Abstract
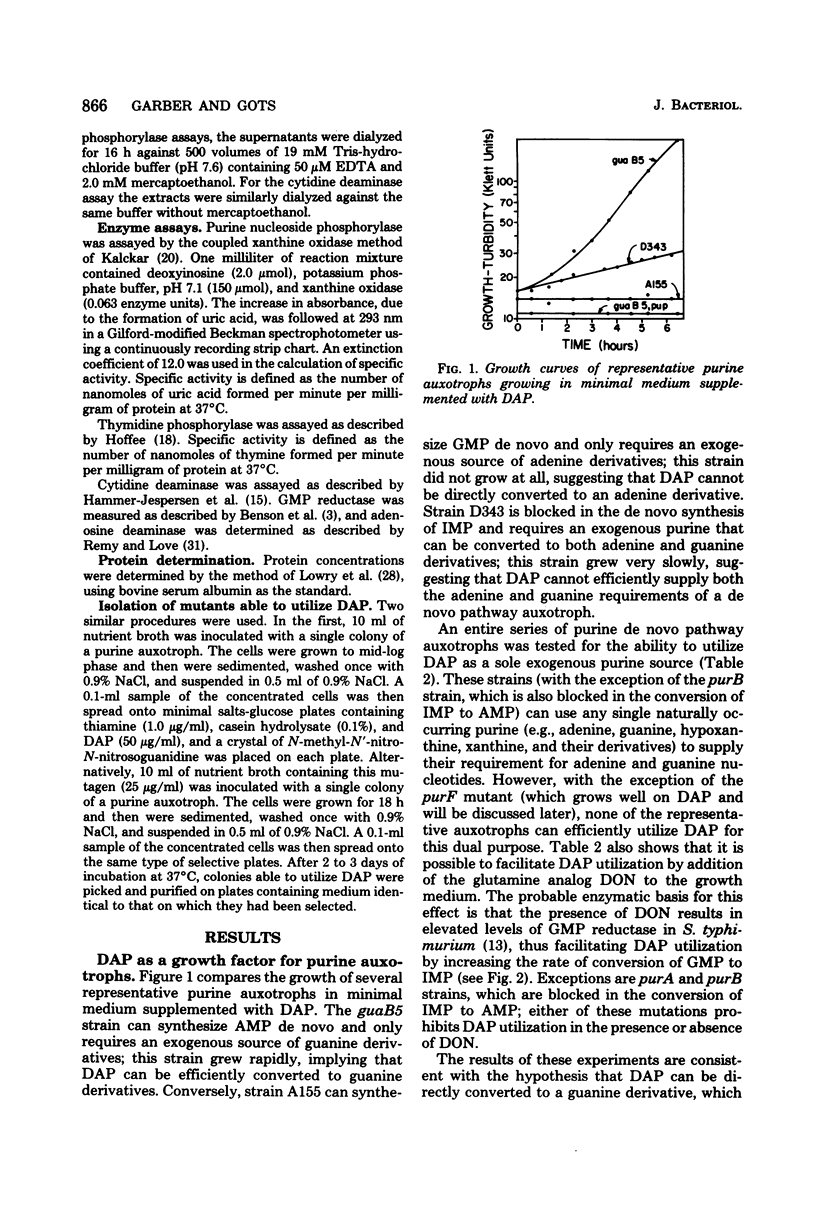
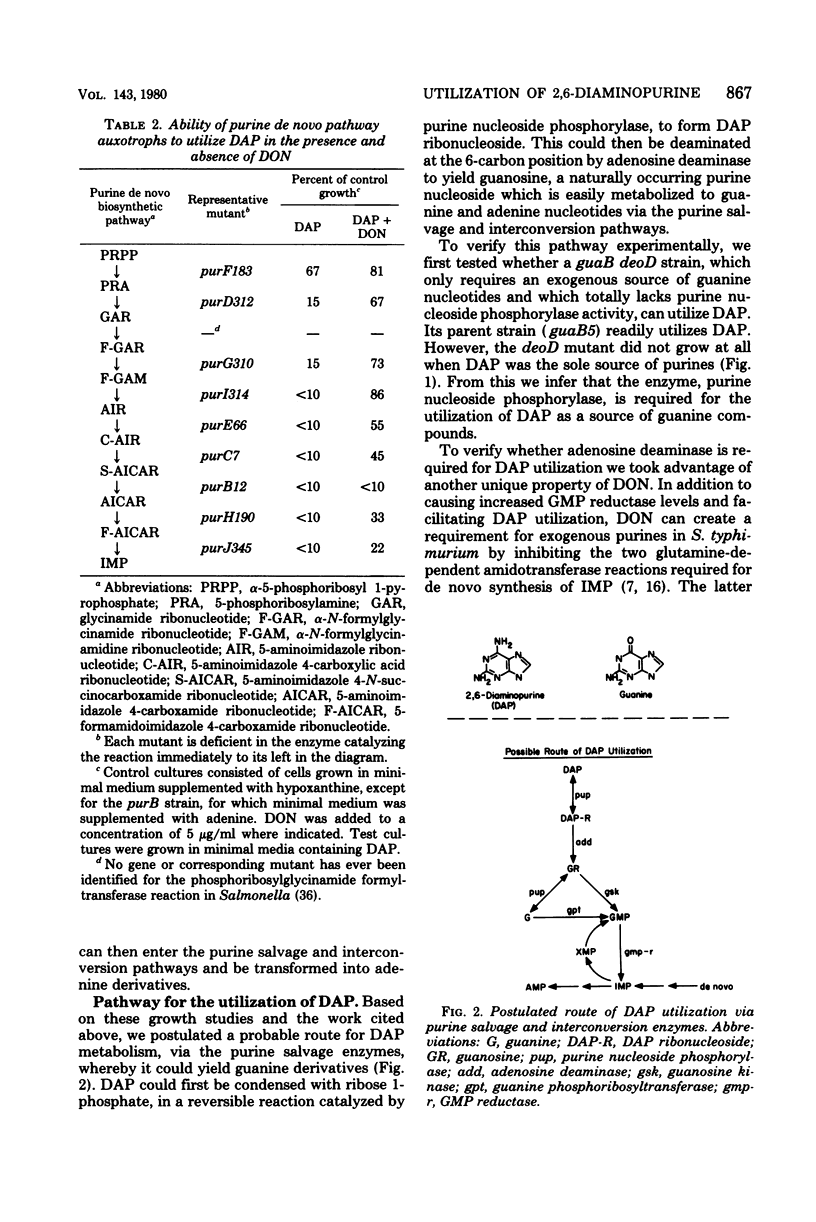
The pathway for the utilization of 2,6-diaminopurine (DAP) as an exogenous purine source in Salmonella typhimurium was examined. In strains able to use DAP as a purine source, mutant derivatives lacking either purine nucleoside phosphorylase or adenosine deaminase activity lost the ability to do so. The implied pathway of DAP utilization was via its conversion to DAP ribonucleoside by purine nucleoside phosphorylase, followed by deamination to guanosine by adenosine deaminase. Guanosine can then enter the established purine salvage pathways. In the course of defining this pathway, purine auxotrophs able to utilize DAP as sole purine source were isolated and partially characterized. These mutants fell into several classes, including (i) strains that only required an exogenous source of guanine nucleotides (e.g., guaA and guaB strains); (ii) strains that had a purF genetic lesion (i.e., were defective in alpha-5-phosphoribosyl 1-pyrophosphate amidotransferase activity); and (iii) strains that had constitutive levels of purine nucleoside phosphorylase. Selection among purine auxotrophs blocked in the de novo synthesis of inosine 5'-monophosphate, for efficient growth on DAP as sole source of purine nucleotides, readily yielded mutants which were defective in the regulation of their deoxyribonucleoside-catabolizing enzymes (e.g., deoR mutants).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALIS M. E., BROOKE M. S., BROWN G. B., MAGASANIK B. The utilization of purines by purineless mutants of Aerobacter aerogenes. J Biol Chem. 1956 Apr;219(2):917–926. [PubMed] [Google Scholar]
- BALIS M. E., LEVIN D. H., BROWN G. B., ELION G. B., VANDERWERFF H., HITCHINGS G. H. The incorporation of exogenous purines into pentose nucleic acid by Lactobacillus casei. J Biol Chem. 1952 May;196(2):729–747. [PubMed] [Google Scholar]
- Benson C. E., Brehmeyer B. A., Gots J. S. Requirement of cyclic AMP for induction of GMP reductase in Escherichia coli. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1089–1094. doi: 10.1016/0006-291x(71)90573-0. [DOI] [PubMed] [Google Scholar]
