SUMMARY
Evaluation of the therapeutic potential of RNAi for HIV infection has been hampered by the challenges of siRNA delivery and lack of suitable animal models. Using a novel delivery method in humanized mice, we show that siRNA treatment can dramatically suppress HIV infection. A CD7-specific single-chain antibody was conjugated to oligo-9-arginine peptide (scFvCD7-9R) for T cell-specific siRNA delivery in NOD/SCIDIL2rγ−/− mice reconstituted with human lymphocytes (Hu-PBL) or CD34+ hematopoietic stem cells (Hu-HSC). In HIV-infected Hu-PBL mice, treatment with anti-CCR5 and antiviral siRNAs complexed to scFvCD7-9R controlled viral replication and prevented the disease-associated CD4 T cell loss. This treatment also suppressed endogenous virus and restored CD4 T cell counts in mice reconstituted with HIV+ PBMC. Moreover, scFvCD7-9R could deliver antiviral siRNAs to naïve T cells in Hu-HSC mice and effectively suppress viremia in infected mice. Thus, siRNA therapy for HIV infection appears to be feasible in a preclinical animal model.
INTRODUCTION
The potency and specificity of gene silencing by RNA interference (RNAi) has raised hopes of developing a new class of drugs to treat several diseases including HIV infection (Manjunath et al., 2006; Rossi et al., 2007; Scherer et al., 2007; Shankar et al., 2005). Many studies have shown the effectiveness of RNAi in suppressing HIV replication in cell lines as well as in primary human T cells and macrophages, the prime targets of HIV (Lee et al., 2005; Novina et al., 2002; ter Brake et al., 2006). Although the propensity of HIV for mutation is a constraint, this can be overcome by using siRNAs that target highly conserved viral sequences and/or host genes important for viral replication but relatively nonessential for immune/cellular function, such as the viral co-receptor CCR5 (Brake et al., 2008; Song et al., 2003a; von Eije et al., 2007).
Despite the promise shown in vitro studies, for RNAi to become clinically useful, many parameters including delivery to susceptible cells, antiviral efficacy, and toxicity need to be tested in vivo. A major impediment for this is the lack of a suitable small animal model that simulates human HIV infection. Immunodeficient mice transplanted with human peripheral blood leukocytes (PBL) or pieces of human fetal tissues containing hematopoietic stem cells (HSC) can support HIV infection (Shacklett, 2008). However, the usefulness of these models is limited by the short time frame of chimerism and the lack of systemic spread of the virus after local infection of tissue implants. Recently, immunodeficient mouse strains bearing a targeted mutation in the common IL-2 receptor gamma chain (IL2rγ−/−) have been shown to serve as excellent models for HIV infection (Berges et al., 2006; Berges et al., 2008). NOD/SCIDIL2rγ−/−mice support long-term multilineage hematopoiesis from transplanted human CD34+ hematopoietic stem/progenitor cells (Hu-HSC model) (Ishikawa et al., 2005; Watanabe et al., 2007), as well as short-term expansion of injected human PBL that become activated in a xenogenic response (Hu-PBL model) (Nakata et al., 2005).
Another challenge is the delivery of siRNA to relevant cell types in vivo. Systemic delivery of siRNA to T cells, the major targets of HIV-1, is particularly difficult because they are resistant to siRNA uptake even by conventional lipid-based transfection in vitro (Goffinet and Keppler, 2006). Although T cells can be transduced by viral vectors expressing shRNA, achieving stable transgene expression is a challenge (Rossi et al., 2007). Moreover, their use carries the risk of induction of immune response to the vector itself, as well as the unpredictable effects of viral integration on host gene expression in the case of retro- and lentiviral vectors. Similar problems can be envisaged in generating T cells from transduced CD34+ HSC. Recently, antibody fragment-protamine fusion proteins were used to deliver siRNAs into tumors implanted in mice engineered to express T cell surface antigens (Peer et al., 2007; Song et al., 2005). However, the applicability of these approaches for siRNA delivery to primary T cells in HIV-1 infection remains untested.
We used a single-chain antibody (scFv) to the pan-T cell surface protein CD7 (Peipp et al., 2002) a surface antigen present on the majority of human T cells. As this receptor is rapidly internalized after antibody binding, it has been exploited for the targeted delivery of several monoclonal antibody (mAb)-toxin conjugates to T cell lymphomas and leukemias in both preclinical studies and clinical trials (Bremer et al., 2005; Frankel et al., 1997; Lazarovits et al., 1993; Peipp et al., 2002). Although the exact function of CD7 is unknown, CD7-deficient murine T lymphocytes respond normally to stimuli (Bonilla et al., 1997), and engaging CD7 on human T-cell lines appears to have no deleterious effect on their proliferation and viability (Bremer et al., 2005; Peipp et al., 2002). In an earlier study, we showed that fusion of 9 arginine residues to a neuronal cell-targeting peptide enabled siRNA delivery to neuronal cells (Kumar et al., 2007). Here, we modified the CD7 scFv to include a Cys residue at its C-terminal end (scFvCD7Cys), which allowed conjugation to a nona-d-arginine (9R) peptide for targeted delivery of siRNA payloads into T cells. We demonstrate the feasibility of this approach for T cell-specific siRNA delivery to suppress HIV infection in humanized mice.
RESULTS
Oligo-9-arginine-conjugated scFvCD7 delivers siRNA specifically to CD7-expressing human T cells
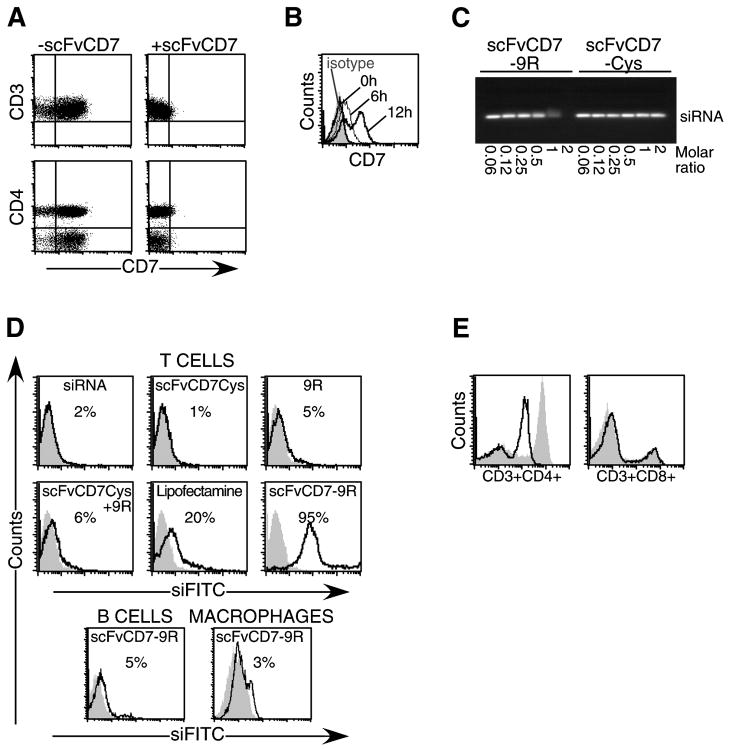
scFvCD7 was expressed with an additional Cys residue at its C-terminal end (scFvCD7Cys) and purified from bacterial lysates. Pretreatment with recombinant scFvCD7Cys completely blocked binding of PE-labeled anti-CD7 antibody but not antibodies to other T cell surface molecules including CD3 and CD4 (Figure 1A). Binding of PE-anti-CD7 was restored by 12 h after treatment with scFvCD7Cys, suggesting rapid internalization and turnover of the receptor (Figure 1B). To enable siRNA binding, scFvCD7Cys was conjugated to a 9R peptide at the C-terminus (scFvCD7-9R). Electrophoretic gel mobility-shift assay confirmed efficient siRNA binding to scFvCD7-9R at a minimal protein to nucleic acid ratio of about 2:1 (Figure 1C).
Figure 1. scFvCD7 binds to CD7 and 9R conjugation allows siRNA binding and delivery to T cells in vitro.
