolarized basal cells. Although speculative, this observation suggests that ATP release occurs through elements that segregate to the apical domain after cell polarization.
Calu-3 cells are thought to be a model of serous cells of the airway submucosal gland (35–37). Hence, it is worth noting that levels of adenine nucleotides and adenosine achieved in the restricted milieu of the gland duct at rest and during physical stress may be greater than those predicted from culture model studies and also greater than those achieved in bronchi and nasal passages. In addition, glandular secretions may be an additional source of nucleotides to the contiguous airway surfaces.
Accumulation of purines in the basolateral compartment of primary cultures of bronchial epithelial cells may be physiologically relevant. ADP under our experimental conditions accumulated in the 20–40 nm concentration range in the basolateral solution of resting HNE and HBE cells. These concentrations may suffice to confer tonic activation to the P2Y1 receptor (38) that is expressed on the basolateral surface of columnar epithelial cells (7).
We can only speculate about the mechanism of release and the subcellular source of extracellular purines. Based on observations suggesting that CFTR expression enhanced extracellular ATP accumulation, it has been proposed that release of cytosolic ATP by airway epithelial cells occurs either through CFTR or through a molecularly distinct CFTR-regulated ATP channel (39–43). However, many studies do not support that conclusion (21, 24, 44–46). By eliminating the potential contribution of ATP hydrolysis to extracellular ATP concentration measurements, our studies add two complementary sets of evidence against the CFTR hypothesis. First, after quantitating the spectrum of adenyl purines in ASL bathing of well differentiated primary cultures of human bronchial epithelial cells, we observed no significant differences in the total content of purines in ASL covering normal versus CF cells, strongly suggesting that CFTR was not involved in the basal release of ATP in resting cells (Fig. 3). Second, by using Calu-3 cells that display high levels of functionally active CFTR (9), we showed that substantial elevation of cellular cyclic AMP levels was not accompanied by changes in the luminal accumulation of ATP or ATP metabolites, including adenosine (Fig. 6). Thus, consistent with previous studies (4, 21, 24, 44–46), our present data suggest that basal ATP release is independent of both CFTR expression and cyclic AMP production.
Aside from the controversy surrounding the role of CFTR and other ABC transporters in ATP release (reviewed in Refs. 17 and 47), the identity of channels that may conduct cytosolic ATP from epithelial cells and potentially other nonexcitatory cells remains unknown (41, 48). Candidate ATP channels include connexin-43, a putative plasmalemmal variant of the mitochondrial voltage-dependent anion channel, and Gd3+-sensitive channels (17). In addition, recent studies (16, 49, 50) with epithelial cells and other cells have indirectly implicated nucleotide release via vesicle-mediated secretion. Given the variety of conditions and cell types in which extracellular ATP and other nucleotides have been detected, several pathways (facilitated and exocytotic) likely are involved in the release of nucleotides by epithelial and nonepithelial cells.
In sum, our present study shows that purines accumulate in physiologically relevant concentrations on apical and basolateral surfaces of resting airways. Our results emphasize that ATP release and metabolism within the ASL constitutes a primary source for the accumulation of adenosine, which provides sustained basal activity to the A2b receptor that regulates CFTR functions, including ASL volume regulation.
Acknowledgments
We are indebted to Robin Davis for assistance with primary cultures and micro-sampling techniques, and to Lisa Brown for editorial assistance. We thank Dr. Maryse Picher for helpful discussions and comments.
Footnotes
This work was supported by National Institutes of Health Grant HL34322 and the Cystic Fibrosis Foundation.
The abbreviations used are: ASL, airway surface liquid; MCC, mucociliary clearance; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; HBSS, Hanks’ balanced salt solution; HPLC, high performance liquid chromatography; ε, etheno; ADO, adenosine; HNE, human nasal epithelial cells; HBE, human bronchial epithelial; 8-SPT, 8-(-sulfophenyl)theophylline; TES, 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid.