- Benson C. E., Gots J. S. Regulation of GMP reductase in Salmonella typhimurium. Biochim Biophys Acta. 1975 Sep 22;403(1):47–57. doi: 10.1016/0005-2744(75)90007-8. [DOI] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank J., Hoffee P. Regulatory mutants of the deo regulon in Salmonella typhimurium. Mol Gen Genet. 1972;116(4):291–298. doi: 10.1007/BF00270086. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Purine phosphoribosyltransferases of Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):1010–1013. doi: 10.1128/jb.112.2.1010-1013.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington A. Some substrates and inhibitors of adenosine deaminase. Biochim Biophys Acta. 1965 Jun 22;99(3):442–451. doi: 10.1016/s0926-6593(65)80198-9. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Shigeura H. T. Dependence of diaminopurine utilization on the mutational site of purine auxotrophy in Bacillus subtilis. 1. Nutritional experiments. J Bacteriol. 1968 Feb;95(2):565–571. doi: 10.1128/jb.95.2.565-571.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELION G. B., HITCHINGS G. H. Antagonists of nucleic acid derivatives. IV. Reversal studies with 2-aminopurine and 2,6-diaminopurine. J Biol Chem. 1950 Dec;187(2):511–522. [PubMed] [Google Scholar]
- Frederiksen S. Specificity of adenosine deaminase toward adenosine and 2'-deoxyadenosine analogues. Arch Biochem Biophys. 1966 Feb;113(2):383–388. doi: 10.1016/0003-9861(66)90202-5. [DOI] [PubMed] [Google Scholar]
- GOTS J. S., GOLLUB E. G. Purine analogs as feedback inhibitors. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:641–643. doi: 10.3181/00379727-101-25045. [DOI] [PubMed] [Google Scholar]
- Garber B. B., Jochimsen B. U., Gots J. S. Glutamine and related analogs regulate guanosine monophosphate reductase in Salmonella typhimurium. J Bacteriol. 1980 Jul;143(1):105–111. doi: 10.1128/jb.143.