(A) Purified human CD3+ T cells were stained with antibodies to CD3, CD4 and CD7 before or after treatment with scFvCD7Cys. (B) CD7 expression was assessed at indicated times after preincubation with scFvCD7Cys. (C) siRNA was incubated with scFvCD7-9R or unconjugated scFvCD7Cys at the indicated molar ratios for 15 min and electrophoresed on 1% agarose gels. The position of the non-bound siRNA is indicated. (D) Purified human CD3+ T cells (upper panels), CD19+ B cells (bottom panel), and differentiated CD14+ monocyte-derived macrophages (bottom panel) were treated with FITC-labeled siRNA alone (grey, filled histograms) or siRNA mixed with the indicated reagents (black, open histograms). (E) PHA-activated PBMC were treated with anti-huCD4 siRNA complexed to scFvCD7-9R. CD4 and CD8 expression levels on CD3+ T cells were monitored 60 h later (black histograms). Grey filled histograms depict control PBMC treated similarly with scFvCD7-9R/siLuc.
scFvCD7-9R was able to transduce FITC-siRNA into primary human CD3+ T cells, with efficiencies of nearly 95% with no apparent toxicity (Figure 1D, upper panels). No uptake was observed with FITC-siRNA alone or when combined with scFvCD7Cys, 9R, or scFvCD7Cys mixed with 9R. Transfection efficiencies with a commercial lipid reagent were 4-fold lower than with scFvCD7-9R. T cell-specific delivery of siRNA was confirmed by the absence of siRNA in similarly treated CD7-negative B cells (CD19+) and monocyte-derived macrophages (CD14+) (Figure 1D, lower panel). When PHA-activated human PBMC were treated with scFvCD7-9R/siCD4 complexes and surface CD4 expression examined 60 h later, the mean fluorescent intensity (MFI) of CD4 was reduced by almost one log unit on CD3+ T cells (Figure 1E). The silencing was specific since CD8 expression remained unaffected. CD4 expression was not reduced with scFvCD7-9R/siLuc, siCD4 alone, or with 9R or scFvCD7Cys (data not shown). Thus, scFvCD7-9R provides a reagent to deliver siRNA and silence target gene expression specifically in human T cells.
Intravenous (iv) administration of scFvCD7-9R/siRNA silences target gene expression in T cells in Hu-PBL mice
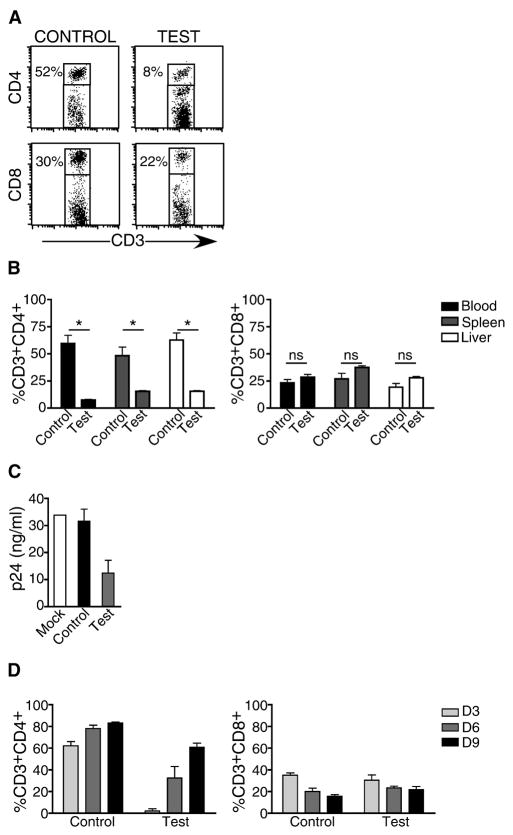
The ability of scFvCD7-9R to deliver siRNA to T cells in vivo was studied in the NOD/SCIDIL2rγ−/− Hu-PBL mouse model, which supports a high level of human peripheral blood leukocyte engraftment as early as 1 week post-transplantation (Figure S1A). Hu-PBL mice were iv injected with scFvCD7-9R/siRNA complexes on 2 consecutive days and CD4 expression on peripheral blood T cells was examined 60 h later. CD4 expression was significantly reduced on siCD4-treated, but not control siLuc-treated mice (Figure 2A, mean level of peripheral CD3+CD4+ T cells was 7.5 ± 0.7% in treated mice and 59.5 ± 10.7% in control mice, n=3, P<0.05). Again, CD8+ T cell levels were unchanged, confirming that silencing was restricted to the targeted gene (28.5 ± 3.5% versus 23.3 ± 4.9% respectively in treated and control mice, n=3, P>0.05). T cells from other organs, including liver and spleen, also showed comparable CD4 knockdown (Figure 2B). When PBMC from scFvCD7-9R/siCD4-treated mice were infected with the T cell-tropic HIVIIIB ex vivo, HIV-1 p24 levels were significantly reduced in the culture supernatants, confirming reduced permissibility to viral infection (Figure 2C). We also determined the duration of gene silencing in vivo. Silencing was maximal during the first 3 days, but was progressively lost and by day 9, CD4 expression returned to 70% of normal levels (Figure 2D).
Figure 2. scFvCD7-9R-mediated siRNA uptake and gene-silencing in T cells in vivo in Hu-PBL mice.
NOD/SCIDIL2rγ−/− mice reconstituted with human PBMC were injected iv with siLuc (control) or siCD4 (test) complexed to scFvCD7-9R twice, 16 h apart and human CD3+ T cells in the peripheral blood, spleen and liver were analyzed for CD4 and CD8 expression 60 h later. Representative dot plots from one mouse (A), and cumulative data from 3 mice (B), are shown. Asterisks indicate significant and “ns” indicates no significant differences between test and control groups. P < 0.05. (C) PBMC isolated from groups of Hu-PBL mice were PHA-stimulated and infected with HIVIIIB. Culture supernatants collected on day 10 after infection were tested for p24 antigen levels in triplicate by ELISA. (D) Mice were treated with siRNA 20 days after reconstitution as in (A) three times at 16 h intervals and CD4 and CD8 expression in peripheral blood T cells were determined on days 3, 6 and 9 after the last injection. Error bars indicate standard deviation.
