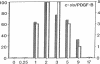
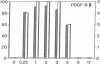
Abstract
The present studies investigated the in vivo expression of the p53 suppressor gene and protein product in response to acute cutaneous injury in swine, along with the parallel expression of the c-sis/PDGF-B mitogen and its receptor beta (PDGF-R beta). p53 expression was shown to be suppressed during the period of active cellular proliferation in the injured tissue and to reemerge during the stages of healing. In contrast, c-sis/PDGF-B and PDGF-R beta were expressed during the early phase of active cellular proliferation and they were suppressed upon healing. This inverse relationship between mitogenic growth factors and p53 suggests the presence of well-controlled physiologic mechanisms that regulate in vivo the processes of normal tissue repair in response to injury. At the stages of tissue regeneration, these mechanisms include both the expression of growth factors that promote cell proliferation and the suppression of p53 that downregulates proliferation. At the stages of healing, the expression of the mitogenic growth factors is suppressed and that of p53 reemerges, reaching its peak at the time of complete epithelialization and healing of the injured tissue. These studies are the first to link the response of p53 protein to physiologic processes of tissue regeneration in vivo.
Full text
PDF




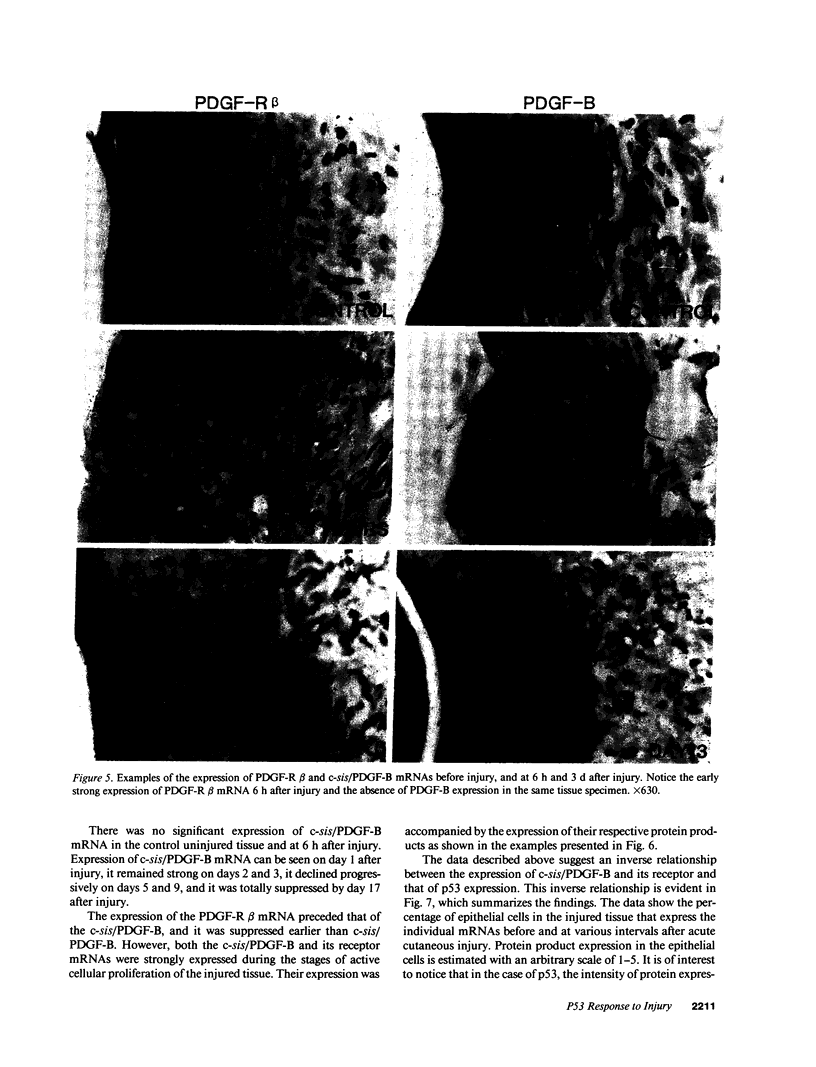
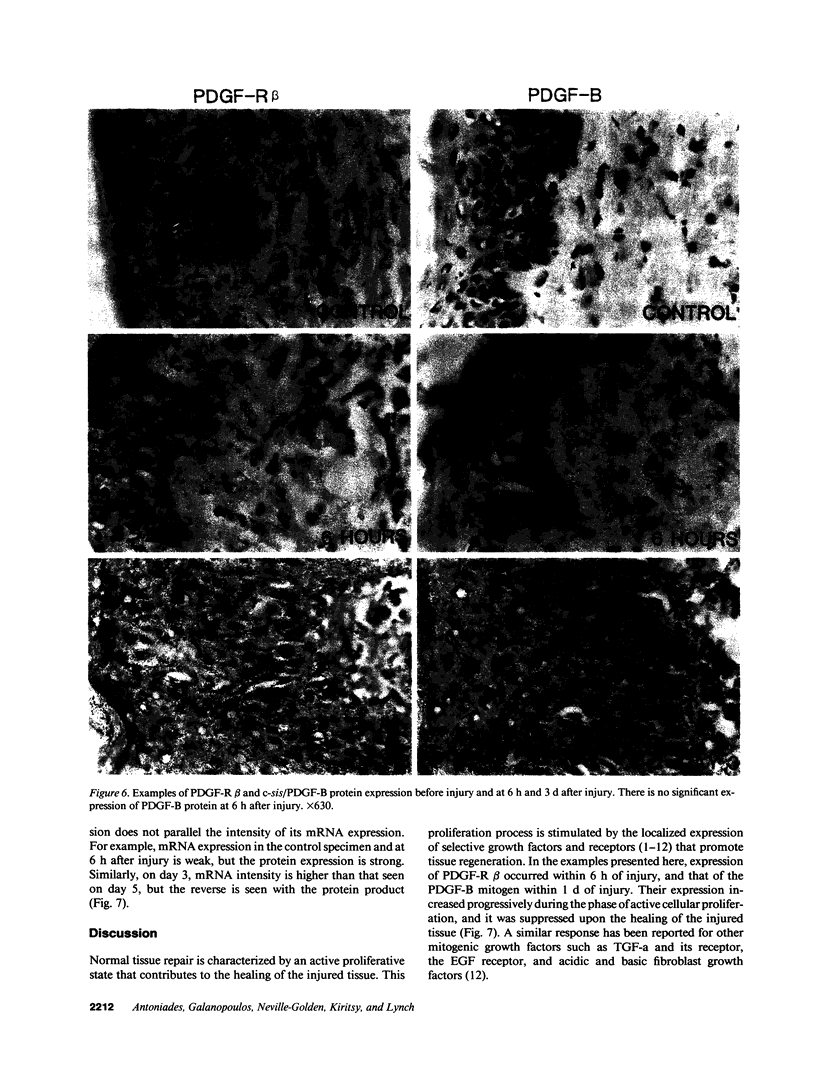


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. Expression of growth factor and receptor mRNAs in skin epithelial cells following acute cutaneous injury. Am J Pathol. 1993 Apr;142(4):1099–1110. [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N. PDGF: a multifunctional growth factor. Baillieres Clin Endocrinol Metab. 1991 Dec;5(4):595–613. doi: 10.1016/s0950-351x(10)80005-9. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bargonetti J., Friedman P. N., Kern S. E., Vogelstein B., Prives C. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell. 1991 Jun 14;65(6):1083–1091. doi: 10.1016/0092-8674(91)90560-l. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Danscher G., Nörgaard J. O. Light microscopic visualization of colloidal gold on resin-embedded tissue. J Histochem Cytochem. 1983 Dec;31(12):1394–1398. doi: 10.1177/31.12.6631001. [DOI] [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Mechta F., Yaniv M., Oren M. Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Michael-Michalovitz D., Ginsberg D., Oren M. Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decreased stability of the protein. Mol Cell Biol. 1991 Jan;11(1):582–585. doi: 10.1128/mcb.11.1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M. A., Au Y. P., Kenagy R. D., Clowes A. W. Growth factor gene expression by intimal cells in healing polytetrafluoroethylene grafts. J Vasc Surg. 1990 Apr;11(4):580–585. [PubMed] [Google Scholar]