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN S. C. THE INTERACTION OF 6-DIAZO-5-OXO-L-NORLEUCINE WITH PHOSPHORIBOSYL PYROPHOSPHATE AMIDOTRANSFERASE. J Biol Chem. 1963 Sep;238:3036–3047. [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A., Schwartz M., Nygaard P. Induction of enzymes involed in the catabolism of deoxyribonucleosides and ribonucleosides in Escherichia coli K 12. Eur J Biochem. 1971 Apr 30;19(4):533–538. doi: 10.1111/j.1432-1033.1971.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Hoffee P. A. 2-deoxyribose gene-enzyme complex in Salmonella typhimurium. I. Isolation and enzymatic characterization of 2-deoxyribose-negative mutants. J Bacteriol. 1968 Feb;95(2):449–457. doi: 10.1128/jb.95.2.449-457.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer J., Neuhard J. Metabolism of exogenous purine bases and nucleosides by Salmonella typhimurium. J Bacteriol. 1971 Apr;106(1):14–24. doi: 10.1128/jb.106.1.14-24.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Alterations in purine nucleotide pyrophosphorylases and resistance to purine analogues. Biochim Biophys Acta. 1961 Oct 14;53:166–173. doi: 10.1016/0006-3002(61)90803-4. [DOI] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Antagonisms between purines and purine analogues in auxotrophs of Salmonella typhimurium. J Bacteriol. 1961 Mar;81:331–337. doi: 10.1128/jb.81.3.331-337.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. GENETIC ALTERATION OF ADENYLIC PYROPHOSPHORYLASE IN SALMONELLA. Science. 1963 Nov 8;142(3593):680–681. doi: 10.1126/science.142.3593.680. [DOI] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Mechanism of resistance to 2,6-diaminopurine in Salmonella typhimurium. Biochim Biophys Acta. 1961 Jul 22;51:130–137. doi: 10.1016/0006-3002(61)91023-x. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic phosphorylation of adenosine and 2,6-diaminopurine riboside. J Biol Chem. 1951 Dec;193(2):481–495. [PubMed] [Google Scholar]
- KORN E. D., BUCHANAN J. M. Biosynthesis of the purines. VI. Purification of liver nucleoside phosphorylase and demonstration of nucleoside synthesis from 4-amino-5-imidazolecarboxamide, adenine, and 2, 6-diaminopurine. J Biol Chem. 1955 Nov;217(1):183–191. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Gal M. L., Le Gal Y., Roche J., Hedegaard J. Purine biosynthesis: enzymatic formation of ribosylamine-5-phosphate from ribose-5-phosphate and ammonia. Biochem Biophys Res Commun. 1967 Jun 23;27(6):618–624. doi: 10.1016/s0006-291x(67)80079-2. [DOI] [PubMed] [Google Scholar]