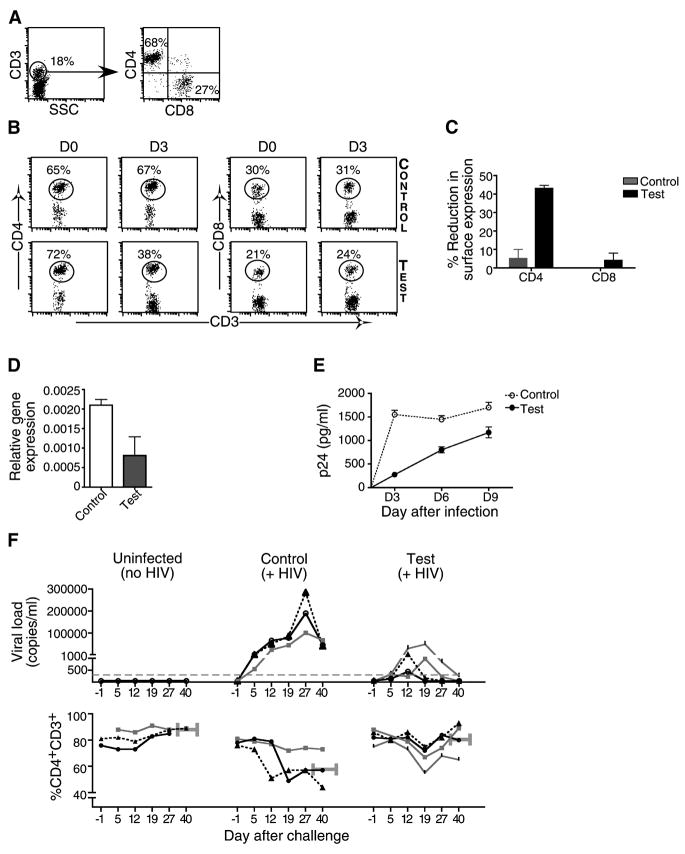
Systemic delivery of antiviral siRNA/scFvCD7-9R complex protects Hu-PBL mice from HIV-1 challenge
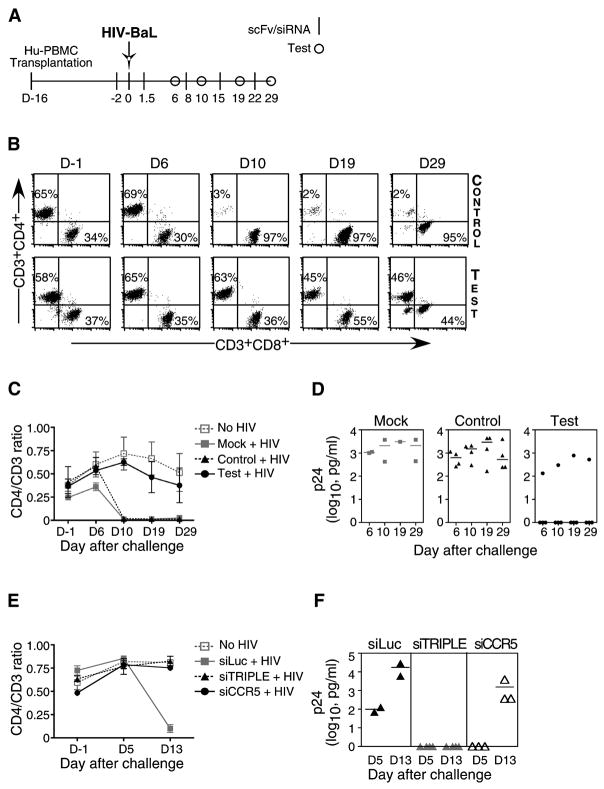
T cells in Hu-PBL mice express CCR5 and are susceptible to R5-tropic strains of HIV (Fais et al., 1999; Nakata et al., 2005), with infection resulting in a progressive loss of CD4 T cells (Berges et al., 2006). A combination of siRNAs targeting the cellular CCR5 and 2–3 conserved viral gene sequences has been proposed as an optimal strategy to prevent the emergence of escape mutants (Brake et al., 2008; von Eije et al., 2007). Thus, Hu-PBL mice were treated with CCR5 siRNA (Song et al., 2003a) to block viral entry, challenged with HIVBaL two days later, and further treated by weekly administration of a combination of siRNAs targeting CCR5 (to prevent viral spread) and conserved target sequences in the viral Vif and Tat genes (to block viral replication) (Lee et al., 2005; Surabhi and Gaynor, 2002) (Figure 3A). All siRNAs were complexed to scFvCD7-9R prior to injection. As early as 10 days post infection, CD4 T cell levels declined precipitously in all of the mock- and control siLuc-treated mice, with CD4+CD3+ T cell percentages dropping to as low as 2% and CD8+CD3+ percentages concomitantly increasing to over 95% (Figures 3B and C). In sharp contrast, in 3/4 antiviral siRNA treated mice, CD4 T cell levels remained essentially normal even 4 weeks post infection (Figures 3B and C). Consistent with changes in the CD4 T cells, viral replication (assessed by serial measurement of serum p24 antigen levels by ELISA) was high in the mock- and control siLuc-treated mice, but undetectable in 3 of the 4 relevant siRNA-treated mice (Figure 3D). In the single test mouse that was not protected, the CD4 T cell loss exhibited slower kinetics (CD4/CD3 ratio of 0.6 at day 10 as opposed to a mean value of 0.016 in control mice) and, correspondingly, the serum p24 levels tended to be lower.
Figure 3. iv treatment with siRNAs complexed to scFvCD7-9R prevents HIV infection in Hu-PBL mice.
(A) Protocol for scFvCD7-9R/siRNA administration and immunological and virological monitoring of Hu-PBL mice infected with HIVBaL. (B-D) Hu-PBL mice were treated iv with siCCR5 or control siLuc 14 days after reconstitution. Two days later, the mice were ip infected with HIVBaL and subsequently either mock-treated (n=2) or treated with a combination of siCCR5/Vif/Tat (test, n=4) or siLuc (control, n=4) complexed to scFvCD7-9R as indicated in (A) and CD3/CD4/CD8 T cell levels were monitored by flow cytometry. Representative dot plots from one test and one control mouse are shown in (B) and cumulative data in (C). Quadrants at each time point were drawn in comparison with corresponding isotype controls. Numbers in (B) represent CD4+ or CD8+ percentages as a proportion of total CD3+ T cells. Error bars indicate standard deviations. (D) Serum p24 levels were measured by ELISA at the indicated times after viral challenge. Horizontal lines indicate median values. (E, F) Hu-PBL mice were treated with siLuc (control) or siCCR5 or siCCR5/Vif/Tat combination (siTRIPLE) complexed to scFvCD7-9R as in (A) and CD4+ T cell ratios and plasma p24 levels tested as above.
In a separate experiment, we also compared the protection afforded by siCCR5 alone versus combination therapy. Hu-PBL mice were treated with scFvCD7-9R complexed to either siCCR5 or the triple siRNA combination as in Figure 3A. All control mice displayed near complete loss of CD4+ T cells and high levels of plasma p24 by day 13, whereas the CD4 ratios were preserved both in mice treated with siCCR5 alone and the triple siRNA combination (Figure 3E). However, unlike the triple siRNA-treated group, plasma p24 antigen became detectable in all mice that received only siCCR5 (Figure 3F), although the mean level was reduced by one log unit compared to control mice (1530 ± 1163 pg/ml as opposed to 17410 ± 11410 pg/ml, P=0.03). Thus, preventing viral entry does contribute to protection but more robust control of viral infection requires virus-specific siRNAs along with siCCR5. Taken together, our results suggest that treatment with scFvCD7-9R/siRNA can prevent HIV replication and the consequent CD4 T cell loss in vivo.
