Abstract
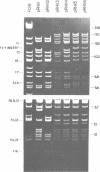
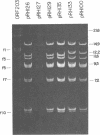
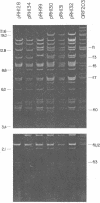
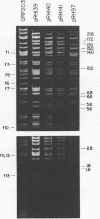
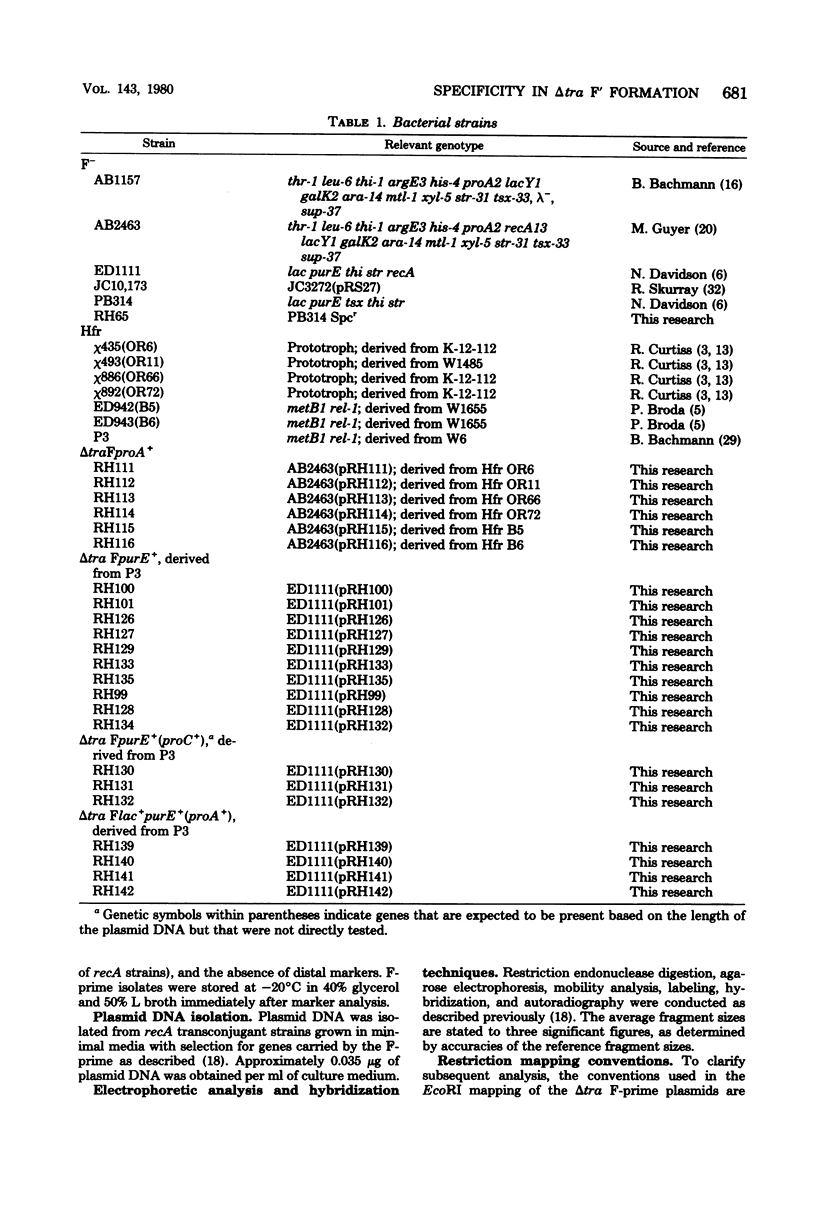
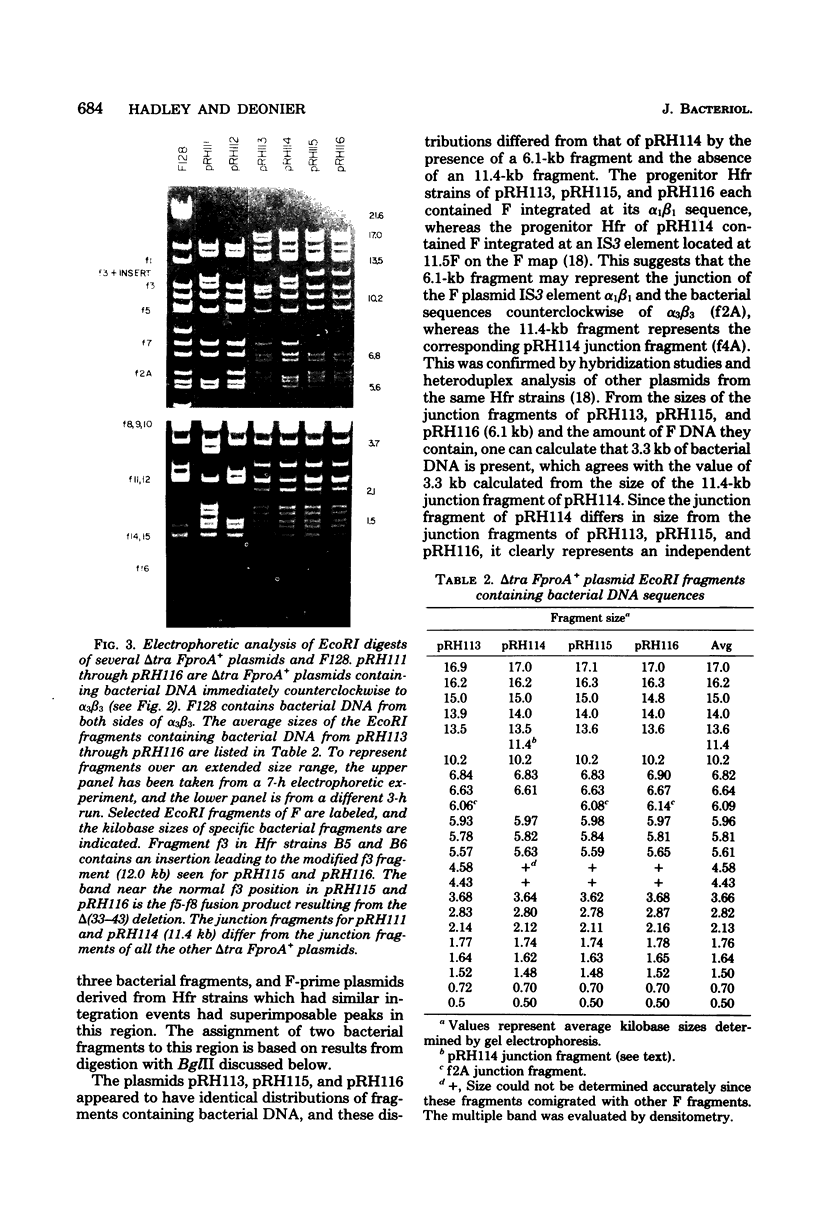
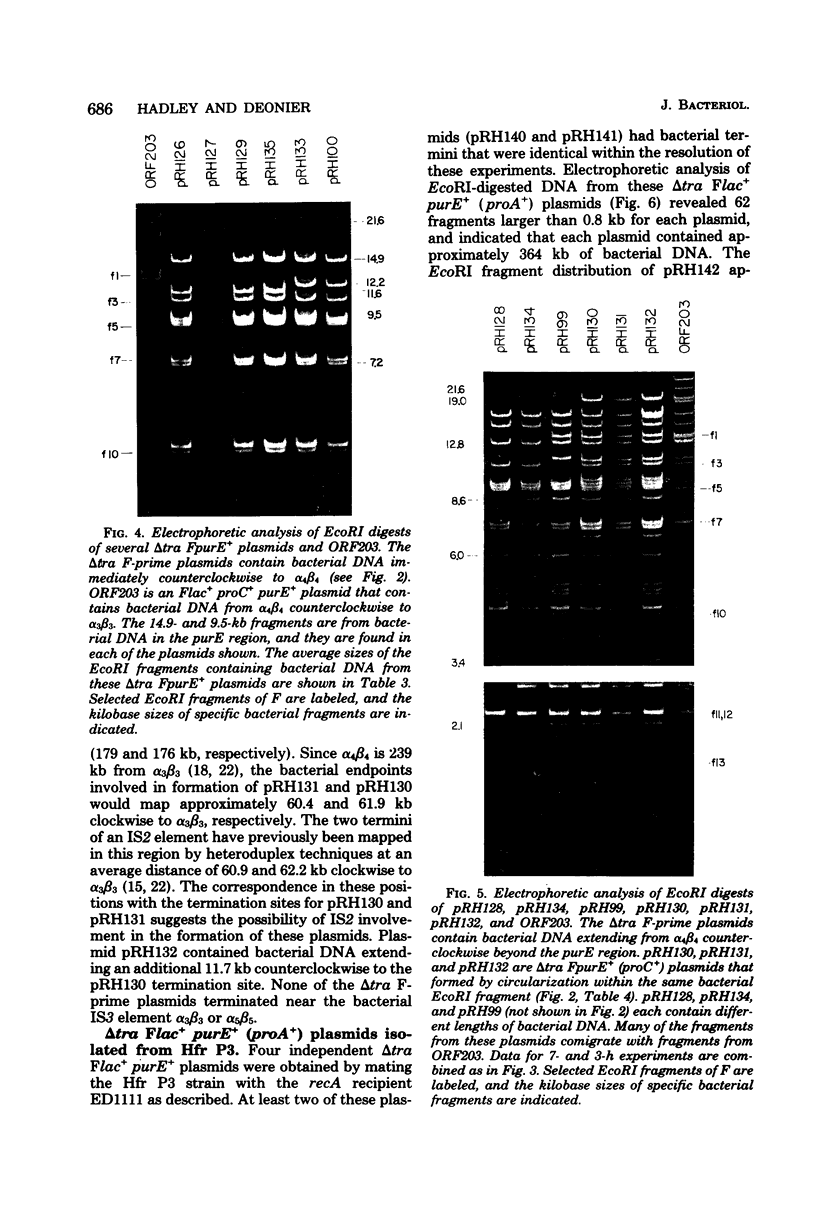
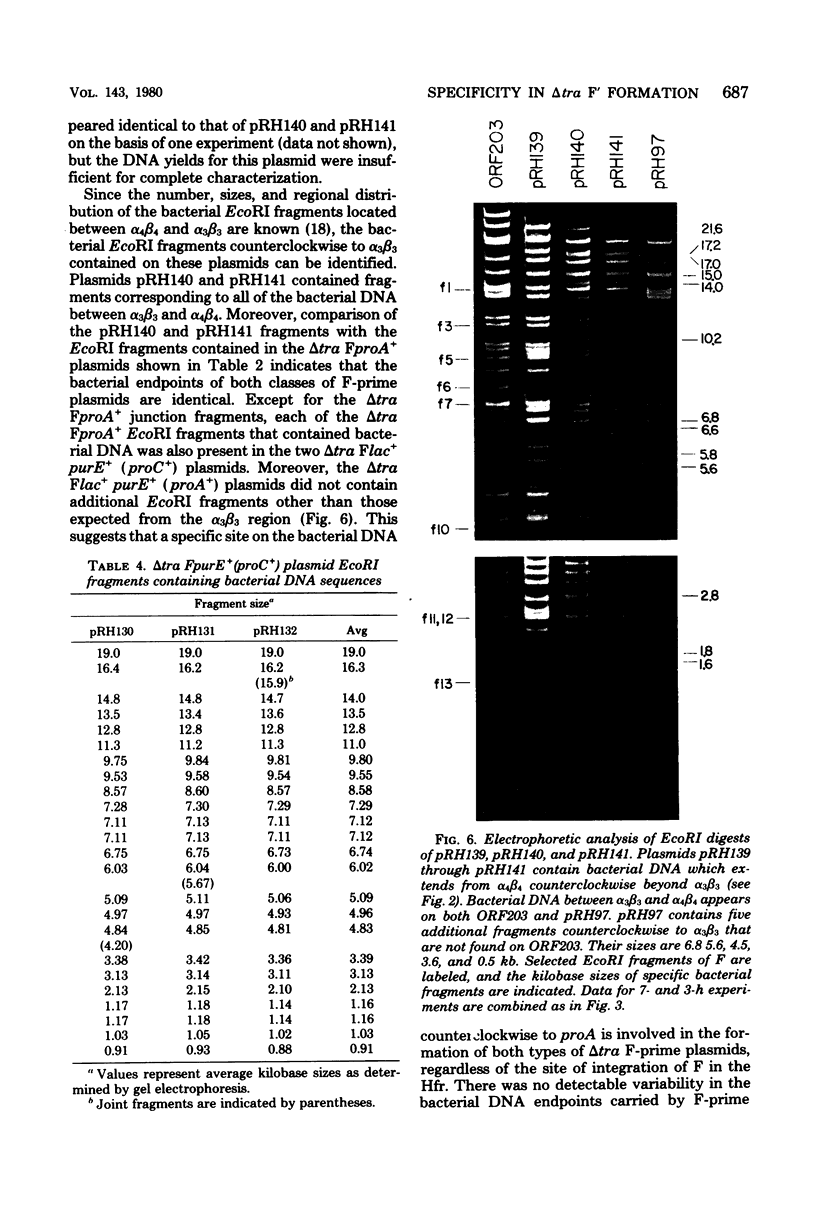
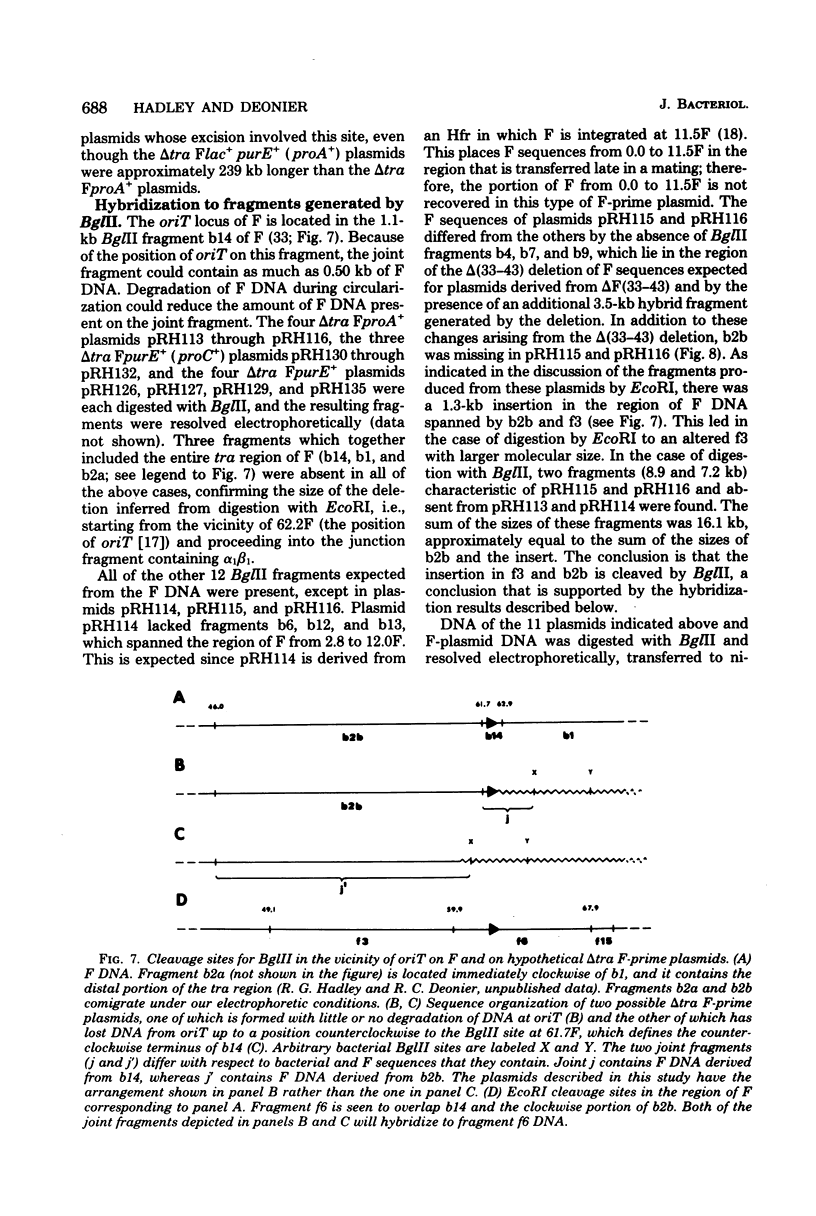
Twenty-three independent delta tra F-prime plasmids from three different Escherichia coli K-12 sublines were isolated from Hfr strains whose points of origin coincided with the IS3 element alpha 3 beta 3 or alpha 4 beta 4 in the lac-purE region of the E. coli chromosome. Electrophoretic analysis of plasmid deoxyribonucleic acid digested with EcoRI and hybridization