REFERENCES
- 1.Knowles MR, Boucher RC. J. Clin. Investig. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher RC. Adv. Drug Delivery Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel SE. In: Current Topics in Membranes: Calcium-activated Chloride Channels. Fuller CM, editor. San Diego: Academic Press; 2002. pp. 193–207. [Google Scholar]
- 4.Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Mol. Med. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarowski ER, Boucher RC, Harden TK. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 6.Lazarowski ER, Boucher RC, Harden TK. Drug Dev. Res. 2001;53:66–71. [Google Scholar]
- 7.Homolya L, Steinberg AD, Boucher RC. J. Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paradiso AM, Ribeiro CMP, Boucher RC. Pediatr. Pulmonol. 2001;22 suppl.:210. [Google Scholar]
- 9.Huang PB, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt B, Head RJ, Westfall DP. Anal. Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- 11.Matsui H, Davis CW, Tarran R, Boucher RC. J. Clin. Investig. 2000;105:1419–1427. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 13.Lazarowski ER, Boucher RC, Harden TK. In: Ecto-ATPases and Related Ectonucleotidases. Vanduffel L, Lemmens R, editors. Maastricht: Shaker Publishing BV; 2000. pp. 283–294. [Google Scholar]
- 14.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. J. Gen. Physiol. 2001;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarran R, Boucher RC. Methods Mol. Med. 2002;70:479–492. doi: 10.1385/1-59259-187-6:479. [DOI] [PubMed] [Google Scholar]
- 16.Dinter A, Berger EG. Histochem. Cell Biol. 1998;109:571–590. doi: 10.1007/s004180050256. [DOI] [PubMed] [Google Scholar]
- 17.Lazarowski ER, Boucher RC, Harden TK. Mol. Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 18.Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picher M, Burch LH, Boucher RC. J. Biol. Chem. 2004;279:20234–20241. doi: 10.1074/jbc.M400305200. [DOI] [PubMed] [Google Scholar]
- 20.Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. J. Biol. Chem. 2003;278:13468–13479. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- 21.Watt WC, Lazarowski ER, Boucher RC. J. Biol. Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- 22.Cobb BR, Fan LJ, Kovacs TE, Sorscher EJ, Clancy JP. Am. J. Respir. Cell Mol. Biol. 2003;29:410–418. doi: 10.1165/rcmb.2002-0247OC. [DOI] [PubMed] [Google Scholar]
- 23.Szkotak AJ, Ng AM, Man SF, Baldwin SA, Cass CE, Young JD, Duszyk M. J. Membr. Biol. 2003;192:169–179. doi: 10.1007/s00232-002-1073-x. [DOI] [PubMed] [Google Scholar]
- 24.Grygorczyk R, Hanrahan JW. Am. J. Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 25.Picher M, Boucher R. Drug Dev. Res. 2001;52:52–75. [Google Scholar]
- 26.Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. Am. J. Physiol. 2002;282:L12–L25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- 27.Lazarowski ER, Mason SJ, Clarke L, Harden TK, Boucher RC. Br. J. Pharmacol. 1992;106:774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. J. Biol. Chem. 1997;272:22265–22271. doi: 10.1074/jbc.272.35.22265. [DOI] [PubMed] [Google Scholar]
- 29.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 30.Beigi R, Kobatake E, Aizawa M, Dubyak GR. Am. J. Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 31.Joseph SM, Buchakjian MR, Dubyak GR. J. Biol. Chem. 2003;278:23342–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 32.Boudreault F, Grygorczyk R. Am. J. Physiol. 2002;282:C219–C226. doi: 10.1152/ajpcell.00317.2001. [DOI] [PubMed] [Google Scholar]
- 33.Devor DC, Pilewski JM. Am. J. Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- 34.Jia Y, Mathews CJ, Hanrahan JW. J. Biol. Chem. 1997;272:4978–4984. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- 35.Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Am. J. Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- 36.Lee MC, Penland CM, Widdicombe JH, Wine JJ. Am. J. Physiol. 1998;274:L450–L453. doi: 10.1152/ajplung.1998.274.3.L450. [DOI] [PubMed] [Google Scholar]
- 37.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. J. Gen. Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Mol. Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- 39.Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF. J. Biol. Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- 40.Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 41.Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, Benos DJ, Schwiebert LM, Fitz JG, Schwiebert EM. J. Biol. Chem. 2001;276:6621–6630. doi: 10.1074/jbc.M005893200. [DOI] [PubMed] [Google Scholar]
- 42.Pasyk EA, Foskett JK. J. Biol. Chem. 1997;272:7746–7751. doi: 10.1074/jbc.272.12.7746. [DOI] [PubMed] [Google Scholar]
- 43.Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. J. Physiol. (Lond.) 2000;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grygorczyk R, Tabcharani JA, Hanrahan JW. J. Membr. Biol. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- 45.Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- 46.Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y. J. Gen. Physiol. 1999;114:525–533. doi: 10.1085/jgp.114.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwiebert EM. Am. J. Physiol. 1999;276:C1–C8. doi: 10.1152/ajpcell.1999.276.1.C1. [DOI] [PubMed] [Google Scholar]
- 48.Roman RM, Lomri N, Braunstein G, Feranchak AP, Simeoni LA, Davison AK, Mechetner E, Schwiebert EM, Fitz JG. J. Membr. Biol. 2001;183:165–173. doi: 10.1007/s00232-001-0064-7. [DOI] [PubMed] [Google Scholar]
- 49.Bodin P, Burnstock G. J. Cardiovasc. Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Knight GE, Bodin P, De Groat WC, Burnstock G. Am. J. Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]