IV treatment with antiviral siRNA/scFvCD7-9R complex prevents CD4 T cell loss in Hu-PBL mice reconstituted with PBMC from HIV+ patients
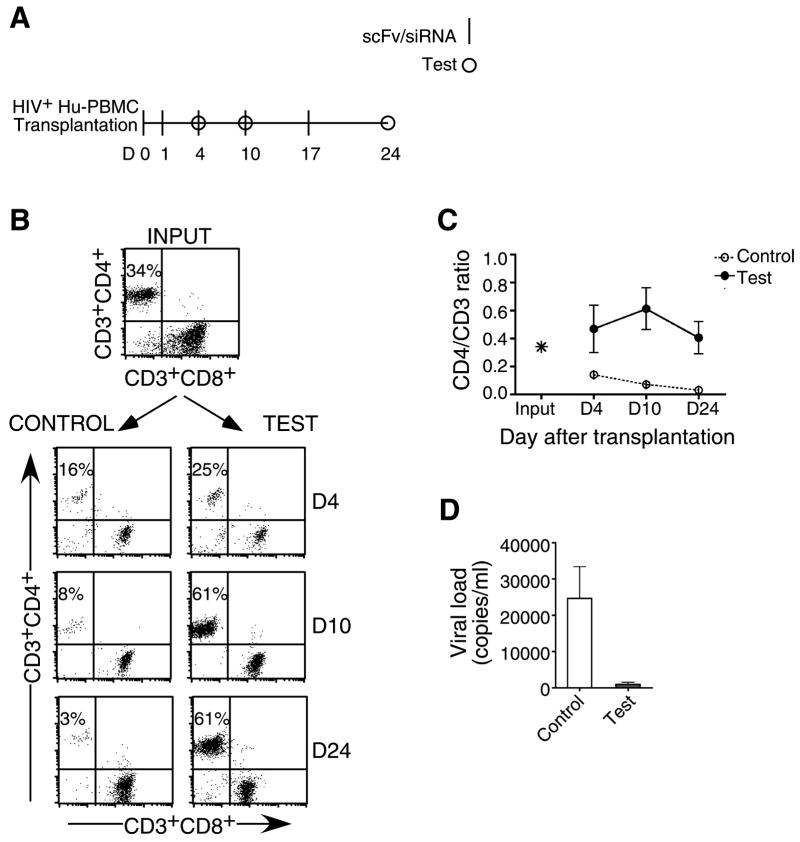
For potential use of RNAi as a therapeutic, it is important to test its efficacy in an established infection. However in the Hu-PBL model exogenous viral challenge leads to a rapid decline in CD4 T cells, making it difficult to assess post-infection treatment efficacy. Thus, as an alternate strategy to mimic established infection, we reconstituted mice with PBL from a HIV seropositive donor (Figure 4A). This approach also enabled us to evaluate whether the siRNAs targeting the conserved vif and tat viral sequences, which could protect against the lab strain of HIV-1, were effective against the multiple viral quasispecies likely to be present in infected individuals. Mice were reconstituted with PBL from a HIV-positive donor who had been on HAART for 4 years and exhibited viral loads below detection and a CD4/CD3 ratio of 0.34 (Figure 4B, input) and treated with a combination of siRNAs targeting CCR5, Vif and Tat using the regimen indicated in Figure 4A. Similar to the experimental infection model, mice treated with control siLuc showed severe CD4 T cell depletion 10 days after engraftment (mean CD4/CD3 ratios of 0.14, n=4) (Figures 4B and C). In contrast, CD4 T cell levels did not decline in the antiviral siRNA-treated mice, but instead expanded due to xenogenic activation resulting in a steady increase in numbers to about 60% of total CD3 T cells up to the 2nd week. In fact, CD4/CD3 ratios 3.5 weeks after transplantation were higher (mean=0.47) than the input (0.34), suggesting that siRNA treatment can potentially reverse the CD4 T cell loss associated with HIV disease. As the serum p24 ELISA levels were below detection even in the control mice (probably due to the low numbers of input CD4 T cells), we measured plasma viral RNA copy numbers. Viral loads were highly reduced in scFvCD7-9R/antiviral siRNA-treated mice as compared to control mice (Figure 4D). Thus, multiplexed siRNAs can serve as an effective antiviral treatment analogous to combination antiretroviral therapy in a clinical setting.
Figure 4. iv treatment with siRNA/scFvCD7-9R complexes prevents CD4 T cell loss and HIV-1 amplification in mice reconstituted with HIV-seropositive donor PBMC.
(A) Protocol for siRNA/scFvCD7-9R administration and immunological and virological monitoring. (B, C) Mice transplanted with PBMC from a HIV-seropositive donor were treated iv with scFvCD7-9R complexed to either siLuc (control) or siCCR5/Vif/Tat (test) as indicated in (A) and CD4 T cell levels were monitored by flow cytometry. Representative dot plots from one mouse in each group are shown in (B) and cumulative data from 4 mice in (C). Numbers indicated in (B) represent CD4+ percentages as a proportion of total CD3+ T cells. (D) Viral copy numbers in plasma were measured by the Amplicor test on day 17 after reconstitution with donor PBMC. Error bars represent standard deviation.
scFvCD7-9R silences target gene expression in naïve T cells in Hu-HSC mice
While Hu-PBL mice offer a suitable acute infection model to test antiviral efficacy because the T cells are activated by xenogenic stimulation, the model precludes testing of siRNA delivery to naïve and resting T cells. Thus, we also tested if scFvCD7-9R is able to deliver siRNA to T cells in mice engrafted with human-HSC. In this model, multilineage immune cell reconstitution occurred 12 weeks after HSC transplantation, with average levels of 50% human CD45+ lymphocytes in the peripheral blood that included CD3+ T cells, CD19+ B cells, CD14+ monocytes and CD11c+ dendritic cells (Figures S1B and 5A). T cells in these mice are predominantly naïve unactivated (CD45RAhi, CCR7hi, CD62Lhi, CD27hi and CCR5lo) in contrast to T cells from Hu-PBL mice, which display a predominantly activated phenotype (Gorantla et al., 2007) (Figure S1C). When Hu-HSC mice were treated with scFvCD7-9R/siCD4, a substantial reduction in CD4 expression was seen in CD3-gated T cells (Figures 5B and C). Moreover, even a single administration of siCCR5 reduced target mRNA levels in splenic T cells harvested from Hu-HSC mice 24 h post-treatment by greater than 50% in comparison to control siLuc-treated mice (Figure 5D). When these cells were PHA stimulated and infected with HIVBaL ex-vivo, the p24 levels in serial culture supernatants were significantly lower in the cell cultures of siCCR5 treated mice (Figure 5E). Thus scFvCD7-9R can mediate siRNA delivery in vivo into naïve human T cells that are normally refractory to nucleic acid uptake.
Figure 5. scFvCD7-9R mediates siRNA delivery to naïve T cells in Hu-HSC mice and suppresses HIV replication in vivo.
(A) Peripheral blood from Hu-HSC mice was examined for the presence of human CD4 and CD8 T cells 12 weeks after reconstitution. (B, C) Hu-HSC mice were iv-injected twice, 16 h apart, with siCD4 (test) or control siLuc complexed to scFvCD7-9R and peripheral blood T cells were tested for CD4 and CD8 expression before and 3 days after treatment. Representative dot plots from one mouse in each group are shown in (B) and cumulative data from 3 mice in (C). Numbers indicated in (B) represent the percentage of total CD3+ T cells. In (C) the reduction in surface CD4 or CD8 levels was calculated as a percentage of the initial expression level before siRNA injection. (D) Splenocytes isolated from Hu-HSC mice 1 day after a single injection with scFvCD7-9R/siLuc (control) or siCCR5 (test) were examined for CCR5 mRNA levels by qPCR using β-actin mRNA levels for normalization. (E) Splenocytes in (D) were PHA-stimulated and infected with HIVBaL at a moi of 3 and p24 antigen levels in culture supernatants were assayed in triplicate by ELISA at the indicated time points. Error bars indicate standard deviation. (F) Hu-HSC mice were either mock-treated (n=3), treated iv with siLuc (Control, n=3) or siVif/Tat (Test, n=4) complexed to scFvCD7-9R 22 weeks after reconstitution. 18 h later, the control and test animals were ip infected with HIVBaL and further treated with scFv/siRNA every 4–5 days. Viral copy numbers in plasma measured by the Amplicor test (upper panel) and CD4+CD3+ T cell percentages monitored by flow cytometry (lower panel) at various times are shown. The grey dotted line in the upper panel represents the limit of detection of the Amplicor test. CD4 T cell ratios were calculated as a ratio of the entire CD3 population (CD4+CD3+:CD3+) and mean ratios (horizontal grey bars) at 40 d post challenge is shown. Individual animals in each group are represented by distinct symbols.
scFvCD7-9R/siRNA treatment controls HIV viremia in Hu-HSC mice
In the Hu-HSC model the constant replenishment of multilineage human hematopoietic cells permits establishment of chronic infection. We therefore tested whether delivery of siRNA to naïve T cells in Hu-HSC mice could confer long-term protection after HIV challenge. Hu-HSC mice were infected with HIVBAL and treated with a combination of two antiviral siRNAs siVif/Tat or control siLuc complexed to scFvCD7-9R with repeat administrations every 4–5 days. All control mice displayed viremia by the first week, which persisted throughout the 7 week observation period (Figure 5F, upper panel). Viral RNA copies as high as 1.92 ± 0.5 × 105/ml plasma were detected in infected mice in conjunction with a decline in peripheral blood human CD4 T cell numbers (Figure 5F, lower panel). However, possibly due to the constant de novo supply of naïve T cells, the extent of CD4 T cell decline was not as rapid or drastic as in the Hu-PBL model. The viremia levels dropped after attaining peak levels between 19–40 days, akin to the establishment of viral set point in chronic persistent HIV infection (Berges et al., 2006). In contrast to control mice, animals that received the siVif/Tat were remarkably competent in controlling infection during the 7 weeks of observation (Figure 5F, upper panel). Correspondingly, the mean CD4 levels in test mice were similar to those in uninfected mice (89 ± 0% versus 81.8 ± 12.4% respectively at day 40 post challenge). Even the single mouse in the test group that recorded a drop in CD4 T cells displayed a peak plasma viral load, nearly 30 fold less than the control siLuc-treated mice. Thus, antiviral siRNAs can effectively control viral infection and T cell loss, which are key features of clinical AIDS. The findings in Hu-HSC mice are particularly relevant from the therapeutic standpoint since resting T cells harboring integrated HIV provirus are an important latent reservoir that can rekindle viral replication after interruption of HAART (Chun et al., 1997; Finzi et al., 1999).