analysis of plasmid deoxyribonucleic acid digested with BglII revealed that at least 14 of these plasmids were formed by processes involving specific bacterial and F loci. Two of the specific bacterial loci involved in delta tra F-prime formation were located at approximately 3.3 and 11.7 min on the E. coli chromosomal map. Two of the delta tra F-prime plasmids contained bacterial deoxyribonucleic acid with circularization endpoints that mapped very near the termini of the IS2 element that is normally located between lac and proC.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M. Genetics of the F sex factor in enterobacteriaceae. Curr Top Microbiol Immunol. 1973;60:79–123. doi: 10.1007/978-3-642-65502-9_3. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S. E., Krivonogov S. V., Lanzov V. A. Scale of the genetic map and genetic control of recombination after conjugation in Escherichia coli K-12. Mol Gen Genet. 1978 Nov 9;166(3):337–346. doi: 10.1007/BF00267627. [DOI] [PubMed] [Google Scholar]
- Broda P., Meacock P. Isolation and characterisation of Hfr strains from a recombination-deficient strain of Escherichia coli. Mol Gen Genet. 1971;113(2):166–173. doi: 10.1007/BF00333190. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G. J., Ohtsubo H., Ohtsubo E., Sonnenberg F., Freundlich M. Restriction endonuclease mapping of the Escherichia coli K12 chromosome in the vicinity of the ilv genes. J Mol Biol. 1977 Nov 25;117(1):175–193. doi: 10.1016/0022-2836(77)90030-4. [DOI] [PubMed] [Google Scholar]
