Abstract
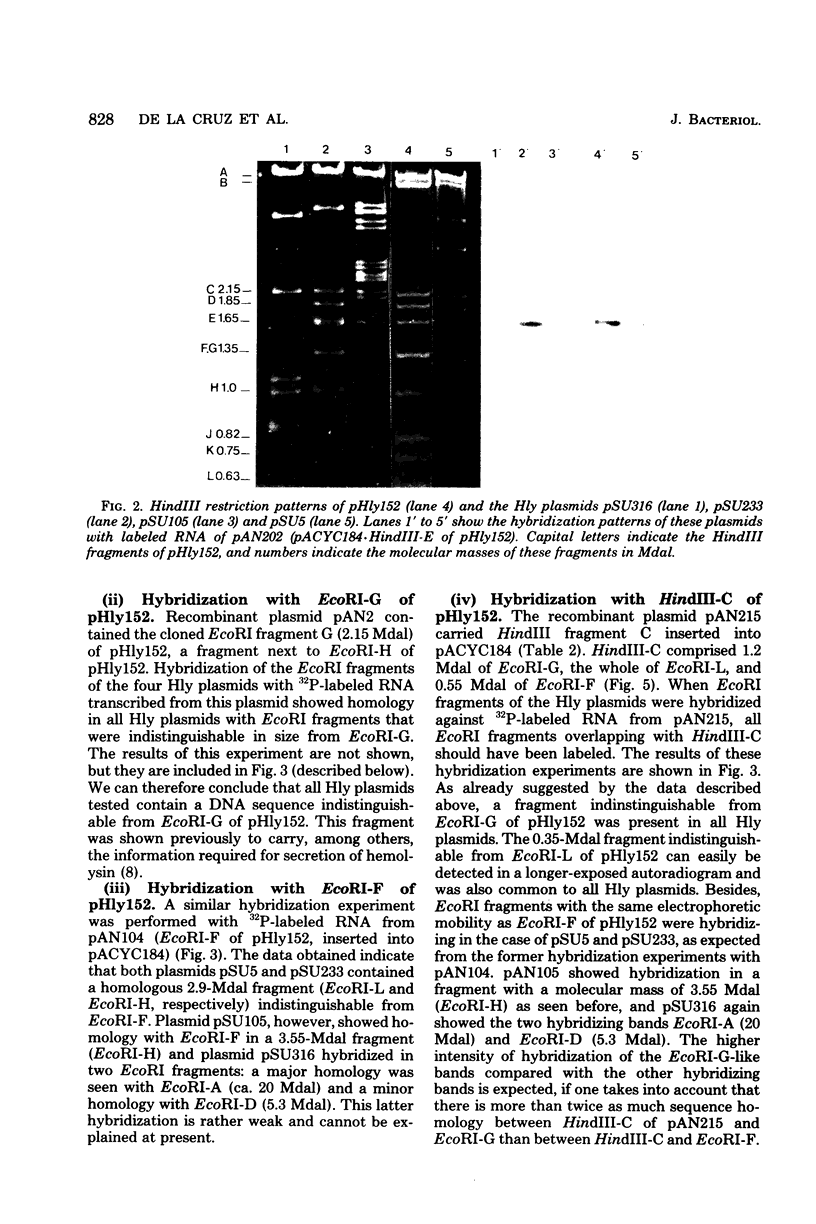
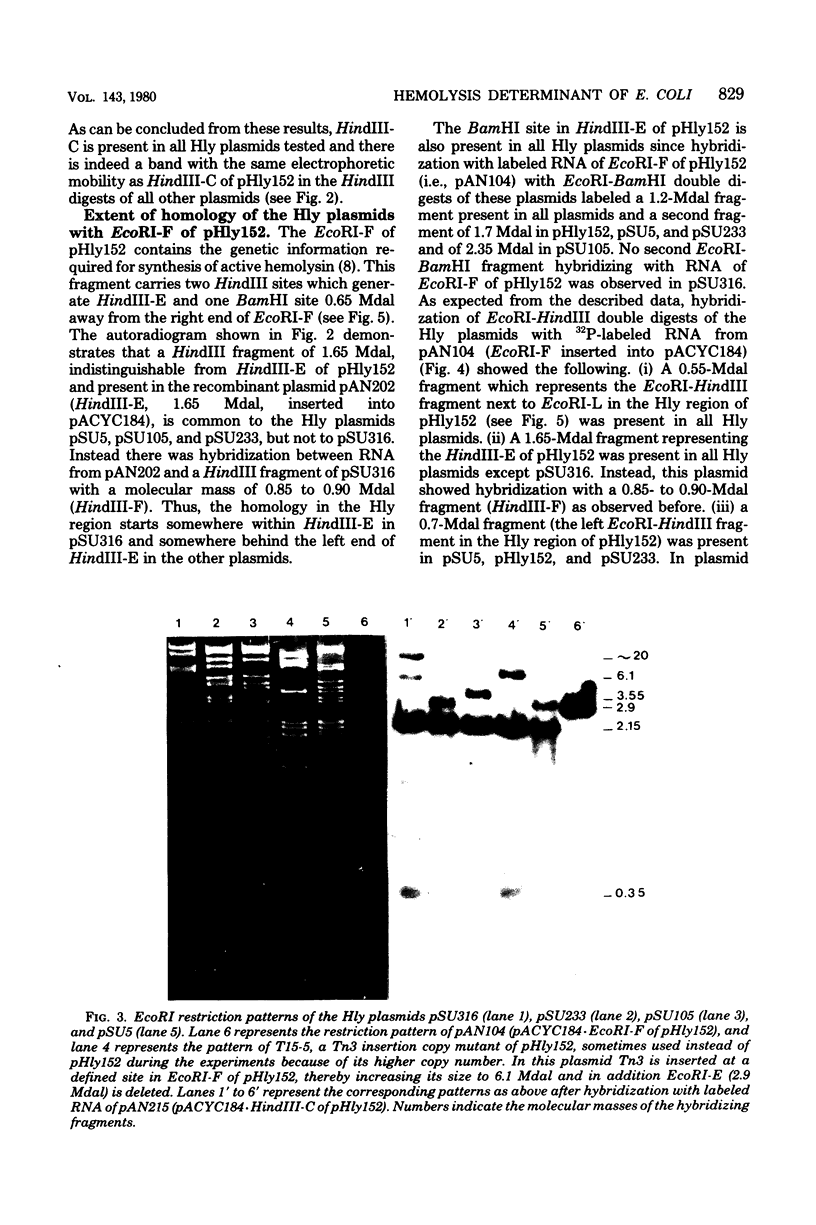
By using cloned deoxyribonucleic acid fragments from the hemolysis determinant of the hemolytic plasmid pHly152 as hybridization probes, a deoxyribonucleic acid segment of about 3.8 megadaltons was identified as a common sequence in several hemolytic (Hly) plasmids of Escherichia coli belonging in four different incompatibility groups. This segment contained the genetic information for the synthesis and secretion of the extracellular toxin alpha-hemolysin of E. coli. With the exception of pSU5, representing a composite plasmid, one part of which seems to be very similar to pHly152, the overall sequence homology of these Hly plasmids with pHly152 seems to be rather restricted. However, the Hly plasmid pSU316 showed sequence homology with pHly152 that did not extend beyond the hemolysis determinant. The two other plasmids, pSU233 and pSU105, also shared homology with pHly152 in the hemolysis determinant as well as in various other parts of this plasmid which did not seem to be directly linked to the hemolysis determinant. This suggests that the hemolysis determinant has spread to presumably unrelated plasmids of E. coli.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Royer-Pokora B., Lindenmaier W., Bujard H. Plasmids controlling synthesis of hemolysin in Escherichia coli: molecular properties. J Bacteriol. 1974 Jun;118(3):964–973. doi: 10.1128/jb.118.3.964-973.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Isolation and characterization of supercoiled circular deoxyribonucleic acid from beta-hemolytic strains of Escherichia coli. J Bacteriol. 