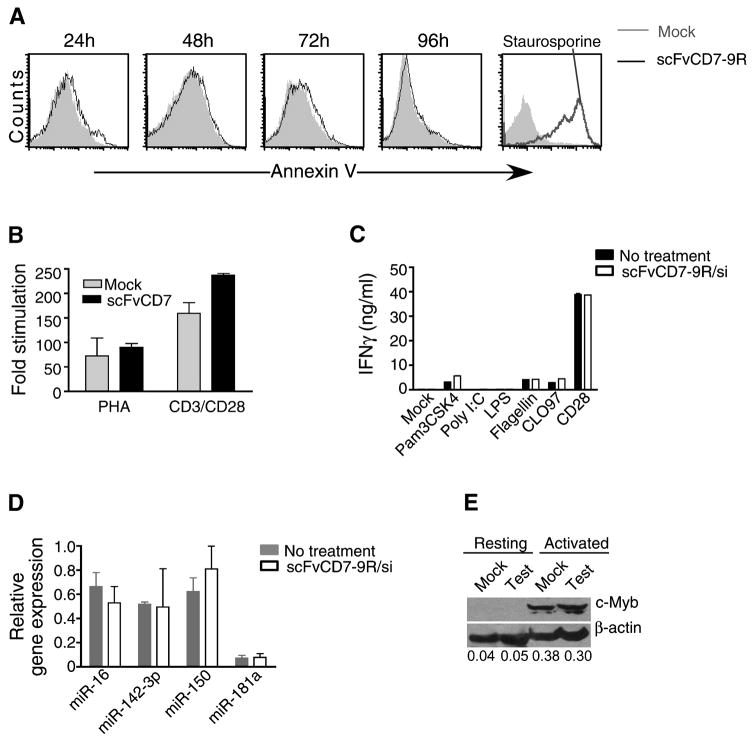
scFvCD7-9R/siRNA complexes do not induce toxicity in target cells
In vitro exposure of PBMC to scFvCD7-9R/siRNA was non-toxic as assessed by lack of Annexin-V positivity (Figure 6A) as well as the normal proliferative response of treated cells to stimulation with PHA or anti-CD3/CD28 beads (Figure 6B). To assess the possible activation of T cell-specific toll-like receptor (TLR) signaling pathways, purified CD4 T cells from a healthy donor were incubated with various TLR agonists under conditions known to induce IFN-γ (Caron et al., 2005) in the presence of scFvCD7-9R/siRNA complexes. No significant differences in IFN-γ levels were discernable in supernatants from treated or control cultures, even with agonists for the endosomally-localized TLRs (Figure 6C).
Figure 6. scFvCD7-9R/siRNA treatment does not induce toxicity.
(A) scFvCD7-9R/siLuc-treated or mock-treated PBMC stimulated with PHA were stained with Annexin-V on 4 consecutive days of culture. 24 h staurosporine-treated cultures served as positive control. (B) PBMC treated with scFvCD7-9R/siLuc were stimulated with PHA or antiCD3/CD28 beads for 3 days and pulsed with 3H-thymidine for 18 h. Fold stimulation was calculated by dividing the counts incorporated in the presence of to those in the absence of stimulating agent. (C) Purified human CD4+ T cells were stimulated with anti-CD3 mAb in the absence (no treatment) or presence of Pam3CSK4 (TLR2 ligand), Poly I:C (TLR3), LPS (TLR4), Flagellin (TLR5), CLO97 (TLR8/9) or anti-CD3/CD28 Dynabeads. IFN-γ was quantified by ELISA in 48 h culture supernatants. Error bars indicate standard deviation of triplicate cultures. (D) Expression profiles of miRNA in CD3+ T cells purified from Hu-HSC mice treated thrice with scFvCD7-9R/siCCR5 are depicted. Expression level was normalized to that of small non-coding RNA U6B. Mean of triplicate runs with two animals each ± SD is shown. (E) CD3+ T cells purified from Hu-HSC mice treated as in (D) were examined for c-Myb protein levels either immediately or after 48 h in culture with PHA. The numbers below represent the ratios of band intensities of c-Myb normalized to that of β-actin. Error bars in all cases represent standard deviation.
We also tested if scFvCD7-9R/siRNA treatment affects the levels of miRNAs predominantly expressed in T cells (Wu et al., 2007.). We could not detect any alterations in the expression levels of miR-142-3p, miR-150, miR-181a and miR-16 in CD3+ T cells purified from Hu-HSC mice after three injections of scFvCD7-9R/siRNA (Figure 6D).
Human T cell-specific gene targets of miRNA have not been definitively identified. However, c-Myb has been shown to be a target for the abundantly expressed miR-150 in mouse T cells (Xiao et al., 2007). Human c-Myb also contains miR-150 seed sequence in the 3′ UTR and is a predicted target for miR150. Normally, c-Myb protein is undetectable in naïve human T cells and is induced upon activation (Lipsick and Boyle, 1987). Thus, we tested for c-Myb protein levels in human CD3+ T cells purified from Hu-HSC mice after three consecutive siRNA administrations, before (resting T cells) and after activation in vitro with PHA. The expression pattern of c-Myb protein was similar in cells from control and treated mice, becoming detectable only after activation and with no difference in the level of expression upon treatment, indicating that siRNA treatment did not perturb the expression pattern of c-Myb protein (Figure 6E). Thus multiple administrations of synthetic siRNA/scFvCD7-9R complex do not appear to affect miRNA regulation in the treated cells.
DISCUSSION
We have developed a new non-viral method for systemic delivery of antiviral siRNAs to T cells. Our results show that scFvCD7-9R is able to mediate efficient siRNA delivery to suppress HIV infection in both activated (Hu-PBL model) and naïve (Hu-HSC model) T cells. These findings overcome a critical barrier of in vivo delivery, significantly enhancing the prospect of siRNA-based therapeutics for HIV infection.
Since the first demonstration of in vivo gene silencing by hydrodynamic injection of siRNA (Song et al., 2003b), there has been a concerted effort to develop more practical delivery strategies suitable for human therapy. A promising approach is to use targeting antibodies that undergo internalization after binding to surface receptors. To carry siRNA, antibodies can be coated on liposomes packaged with siRNA or fused to positively charged proteins/peptides that bind nucleic acids by charge interactions. Accordingly, an immunoliposome coated with antitransferrin scFv has been used to deliver HER-2 siRNA to tumor cells both in vitro and in vivo (Hogrefe et al., 2006). Similarly, a HIV gp140 scFv fused to protamine could deliver siRNA to HIV-infected targets including primary T cells in vitro (Song et al., 2005). A scFv-protamine fusion protein targeting the leukocyte-specific LFA-1 also delivered siRNA to primary human T cells in vitro (Peer et al., 2007). In both studies, siRNA delivery to transplanted tumor cells expressing the targeted ligands was demonstrated in vivo, suggesting that the antibody-based approach may make in vivo siRNA delivery feasible. Our study confirms and extends these observations by using this strategy to deliver anti-viral siRNA in the context of an actual HIV infection. Our results show that siRNA binding capability can be conferred to scFvs by external disulphide conjugation to a 9R moiety. In addition to being relatively simple, this approach may also have an edge over recombinant fusion proteins, as expression of the positively charged residues might interfere with proper folding of the antibody during purification. Moreover, it allows use of the d-isoform of the peptide, which is relatively resistant to degradation by serum proteases (Hamamoto et al., 2002). It is noteworthy that conjugation of the anti-CD7 antibody to 9R, a cell penetrating peptide (Kim et al., 2006), did not affect its high level of T cell selectivity. Thus, it appears that after siRNA binding, the 9R component itself has no role in siRNA delivery, which is an advantage as non-specific transport into unintended cells is avoided.