- Coombs D. H., Pearson G. D. Filter-binding assay for covalent DNA-protein complexes: adenovirus DNA-terminal protein complex. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5291–5295. doi: 10.1073/pnas.75.11.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Stallions D. R. Probability of F integration and frequency of stable Hfr donors in F+ populations of Escherichia coli K-12. Genetics. 1969 Sep;63(1):27–38. doi: 10.1093/genetics/63.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonier R. C., Hadley R. G. IS2-IS2 and IS3-IS3 relative recombination frequencies in F integration. Plasmid. 1980 Jan;3(1):48–64. doi: 10.1016/s0147-619x(80)90033-5. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Oh G. R., Hu M. Further mapping of IS2 and IS3 in the lac-purE region of the Escherichia coli K-12 genome: structure of the F-prime ORF203. J Bacteriol. 1977 Feb;129(2):1129–1140. doi: 10.1128/jb.129.2.1129-1140.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S., Davidson N., Clark A. J. Heteroduplex analysis of tra delta f' plasmids and the mechanism of their formation. J Bacteriol. 1977 Sep;131(3):970–980. doi: 10.1128/jb.131.3.970-980.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
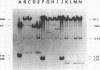
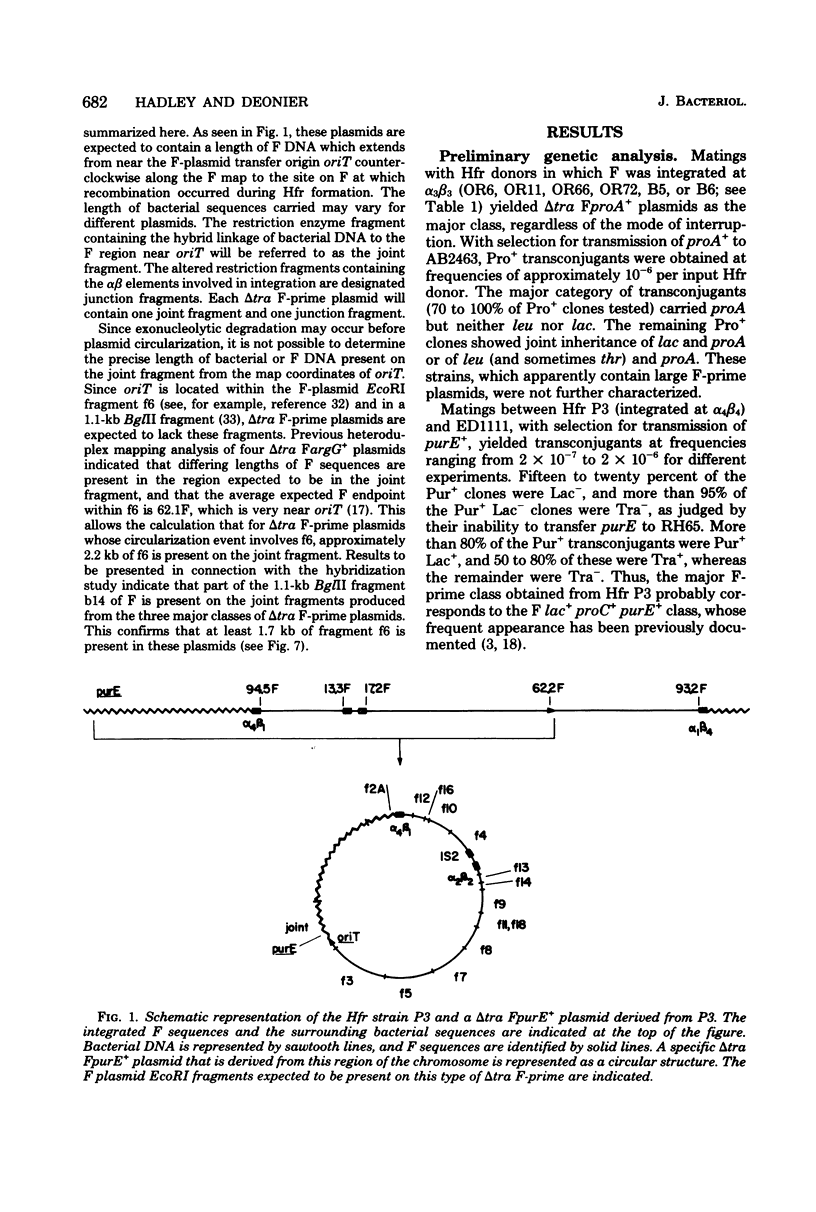
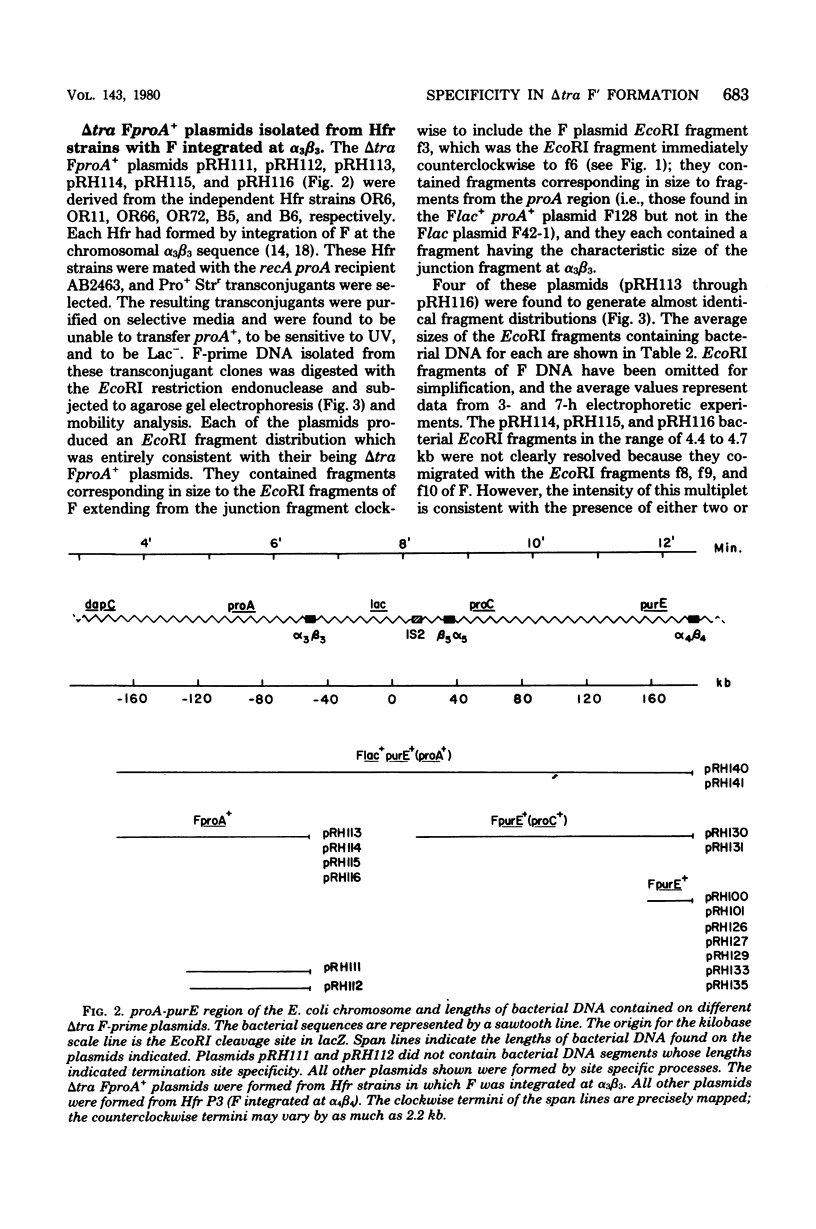