- Grotendorst G. R., Grotendorst C. A., Gilman T. Production of growth factors (PDGF & TGF-beta) at the site of tissue repair. Prog Clin Biol Res. 1988;266:47–54. [PubMed] [Google Scholar]
- Grotendorst G. R., Soma Y., Takehara K., Charette M. EGF and TGF-alpha are potent chemoattractants for endothelial cells and EGF-like peptides are present at sites of tissue regeneration. J Cell Physiol. 1989 Jun;139(3):617–623. doi: 10.1002/jcp.1041390323. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Jennische E., Skottner A. Regenerating endothelial cells express insulin-like growth factor-I immunoreactivity after arterial injury. Cell Tissue Res. 1987 Dec;250(3):499–505. doi: 10.1007/BF00218940. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Jonsson R., Petruson K. Transiently increased insulin-like growth factor. I. Immunoreactivity in UVB-irradiated mouse skin. J Invest Dermatol. 1988 Oct;91(4):328–332. doi: 10.1111/1523-1747.ep12475660. [DOI] [PubMed] [Google Scholar]
- Hoefler H., Childers H., Montminy M. R., Lechan R. M., Goodman R. H., Wolfe H. J. In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J. 1986 Nov-Dec;18(11-12):597–604. doi: 10.1007/BF01675295. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Jennische E., Skottner A., Hansson H. A. Dynamic changes in insulin-like growth factor I immunoreactivity correlate to repair events in rat ear after freeze-thaw injury. Exp Mol Pathol. 1987 Oct;47(2):193–201. doi: 10.1016/0014-4800(87)90074-8. [DOI] [PubMed] [Google Scholar]
- Kane C. J., Knapp A. M., Mansbridge J. N., Hanawalt P. C. Transforming growth factor-beta 1 localization in normal and psoriatic epidermal keratinocytes in situ. J Cell Physiol. 1990 Jul;144(1):144–150. doi: 10.1002/jcp.1041440119. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Key G., Becker M. H., Baron B., Duchrow M., Schlüter C., Flad H. D., Gerdes J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest. 1993 Jun;68(6):629–636. [PubMed] [Google Scholar]
- Kley N., Chung R. Y., Fay S., Loeffler J. P., Seizinger B. R. Repression of the basal c-fos promoter by wild-type p53. Nucleic Acids Res. 1992 Aug 11;20(15):4083–4087. doi: 10.1093/nar/20.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Lin D., Shields M. T., Ullrich S. J., Appella E., Mercer W. E. Growth arrest induced by wild-type p53 protein blocks cells prior to or near the restriction point in late G1 phase. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9210–9214. doi: 10.1073/pnas.89.19.