Abstract
Early epithelial restitution is an important repair modality in the gut mucosa and occurs as a consequence of epithelial cell migration. Canonical transient receptor potential-1 (TRPC1) functions as a store-operated Ca2+ channel (SOCs) in intestinal epithelial cells (IECs) and regulates intestinal restitution, but the exact upstream signals initiating TRPC1 activation after mucosal injury remain elusive. Stromal interaction molecule 1 (STIM1) is a single membrane-spanning protein and is recently identified as essential components of SOC activation. The current study was performed to determine whether STIM1 plays a role in the regulation of intestinal epithelial restitution by activating TRPC1 channels. STIM1 translocation to the plasma membrane increased after wounding, which was followed by an increase in IEC migration to reseal wounds. Increased STIM1 levels at the plasma membrane by overexpressing EF-hand mutant STIM1 enhanced Ca2+ influx through SOCs and stimulated IEC migration after wounding. STIM1 interacted with TRPC1 and formed STIM1/TRPC1 complex, whereas inactivation of STIM1 by STIM1 silencing decreased SOC-mediated Ca2+ influx and inhibited epithelial restitution. In cells overexpressing EF-hand mutant STIM1, TRPC1 silencing also decreased STIM1/TRPC1 complex, reduced SOC-mediated Ca2+ influx, and repressed cell migration after wounding. Our findings demonstrate that induced STIM1 translocation to the plasma membrane promotes IEC migration after wounding by enhancing TRPC1-mediated Ca2+ signaling and provide new insight into the mechanism of intestinal epithelial restitution.
Keywords: mucosal injury, early rapid repair, cell migration, capacitative Ca2+ entry, intestinal epithelial cells, surface biotinylation
early mucosal restitution is an important repair modality in the gastrointestinal tract, and its defective regulation is implicated in various critical pathological states such as mucosal bleeding and ulcers, disruption of epithelial integrity, and barrier dysfunction (9, 10, 21, 43). Epithelial restitution occurs rapidly after injury as a consequence of intestinal epithelial cell (IEC) migration to reseal superficial wounds, which is independent of cell proliferation (7, 21, 37, 39, 50). This reepithelialization is a complex process that is highly regulated by numerous extracellular and intracellular factors. A significant body of evidence indicates that cytosolic free Ca2+ ([Ca2+]cyt) plays a critical role in regulating IEC migration after injury and that increasing [Ca2+]cyt enhances epithelial restitution (28–35). Ca2+ entry due to store depletion is referred to as capacitative Ca2+ entry (CCE) and is mediated by Ca2+-permeable channels termed store-operated Ca2+ channels (SOCs) (23, 24). CCE through SOCs contributes to maintaining a sustained increase in intracellular [Ca2+]cyt and the refilling of Ca2+ into the stores (5, 19, 23). Our previous studies show that voltage-gated K+ (Kv) channels regulates Ca2+ influx by regulating the membrane potential (Em) that governs the driving force for Ca2+ influx in IECs (29, 33, 53) and that canonical transient receptor potential-1 (TRPC1) protein functions as a Ca2+-permeable channel mediating store-operated Ca2+ entry (SOCE) and regulates intestinal epithelial restitution after injury (32). However, the exact upstream signals initiating TRPC1 activation after mucosal injury remain to be elucidated.
Recently, there is emerging evidence showing that the single membrane-spanning protein stromal interaction molecule 1 (STIM1) plays an essential role in the activation of SOCs in certain types of cells (2, 15, 20, 44, 55). The STIM1 is proposed primarily as a sensor of Ca2+ within the store, and a single EF-hand Ca2+-binding motif located near the intraluminal NH2-terminus of STIM1 is able to sense the decrease in store free Ca2+ concentration (8, 56). In unstimulated cells, STIM1 is predominantly located in the endoplasmic reticulum (ER), but it rapidly translocates to the plasma membrane in response to Ca2+ store depletion or various biological stimuli (2, 3, 15, 38). Inhibition of the expression of STIM1 reduces SOC activation in response to store depletion, whereas overexpression of a constitutively active EF-hand motif mutant STIM1 increases its levels at the plasma membrane region and activates SOCs without store depletion (6, 56). Although these induced densely labeled STIM1 puncta by store depletion are close to the plasma membrane (2, 15), the STIM1 is not inserted into the membrane (55). Several studies also suggest that increased STIM1 translocation to the plasma membrane following store depletion regulates Ca2+ influx by directly interacting with the highly selective SOCs in cell type-dependent manner (2, 15, 56). In this regard, STIM1 is found to activate members of the Orai family of SOCs, resulting in an increase in Ca2+ influx in smooth muscle, HEK293, and salivary gland cells (4, 22, 57). However, the exact role of STIM1 in the regulation of TRPC1 channel in IECs and intestinal epithelial restitution after injury remains unknown.
In this study, we tested the hypothesis that STIM1 regulates intestinal epithelial restitution after wounding by altering TRPC1-mediated Ca2+ signaling. First, we examined the patterns of subcellular distribution of STIM1 during restitution after wounding in IECs. Second, we determined the changes in CCE and epithelial restitution in IECs stably overexpressing either wild-type or EF-hand mutant STIM1. Finally, we investigated whether increased STIM1 at the plasma membrane after wounding increased Ca2+ influx through SOCs by interacting with TRPC1 channels. The results presented herein indicate that relocalization of STIM1 to the plasma membrane increases remarkably after wounding and that induced STIM1 functions within the plasma membrane to promote Ca2+ influx through SOCE, thus enhancing intestinal epithelial restitution. Our results also show that STIM1 physically interacts with TRPC1 in IECs and that the formation of STIM1/TRPC1 complex is necessary for the activation of TRPC1 channels during restitution after wounding. Some of these data have been published in abstract form (36).