- Hadley R. G., Deonier R. C. Specificity in formation of type II F' plasmids. J Bacteriol. 1979 Sep;139(3):961–976. doi: 10.1128/jb.139.3.961-976.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. Models of genetic recombination. Annu Rev Microbiol. 1974;28(0):445–468. doi: 10.1146/annurev.mi.28.100174.002305. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. T., Davidson N. Structure of inserted bacteriophage Mu-1 DNA and physical mapping of bacterial genes by Mu-1 DNA insertion. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2823–2827. doi: 10.1073/pnas.69.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Ohtsubo E., Hsu M. T. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F100, F152, and F8 and mapping of the Escherichia coli chromosomal region fep-supE-gal-attlambda-uvrB. J Bacteriol. 1978 Jun;134(3):778–794. doi: 10.1128/jb.134.3.778-794.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Construction and BamHL analysis of chimeric plasmids containing EcoRL DNA fragments of the F sex factor. Plasmid. 1978 Feb;1(2):174–186. doi: 10.1016/0147-619x(78)90037-9. [DOI] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., Botstein D. Regional specificity of illegitimate recombination associated with the translocatable ampicillin-resistance element Tn1. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1209–1215. doi: 10.1101/sqb.1979.043.01.137. [DOI] [PubMed] [Google Scholar]
- Willetts N. The genetics of transmissible plasmids. Annu Rev Genet. 1972;6:257–268. doi: 10.1146/annurev.ge.06.120172.001353. [DOI] [PubMed] [Google Scholar]