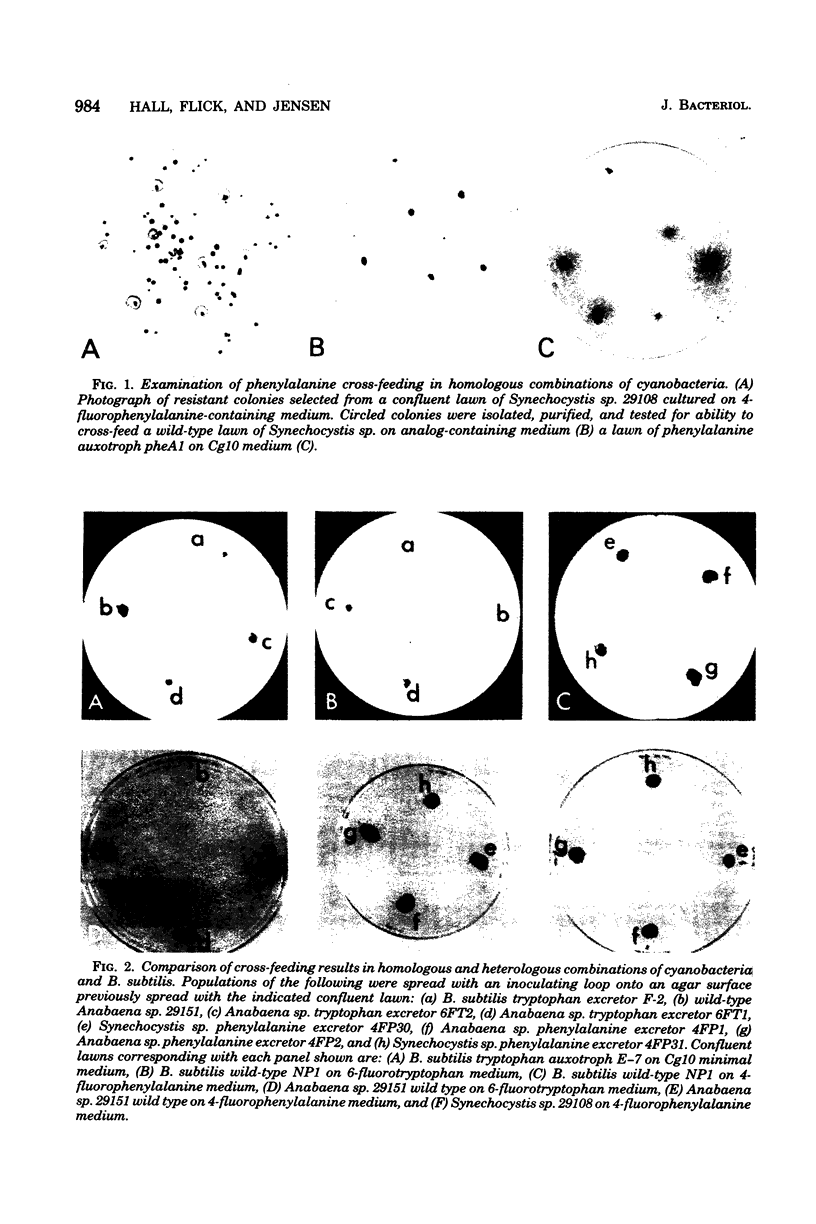
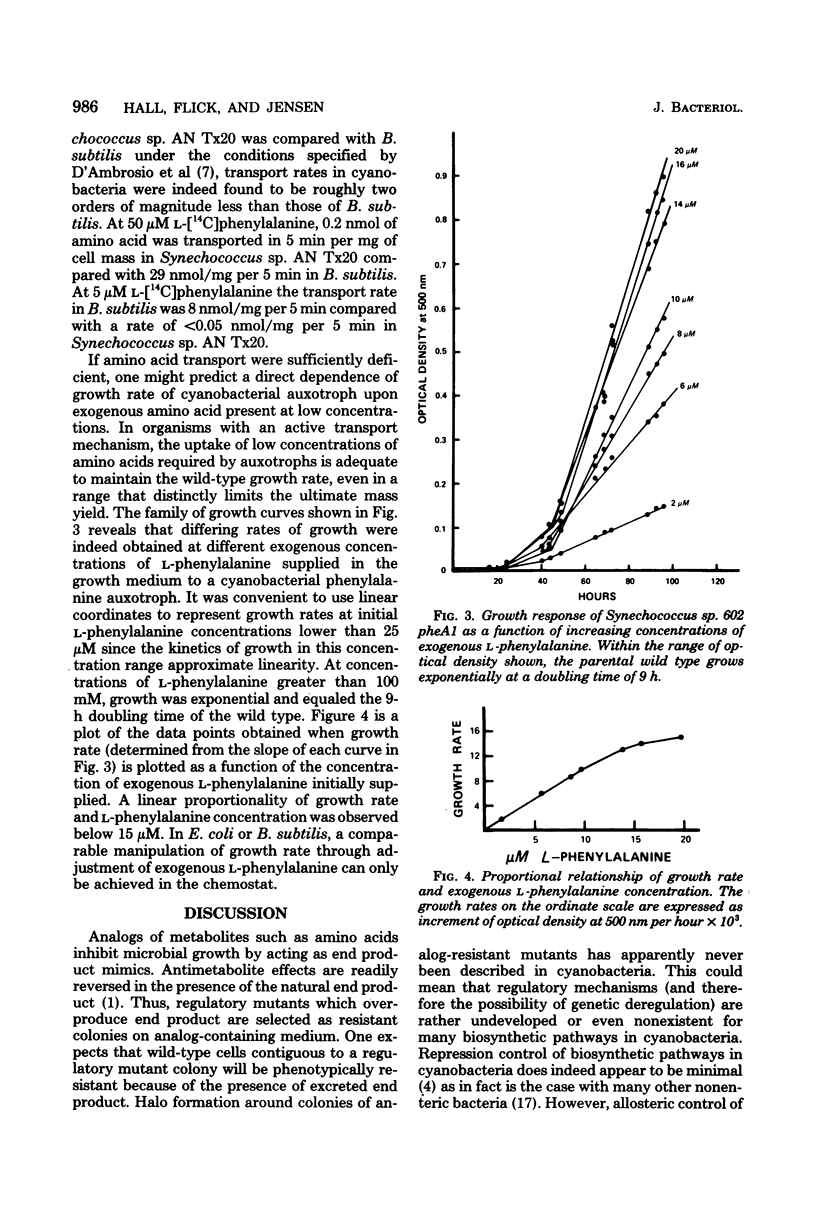
Abstract
Antimetabolite analogs of essential amino acids are useful as selective agents for isolation of regulatory mutants of cyanobacteria, although we observed striking microbiological differences from other widely used eubacterial systems. Regulatory mutants shown to overproduce and excrete tryptophan, phenylalanine, tyrosine, methionine, or arginine were isolated from four cyanobacteria: Anabaena sp. 29151, Synechococcus sp. 602, Synechococcus sp. AN Tx20, and Synechocystis sp. 29108. Surprisingly, regulatory-mutant colonies did not support a halo of cross-fed wild-type growth on selective medium. Since regulatory mutants were shown to excrete substantial levels of amino acids, it was deduced that poor cross-feeding must reflect a generally low nutritional responsiveness of the cyanobacterial background. This conclusion was confirmed by results which showed that regulatory-mutant cells of cyanobacteria dispersed among wild-type populations of Bacillus subtilis did produce halo colonies on solid analog-containing medium. Cross-feeding between one cyanobacterial pair (a phenylalanine excretor and a phenylalanine auxotroph) was successfully demonstrated in the absence of the analog under conditions in which relatively large masses of each cell population type were spread near one another on agar plates. These results suggest that amino acid excreted by regulatory mutants of cyanobacteria on analog-containing selective medium is transported into nearby wild-type cells too inefficiently to overcome the antimetabolite effects of the analog, thereby failing to generate halos of physiologically resistant background cells. Consistent with this interpretation was the finding that the pheA1 auxotroph from Synechococcus sp. 602 exhibited a linearly proportional dependence of growth rate upon exogenous concentration of l-phenylalanine (below 20 μM). Wild-type B. subtilis serves as a convenient and sensitive test lawn for screening obvious regulatory mutants from among collections of analog-resistant cyanobacterial mutants. Appropriate B. subtilis auxotrophs can be used as convenient indicator strains for the identification of regulatory mutants in cyanobacteria through the observation of syntrophic growth responses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A. Selection of bacterial mutants which excrete antagonists of antimetabolites. J Bacteriol. 