Animal models for HIV-1 have suffered from either the lack of a system that precisely mirrors human HIV infection or, in the case of primate models, scarcity of the species, high cost, and the need to use the related but distinct simian virus for infection. We (L.S.) and others have recently developed gamma chain null mice that support long lasting HIV infection with both macrophage and T-cell tropic strains of HIV (Berges et al., 2008; Watanabe et al., 2007). Using the NOD/SCIDIL2γ−/− mice, we found that HIV infection could be controlled both in a prophylactic setting, where viral challenge was performed after initiation of siRNA treatment, as well as in a post-infection therapeutic setting, where mice were reconstituted with PBLs from a human subject with an established HIV infection. Of note, knocking down CCR5 before viral challenge was not enough to completely block viral infection underscoring the importance of blocking multiple stages of viral replication by combinations of siRNAs targeting both host and viral genes. In a therapeutic setting, delivery to naïve/resting T cells will be important to ensure that siRNA is present in cells at the time of activation when they become most vulnerable to infection. This is also important for controlling viral resurgence in latently infected memory T cells. Thus, the successful delivery of siRNA to naïve T cells to control HIV infection in Hu-HSC mice attests to the versatility of our delivery strategy for clinical application.
It has been suggested that for a chronic infection like AIDS, a sustained antiviral state is best achieved by a gene therapy approach where vector-mediated delivery of shRNA to hematopoietic stem cells allows stable endogenous synthesis of siRNA in the repopulating progeny cells (Rossi et al., 2007). HIV resistance has been demonstrated ex vivo in progeny T cells derived from shRNA transduced HSCs transplanted into SCID/Hu mice (Anderson et al., 2007; Banerjea et al., 2003; Brake et al., 2008; Lee et al., 2005; Rossi et al., 2007; Scherer et al., 2007; ter Brake et al., 2006). However, obtaining stable transgene expression in sufficient numbers of expanded progeny which is critical for HIV-resistance has proved difficult to achieve in vivo (Levine et al., 2006; Rossi et al., 2007). A phase I clinical trial of a triple combination vector expressing an anti–tat/rev shRNA, a nucleolar localizing TAR decoy and an anti-CCR5 ribozyme has been launched recently which should shed light on the effectiveness of this approach and clarify concerns about toxicity related to shRNA expression, vector integration and the induction of interferon responses (Anderson et al., 2007). Given the high mutability of HIV, another obvious disadvantage of vector-driven expression of a few specifically selected, but fixed shRNA sequences is that the protection would be compromised if escape mutants arise. In contrast, exogenous delivery of siRNA using the strategy described here not only delivers siRNA to a large proportion of T cells but also provides freedom to vary siRNA combinations to keep pace with the mutating virus if the need arises.
Nonspecific activation of the immune system and off-targeting effects have been reported with synthetic siRNA, however recent studies suggest that this can be overcome by optimizing the sequence or by chemical modifications (Svoboda, 2007). Although over expression of shRNA in vivo has been reported to affect miRNA biogenesis and function leading to lethality in mice (Grimm et al., 2006), a recent study suggests that repeated administration of synthetic siRNA targeting the liver did not affect liver-specific miRNA expression or function (John et al., 2007). We also found that siRNA treatment did not affect the expression of several T cell expressed miRNAs. However, unlike for mouse liver-specific siRNAs, the gene targets for human T cell expressed miRNAs have not been definitively identified; hence we could only test one predicted target of miR-150 and found no changes in c-Myb protein levels after siRNA treatment. Thus, the risk of saturating endogenous miRNA pathway by exogenous siRNA appears to be minimal, although confirmation by a more comprehensive microarray/proteome analysis may be required. The possibility generating an immune response to the antibody used as the delivery vehicle is also a concern that needs to be addressed. However, many mAbs have been successfully used in clinical therapy without adverse effects and can also been ‘humanized’ in order to reduce potential toxicity (Marasco and Sui, 2007). Further, since liposomal or polymeric nanoparticles can accommodate a lot more siRNA, use of siRNA encapsulated nanoparticles coated with CD7 scFv as a targeting agent could reduce the number of injections/dosage. While our own preparation did not induce TLR activation, the data needs to be reinforced by further testing in nonhuman primate models.
Another important issue in the treatment of HIV infection is the ability to target macrophages and dendritic cells. In this context, it has been recently reported that an antibody to LFA-1 may be able to target all leukocytes, although its potential for efficient siRNA delivery in vivo without adverse effects on leukocyte function remains to be tested (Peer et al., 2007). Similarly, targeting approaches for siRNA delivery to other HIV-susceptible cell types could conceivably be used in combination with scFvCD7-9R. The availability of a preclinical animal model for HIV infection, as shown in this study, should allow rapid testing of these strategies, as well as other potential problems, such as viral escape and toxicity that have to be resolved before RNAi therapy can be translated for clinical use.
EXPERIMENTAL PROCEDURES
siRNAs
siRNAs targeting firefly luciferase (siLuc) (Kumar et al., 2007), the HIV genes Vif (Lee et al., 2005) and Tat (Surabhi and Gaynor, 2002), the human T cell receptor CD4 (Novina et al., 2002) and coreceptor CCR5 (Song et al., 2003a) were purchased from Dharmacon, Inc.
Purification of scFvCD7 single chain antibody and conjugation to oligo-9R
scFvCD7 coding sequence was PCR-amplified from the pAK400scFvCD7-GFP plasmid (Peipp et al., 2002) using primers to introduce a C-terminal cysteine residue and the amplified scFvCD7Cys was cloned into the pET 26b(+) vector (Novagen Inc.). The recombinant protein was purified by FPLC using Bio Scale Mini Profinity immobilized metal affinity chromatography (Bio-Rad) and then refolded as described (Wan et al., 2006). Cell-specific binding was verified by pre-incubating 5 × 105 CD3+ T cells purified from human PBMC for 30 min on ice with purified scFvCD7Cys (20 μg/ml). Cells were then washed and stained with anti-human CD7-PE, CD3-FITC and CD4-PECy5 antibodies (BD-Pharmingen), followed by flow cytometric analysis. In some experiments, the scFvCD7Cys treated cells were cultured at 37°C and stained at different times for surface CD7 expression with anti-CD7-PECy5.
To generate scFvCD7-9R, scFvCD7Cys (1mg/ml) was mixed with Cys(Npys)-(D-Arg)9 peptide (9R, Anaspec) in 0.1M phosphate buffer (pH 5.5) at a molar ratio of 10 to 1 and gently stirred for 4 h at room temperature (Zeng et al., 2006). Unconjugated 9R was removed by dialysis using a membrane with a MWCO of 10,000. Typically, conjugation efficiencies of around 75% were achieved as measured by a thiol and sulfide quantization assay kit (Molecular probes, data not shown).
siRNA binding and silencing experiments
To test siRNA binding, 100 pmole of siRNA was incubated with different amounts of scFv CD7-9R for 15 min and analyzed by agarose gel electrophoresis. To test delivery, PBMC derived CD3+ T cells, CD19+ B cells or CD14+ monocyte-derived macrophages were seeded in 96 well-plates at 2×105 cells/well and treated 24 hours later with 200 pmol siFITC bound to scFvCD7-9R at a molar ratio 5:1. After 4 hours the cells were washed and incubated for an additional 16 h at 37°C and subjected to flow cytometry. For gene silencing experiments, scFvCD7-9R complexed with 400 pmole of siCD4 at a molar ration of 5:1 was added to 5×105 PHA-stimulated PBMC, and surface CD3, CD4 and CD8 levels were assessed after 60 h of treatment.