- NIERLICH D. P., MAGASANIK B. PHOSPHORIBOSYLGLYCINAMIDE SYNTHETASE OF AEROBACTER AEROGENES. PURIFICATION AND PROPERTIES, AND NONENZYMATIC FORMATION OF ITS SUBSTRATE 5'-PHOSPHORIBOSYLAMINE. J Biol Chem. 1965 Jan;240:366–374. [PubMed] [Google Scholar]
- Nygaard P. Nucleoside-catabolizing enzymes in Salmonella typhimurium. Introduction by ribonucleosides. Eur J Biochem. 1973 Jul 2;36(1):267–272. doi: 10.1111/j.1432-1033.1973.tb02909.x. [DOI] [PubMed] [Google Scholar]
- REMY C. N., SMITH M. S. Metabolism of 2, 6-diaminopurine; conversion to 5'-phosphoribosyl-2-methylamino-beta-aminopurine by enzymes of Escherichia coli. J Biol Chem. 1957 Sep;228(1):325–338. [PubMed] [Google Scholar]
- Remy C. N., Love S. H. Induction of adenosine deaminase in Escherichia coli. J Bacteriol. 1968 Jul;96(1):76–85. doi: 10.1128/jb.96.1.76-85.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeura H. T., Demain A. L. Dependence of diaminopurine utilization on the mutational site of purine auxotrophy in Bacillus subtilis. II. Tracer experiments. J Bacteriol. 1968 Feb;95(2):572–577. doi: 10.1128/jb.95.2.572-577.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Valentin-Hansen P., Hammer-Jespersen K., Buxton R. S. Evidence for the existence of three promoters for the deo operon of Escherichia coli K12 in vitro. J Mol Biol. 1979 Sep 5;133(1):1–17. doi: 10.1016/0022-2836(79)90248-1. [DOI] [PubMed] [Google Scholar]
- Westby C. A., Gots J. S. Genetic blocks and unique features in the biosynthesis of 5'-phosphoribosyl-N-formylglycinamide in Salmonella typhimurium. J Biol Chem. 1969 Apr 25;244(8):2095–2102. [PubMed] [Google Scholar]
- Wheeler G. P., Bowdon B. J. Identification of analogues of nicotinamide adenine dinucleotide among the metabolites of 2,6-diaminopurine in mammalian cells. J Biol Chem. 1966 Mar 10;241(5):1114–1121. [PubMed] [Google Scholar]