1971 May;106(2):311–317. doi: 10.1128/jb.106.2.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minor S., Le Coueffic E. Etude sur les hémolysines des enterobacteriaceae. Ann Microbiol (Paris) 1975 Oct-Nov;126(3):313–332. [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Springer W., Goebel W. Plasmid cistrons controlling synthesis and excretion of the exotoxin alpha-haemolysin of Escherichia coli. Mol Gen Genet. 1979 Oct 1;175(3):343–350. doi: 10.1007/BF00397234. [DOI] [PubMed] [Google Scholar]
- Oertel W., Kollek R., Beck E., Goebel W. The nucleotide sequence of a DNA fragment from the replication origin of the antibiotic resistance factor R1drd19. Mol Gen Genet. 1979 Mar 27;171(3):277–285. doi: 10.1007/BF00267582. [DOI] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Goebel W. Plasmids controlling synthesis of hemolysin in Escherichia coli. II. Polynucleotide sequence relationship among hemolytic plasmids. Mol Gen Genet. 1976 Mar 22;144(2):177–183. doi: 10.1007/BF02428106. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- So M., Heffron F., McCarthy B. J. The E. coli gene encoding heat stable toxin is a bacterial transposon flanked by inverted repeats of IS1. Nature. 1979 Feb 8;277(5696):453–456. doi: 10.1038/277453a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Zabala J. C., Ortiz J. M. Incompatibility among alpha-hemolytic plasmids studied after inactivation of the alpha-hemolysin gene by transposition of Tn802. Plasmid. 1979 Oct;2(4):507–519. doi: 10.1016/0147-619x(79)90050-7. [DOI] [PubMed] [Google Scholar]