To assess possible toxicity PBMC were PHA activated (4 μg/ml) in the absence or presence of scFvCD7-9R/siLuc and stained with Annexin V at different time points. Staurosporine (Sigma) treatment (1 μM for 24 h) was used as positive control. To evaluate effect on cell proliferation, PBMCs were activated with either PHA or CD3/CD28 T cell Expander Dynabeads (Invitrogen,1 bead per cell) in the absence or presence of scFVCD7-9R/siLuc for 3 days and tested for 3H-thymidine incorporation.
Generation of Hu-PBL and Hu-HSC mice
All work with animals was approved by the Institutional Review Board at the Immune Disease Institute. NOD.cg-PrkdcscidIL2rgtm/Wjl/Sz (NOD/SCIDIL2rγ−/−) mice were from the Jackson Laboratory (Bar Harbor, ME). Hu-PBL mice were generated as described (Nakata et al., 2005). Briefly, 107 PBMC freshly isolated from HIV-seronegative donors were injected ip (in 0.5 ml RPMI) into 4–6 week old mice. In some experiments, the mice were injected with PBMC from HIV-seropositive donors. Cell engraftment was tested 3–5 days after transplantation by staining the mice PBMC for human CD45+, CD3+, CD4+ and CD8+ positive cells.
Hu-HSC mice were generated as described (Ishikawa et al., 2005). 1–2 day old neonatal mice were irradiated (100 rads) and injected intravenously with T cell-depleted cord blood cells containing 3×104 CD34+ cells per mouse. Transplanted mice were tested for engraftment 12 weeks later as described above.
Mouse experiments with scFvCD7/siRNA delivery
Human cell engrafted mice were iv-injected with scFvCD7-9R/siRNA complexes at a 5:1 molar ratio at a dose of 50 μg siRNA per injection in 5% glucose in a volume of 200 μl. In infection experiments, Hu-PBL mice were ip injected on day 16 post-transplantation with 10000 TCID50 of HIVBaL in a 100 μl volume. Hu-HSC mice were infected similarly with 30000 TCID50 22 weeks after transplantation with HSC. PBMC recovered from the mice at different times were analyzed by flow cytometry for gene silencing and antiviral effects. Quantitative PCR for CCR5 mRNA levels in T cells from Hu-PBL mice isolated using the Dynal T cell Negative Isolation kit (Invitrogen) was performed with the primers listed in Table S1. Plasma p24 levels were measured using the p24 antigen ELISA kit (NEN, Perkin Elmer). Viral loads in EDTA-treated plasma samples were determined with the Roche Amplicor Monitor v1.5 assay (Roche Diagnostics).
HIV-1 infection of primary cells in vitro
Human cells isolated from the spleens of reconstituted mice were PHA activated for 3 days prior to infection with HIV-1BaL or HIV-1IIIB at a moi of 3. Supernatants were assayed by p24 ELISA at different times.
Analysis of TLR pathway activation
CD4+ T cells purified from healthy donor PBMC by negative selection using MACS technology were cultured in 48 well plates at 1 × 106 cells/ml in the presence of 2 μg/ml anti-CD3 mAb (clone OKT3, e-Bioscience) with 5 μg/ml Pam3CSK4, 10 μg/ml Flagellin, 5 μg/ml CLO97 (all from Invivogen), 10 μg/ml poly I:C, 10 μg/ml LPS (from Sigma Aldrich) or CD3/CD28 Dynabeads at 1 bead/T cell as described (Caron et al., 2005). scFvCD7-9R complexed to 800 pmol siLuc was added to one set of cultures. Levels of IFN-γ in 48 h culture supernatants were measured using the Quantikine Human IFN-γ Immunoassay (R&D Systems).
Analysis of cellular miRNA levels and function
CD3+ T cells were isolated from the spleens of Hu-HSC mice untreated or given three administrations, 16 h apart of scFvCD7-9R/siCCR5 (50 μg/injection) using the Dynal T cell Negative Isolation kit and small RNAs extracted using the miRNeasy mini kit (Qiagen). The small RNAs were poly(A) tailed and subjected to 3′RACE RT-PCR based real time PCR with miR RT-oligo dT and miRNA-specific or U6B RNA-specific primers as described (Wu et al., 2007.). Table S1 shows the primers used in this assay. For estimation of intracellular c-Myb levels as a measure of miR-150 function, whole cell lysates from splenic CD3+ T cells before or after activation with PHA (4 μg/ml) for 48 h were electrophoresed on 10% SDS-polyacrylamide gels (20 μg protein/lane). Western blotting was performed with antibodies to human β-actin (Cell Signaling Technology) and human c-Myb (Santa Cruz Biotechnology, Inc.) and band intensities estimated using the NIH Image J 1.37v software.
Statistical analysis
All statistical analyses comparing groups of mice were performed by one-way analysis of variance followed by Bonferroni’s post hoc test. Students t and Mann Whitney tests were used for other experiments. P<0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Changseon Choi and Nahyun Kim for technical assistance and Luke Jasenosky for editing the manuscript. This work was supported by the following grants: NIH/NIAID RO1 A1071882 and R21 AI06532-01A1 to PS; Korea Ministry of Education and Science Technology R01-2006-000-10506-0 and F104AA010005-07A0101-00510 and Seoul R&BD CR070027 to SKL, CFAR fellowship P30 A060354 to PK, NIH/NIAID Autoimmunity Prevention Center grant to DLG, NIH CA34196 to LDS, JDRF to DLG, LDS and TP, MOEHRD KRF-2006-352-D00070 to SSK, NIH/NIAID UO1 AI075419 to MN and a Wilhelm Sander Foundation research grant to GHF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson J, Li MJ, Palmer B, Remling L, Li S, Yam P, Yee JK, Rossi J, Zaia J, Akkina R. Safety and efficacy of a lentiviral vector containing three anti-HIV genes--CCR5 ribozyme, tat-rev siRNA, and TAR decoy--in SCID-hu mouse-derived T cells. Mol Ther. 2007;15:1182–1188. doi: 10.1038/sj.mt.6300157. [DOI] [PubMed] [Google Scholar]
- Banerjea A, Li MJ, Bauer G, Remling L, Lee NS, Rossi J, Akkina R. Inhibition of HIV-1 by Lentiviral Vector-Transduced siRNAs in T Lymphocytes Differentiated in SCID-hu Mice and CD34+ Progenitor Cell-Derived Macrophages. Mol Ther. 2003;8:62–71. doi: 10.1016/s1525-0016(03)00140-0. [DOI] [PubMed] [Google Scholar]
- Berges B, Wheat W, Palmer B, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gammac−/− (RAG-hu) mouse model. Retrovirology. 2006;3:76. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges BK, Akkina SR, Folkvord JM, Connick E, Akkina R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2(−/−)gammac(−/−) (RAG-hu) mice. Virology. 2008;17:17. doi: 10.1016/j.virol.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla FA, Kokron CM, Swinton P, Geha RS. Targeted gene disruption of murine CD7. Int Immunol. 1997;9:1875–1883. doi: 10.1093/intimm/9.12.1875. [DOI] [PubMed] [Google Scholar]
- Brake OT, Hooft KT, Liu YP, Centlivre M, Jasmijn von Eije K, Berkhout B. Lentiviral Vector Design for Multiple shRNA Expression and Durable HIV-1 Inhibition. Mol Ther. 2008;8:8. doi: 10.1038/sj.mt.6300382. [DOI] [PubMed] [Google Scholar]
- Bremer E, Samplonius DF, Peipp M, van Genne L, Kroesen BJ, Fey GH, Gramatzki M, de Leij LFMH, Helfrich W. Target Cell-Restricted Apoptosis Induction of Acute Leukemic T Cells by a Recombinant Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Fusion Protein with Specificity for Human CD7. Cancer Res. 2005;65:3380–3388. doi: 10.1158/0008-5472.CAN-04-2756. [DOI] [PubMed] [Google Scholar]
- Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct Stimulation of Human T Cells via TLR5 and TLR7/8: Flagellin and R-848 Up-Regulate Proliferation and IFN-{gamma} Production by Memory CD4+ T Cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JAM, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S, Lapenta C, Santini SM, Spada M, Parlato S, Logozzi M, Rizza P, Belardelli F. Human Immunodeficiency Virus Type 1 Strains R5 and X4 Induce Different Pathogenic Effects in hu-PBL-SCID Mice, Depending on the State of Activation/Differentiation of Human Target Cells at the Time of Primary Infection. J Virol. 1999;73:6453–6459. doi: 10.1128/jvi.73.8.6453-6459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- Frankel AE, Laver JH, Willingham MC, Burns LJ, Kersey JH, Vallera DA. Therapy of patients with T-cell lymphomas and leukemias using an anti-CD7 monoclonal antibody-ricin A chain immunotoxin. Leuk Lymphoma. 1997;26:287–298. doi: 10.3109/10428199709051778. [DOI] [PubMed] [Google Scholar]