1958 Sep;76(3):326–326. doi: 10.1128/jb.76.3.326-326.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Champney W. S., Jensen R. A. D-Tyrosine as a metabolic inhibitor of Bacillus subtilis. J Bacteriol. 1969 Apr;98(1):205–214. doi: 10.1128/jb.98.1.205-214.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. N. Regulation of enzyme activity in microorganisms. Annu Rev Microbiol. 1965;19:105–126. doi: 10.1146/annurev.mi.19.100165.000541. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Glover G. I., Nelson S. O., Jensen R. A. Specificity of the tyrosine-phenylalanine transport system in Bacillus subtilis. J Bacteriol. 1973 Aug;115(2):673–681. doi: 10.1128/jb.115.2.673-681.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Metabolic interlock. Regulatory interactions exerted between biochemical pathways. J Biol Chem. 1969 Jun 10;244(11):2816–2823. [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. Metabolic interlock. The influence of histidine on tryptophan biosynthesis in Bacillus subtilis. J Biol Chem. 1970 May 10;245(9):2384–2390. [PubMed] [Google Scholar]
- Pelroy R. A., Rippka R., Stanier R. Y. Metabolism of glucose by unicellular blue-green algae. Arch Mikrobiol. 1972;87(4):303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- Phares W., Chapman L. F. Anacystis nidulans mutants resistant to aromatic amino acid analogues. J Bacteriol. 1975 Jun;122(3):943–948. doi: 10.1128/jb.122.3.943-948.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello J. L., Jensen R. A. Metabolic interlock. The multi-metabolite control of prephenate dehydratase activity in Bacillus subtilis. J Biol Chem. 1970 Aug 10;245(15):3738–3744. [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Specialist phototrophs, lithotrophs, and methylotrophs: a unity among a diversity of procaryotes? Bacteriol Rev. 1977 Jun;41(2):419–448. doi: 10.1128/br.41.2.419-448.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG P. W., O'FLYNN M. E., INOUYE T. MICROMETHODS FOR MEASURING PHENYLALANINE AND TYROSINE IN SERUM. Clin Chem. 1964 Dec;10:1098–1104. [PubMed] [Google Scholar]