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. E., Colvin R. B., Antoniades H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989 Aug;84(2):640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Lamb P., Pim D., Peacock J., Crawford L., Benchimol S. Isolation and characterization of a human p53 cDNA clone: expression of the human p53 gene. EMBO J. 1984 Dec 20;3(13):3257–3262. doi: 10.1002/j.1460-2075.1984.tb02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Shields M. T., Amin M., Sauve G. J., Appella E., Romano J. W., Ullrich S. J. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Shields M. T., Lin D., Appella E., Ullrich S. J. Growth suppression induced by wild-type p53 protein is accompanied by selective down-regulation of proliferating-cell nuclear antigen expression. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1958–1962. doi: 10.1073/pnas.88.5.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Milner J., Cook A., Sheldon M. A new anti-p53 monoclonal antibody, previously reported to be directed against the large T antigen of simian virus 40. Oncogene. 1987;1(4):453–455. [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M., Wang Y., Mayr G., Anderson M. E., Schwedes J. F., Tegtmeyer P. p53 domains: suppression, transformation, and transactivation. Gene Expr. 1993;3(1):95–107. [PMC free article] [PubMed] [Google Scholar]
- Santhanam U., Ray A., Sehgal P. B. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S. J., Anderson C. W., Mercer W. E., Appella E. The p53 tumor suppressor protein, a modulator of cell proliferation. J Biol Chem. 1992 Aug 5;267(22):15259–15262. [PubMed] [Google Scholar]
- Unger T., Mietz J. A., Scheffner M., Yee C. L., Howley P. M. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol Cell Biol. 1993 Sep;13(9):5186–5194. doi: 10.1128/mcb.13.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T., Nau M. M., Segal S., Minna J. D. p53: a transdominant regulator of transcription whose function is ablated by mutations occurring in human cancer. EMBO J. 1992 Apr;11(4):1383–1390. doi: 10.1002/j.1460-2075.1992.tb05183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Werner S., Peters K. G., Longaker M. T., Fuller-Pace F., Banda M. J., Williams L. T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991 Sep;147(1):207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti G. P., Bargonetti J., Walker K., Prives C., Levine A. J. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 1992 Jul;6(7):1143–1152. doi: 10.1101/gad.6.7.1143. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Levine A. J. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993 Jul;7(10):855–865. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]