- Goffinet C, Keppler OT. Efficient nonviral gene delivery into primary lymphocytes from rats and mice. FASEB J. 2006:05–4651fje. doi: 10.1096/fj.05-4651fje. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, Wood C, Dewhurst S, Gendelman HE, Poluektova L. Human Immunodeficiency Virus Type 1 Pathobiology Studied in Humanized BALB/c-Rag2−/−{gamma}c−/− Mice. J Virol. 2007;81:2700–2712. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Hamamoto K, Kida Y, Zhang Y, Shimizu T, Kuwano K. Antimicrobial activity and stability to proteolysis of small linear cationic peptides with D-amino acid substitutions. Microbiol Immunol. 2002;46:741–749. doi: 10.1111/j.1348-0421.2002.tb02759.x. [DOI] [PubMed] [Google Scholar]
- Hogrefe RI, Lebedev AV, Zon G, Pirollo KF, Rait A, Zhou Q, Yu W, Chang EH. Chemically modified short interfering hybrids (siHYBRIDS): nanoimmunoliposome delivery in vitro and in vivo for RNAi of HER-2. Nucleosides Nucleotides Nucleic Acids. 2006;25:889–907. doi: 10.1080/15257770600793885. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chainnull mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M, Constien R, Akinc A, Goldberg M, Moon YA, Spranger M, Hadwiger P, Soutschek J, Vornlocher HP, Manoharan M, et al. Effective RNAi-mediated gene silencing without interruption of the endogenous microRNA pathway. Nature. 2007;449:745–747. doi: 10.1038/nature06179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim YH, Kim SW. Cholesteryl Oligoarginine Delivering Vascular Endothelial Growth Factor siRNA Effectively Inhibits Tumor Growth in Colon Adenocarcinoma. Mol Ther. 2006;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung KE, Hee Kim M, Davidson BL, Kyung Lee S, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Lazarovits AI, Rochon J, Banks L, Hollomby DJ, Muirhead N, Jevnikar AM, White MJ, Amlot PL, Beauregard-Zollinger L, Stiller CR. Human mouse chimeric CD7 monoclonal antibody (SDZCHH380) for the prophylaxis of kidney transplant rejection. J Immunol. 1993;150:5163–5174. [PubMed] [Google Scholar]
- Lee SK, Dykxhoorn DM, Kumar P, Ranjbar S, Song E, Maliszewski LE, Francois-Bongarcon V, Goldfeld A, Swamy NM, Lieberman J, et al. Lentiviral delivery of short hairpin RNAs protects CD4 T cells from multiple clades and primary isolates of HIV. Blood. 2005;106:818–826. doi: 10.1182/blood-2004-10-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, Binder GK, Slepushkin V, Lemiale F, Mascola JR, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proceedings of the National Academy of Sciences. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsick JS, Boyle WJ. c-myb protein expression is a late event during T-lymphocyte activation. Mol Cell Biol. 1987;7:3358–3360. doi: 10.1128/mcb.7.9.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N, Kumar P, Lee SK, Shankar P. Interfering antiviral immunity: application, subversion, hope? Trends Immunol. 2006;27:328–335. doi: 10.1016/j.it.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotech. 2007;25:1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Maeda K, Miyakawa T, Shibayama S, Matsuo M, Takaoka Y, Ito M, Koyanagi Y, Mitsuya H. Potent Anti-R5 Human Immunodeficiency Virus Type 1 Effects of a CCR5 Antagonist, AK602/ONO4128/ GW873140, in a Novel Human Peripheral Blood Mononuclear Cell Nonobese Diabetic-SCID, Interleukin-2 Receptor {gamma}-Chain-Knocked-Out AIDS Mouse Model. J Virol. 2005;79:2087–2096. doi: 10.1128/JVI.79.4.2087-2096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK, Collman RG, Lieberman J, Shankar P, Sharp PA. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8:681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc Natl Acad Sci U S A. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipp M, Kupers H, Saul D, Schlierf B, Greil J, Zunino SJ, Gramatzki M, Fey GH. A Recombinant CD7-specific Single-Chain Immunotoxin Is a Potent Inducer of Apoptosis in Acute Leukemic T Cells. Cancer Res. 2002;62:2848–2855. [PubMed] [Google Scholar]
- Rossi JJ, June CH, Kohn DB. Genetic therapies against HIV. Nat Biotech. 2007;25:1444–1454. doi: 10.1038/nbt1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer L, Rossi JJ, Weinberg MS. Progress and prospects: RNA-based therapies for treatment of HIV infection. Gene Ther. 2007;14:1057–1064. doi: 10.1038/sj.gt.3302977. [DOI] [PubMed] [Google Scholar]
- Shacklett BL. Can the New Humanized Mouse Model Give HIV Research a Boost. PLoS Medicine. 2008;5:e13. doi: 10.1371/journal.pmed.0050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P, Manjunath N, Lieberman J. The prospect of silencing disease using RNA interference. Jama. 2005;293:1367–1373. doi: 10.1001/jama.293.11.1367. [DOI] [PubMed] [Google Scholar]
- Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, Crawford K, Cerny J, Sharp PA, Lieberman J, Manjunath N, et al. Sustained Small Interfering RNA-Mediated Human Immunodeficiency Virus Type 1 Inhibition in Primary Macrophages. J Virol. 2003a;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003b;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotech. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- Surabhi RM, Gaynor RB. RNA interference directed against viral and cellular targets inhibits human immunodeficiency Virus Type 1 replication. J Virol. 2002;76:12963–12973. doi: 10.1128/JVI.76.24.12963-12973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P. Off-targeting and other non-specific effects of RNAi experiments in mammalian cells. Curr Opin Mol Ther. 2007;9:248–257. [PubMed] [Google Scholar]
- ter Brake O, Konstantinova P, Ceylan M, Berkhout B. Silencing of HIV-1 with RNA interference: a multiple shRNA approach. Mol Ther. 2006;14:883–892. doi: 10.1016/j.ymthe.2006.07.007. [DOI] [PubMed] [Google Scholar]
- von Eije KJ, ter Brake O, Berkhout B. HIV-1 escape is restricted when conserved genome sequences are targeted by RNA interference. J Virol. 2007:JVI.02035–02007. doi: 10.1128/JVI.02035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Zeng L, Chen L, Huang Q, Li S, Lu Y, Li Y, Cheng J, Lu X. Expression, purification, and refolding of a novel immunotoxin containing humanized single-chain fragment variable antibody against CTLA4 and the N-terminal fragment of human perforin. Protein Expression and Purification. 2006;48:307–313. doi: 10.1016/j.pep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Ohta S, Yajima M, Terashima K, Ito M, Mugishima H, Fujiwara S, Shimizu K, Honda M, Shimizu N, et al. Humanized NOD/SCID/IL2Rgamma(null) mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol. 2007;81:13259–13264. doi: 10.1128/JVI.01353-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, Manjunath N. miRNA Profiling of Native, Effector and Memory CD8 T Cells. PLoS ONE. 2007;2:e1020. doi: 10.1371/journal.pone.0001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Zeng F, Peritz T, Kannanayakal TJ, Kilk K, Eiriksdottir E, Langel U, Eberwine J. A protocol for PAIR: PNA-assisted identification of RNA binding proteins in living cells. Nat Protocols. 2006;1:920–927. doi: 10.1038/nprot.2006.81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.