MATERIALS AND METHODS
Chemicals and supplies.
Disposable culture ware was purchased from Corning Glass Works (Corning, NY). Tissue culture media, isopropyl-β-d-thiogalactopyranoside (IPTG), LipofectAMINE 2000, and fetal bovine serum (FBS) were obtained from Invitrogen (Carlsbad, CA), and other biochemicals were obtained from Sigma (St. Louis, MO). The affinity-purified rabbit polyclonal antibody against TRPC1 was purchased from Alomone Laboratories (Jerusalem, Israel), and mouse monoclonal antibody against STIM1 was from BD biosciences (San Jose, CA). Fluorescein-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture.
The IEC-6 cell line was purchased from the American Type Culture Collection (ATCC) at passage 13. IEC-6 cells were derived from normal rat intestinal crypt cells and were developed and characterized by Quaroni et al. (25). Stock cells were maintained in T-150 flasks in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate. Flasks were incubated at 37°C in a humidified atmosphere of 90% air-10% CO2, and passages 15–20 were used in the experiments. Stable Cdx2-transfected IEC-6 cells were developed and characterized by Suh and Traber (47) and were a kind gift from Dr. Peter G. Traber (48) (Baylor College of Medicine, Houston, TX). Stock-stable Cdx2-transfected IEC-6 (IEC-Cdx2L1) cells were grown in DMEM supplemented with 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate. Before experiments, IEC-Cdx2L1 cells were grown in DMEM containing 4 mM IPTG for 16 days to induce cell differentiation as described in our earlier publications (26, 31). The stable TRPC1-transfected IEC-6 cells (IEC-TRPC1) were developed and characterized as described in our recent publications (32) and cultured in DMEM medium was used for growing IEC-6 cells.
RNA interference.
The small interfering (si)RNAs that were designed to specifically target the coding region of STIM1 (siSTIM1) or TRPC1 (siTRPC1) mRNA were synthesized and purchased from Dharmacon (Lafayette, CO). Scrambled control siRNA (C-siRNA), which had no sequence homology to any known genes, was used as the control. The siSTIM1, siTRPC1, and C-siRNA were transfected into cells as previously described (32, 35). Briefly, for each 60-mm cell culture dish, 20 μl of the 5 μM stock siSTIM1, siTRPC1, or C-siRNA was mixed with 500 μl of Opti-MEM medium (Invitrogen). This mixture was added to a solution containing LipofectAMINE 2000 in 500 μl of Opti-MEM. The solution was incubated for 20 min at room temperature and gently overlaid onto monolayers of cells in 3 ml of medium, and cells were harvested for various assays after 48 h incubation.
Plasmid construction and transfection.
The ∼2.1-kb cDNA encoding the full-length wild-type (WT) STIM1 or constitutively active STIM1 EF-hand motif mutants (EF1A3A and EF12Q) were described previously (56) and kindly provided by Dr. Cahalan group (University of California, San Diego, CA). To construct the STIM1 expression vectors in IECs, the WT-STIM1 or STIM1 mutant cDNAs were subcloned into the Xho1 and HindIII sites of an expression vector pcDNA3.1(+) (Invitrogen) with the cytomegalovirus immediate-early promoter, and resulting clones were sequenced for the confirmation of successful subcloning of all three STIM1 cDNAs. The IEC-6 cells were transfected with the STIM1 expression vectors or control vectors containing no STIM1 cDNA (Null) by using the LipofectAMINE 2000 and performed as recommended by the manufacturer (Invitrogen). After the 5-h period of incubation, the transfection medium was replaced by the standard growth medium containing 5% FBS for 2 days before exposure to the selection medium. These transfected cells were selected for STIM1 integration by incubation with the selection medium containing 0.6 mg/ml of G418, and clones resistant to the selection medium were isolated, cultured, and screened for STIM1 expression by Western blot analysis with the specific anti-STIM1 antibody.
Immunoprecipitation and immunoblotting analysis.
Cell samples, dissolved in ice-cold RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM DTT, 0.5 mM EDTA, 1.0% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM phenylmethyl-sulfonyl fluoride, 20 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 mM sodium orthovanadate), were sonicated and centrifuged at 4°C, and the supernatants were collected for immunoprecipitation. Equal amounts of proteins (500 μg) for each sample were incubated with the specific antibody against STIM1 or TRPC1 (4 μg) at 4°C for 3 h, and protein A/G-PLUS-Agarose was added and incubated overnight at 4°C. The precipitates were washed five times with ice-cold D-PBS, and the beads were resuspended in SDS sample buffer. For immunoblotting, samples were subjected to electrophoresis on PAGE gels described previously (14, 35, 52). Briefly, after the transfer of protein onto nitrocellulose membranes, the membranes were incubated for 1 h in 5% nonfat dry milk in 1× TBS-T buffer (Tris-buffered saline, pH 7.4, with 0.1% Tween-20). Immunologic evaluation was then performed overnight at 4°C in 5% nonfat dry milk/TBS-T buffer containing a specific antibody against STIM1 or TRPC1. The membranes were subsequently washed with 1× TBS-T and incubated with the secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. The immunocomplexes on the membranes were reacted for 1 min with Chemiluminiscence reagent (NEL-100 DuPont NEN).
Surface biotinylation assay.
Cell surface STIM1 protein was detected by cell surface biotinylation (56) using purification kit (Pierce, Rockford, IL) and performed according to manufacturer instructions. Briefly, after cells were washed twice with ice-cold PBS, they were biotinylated with 0.5 mg/ml sulfo-NHS-SS-biotin/PBS at 4°C for 45 min. After quench of unreacted biotinylation reagent, the cells were washed and then lysed. After insoluble materials were removed by centrifugation, biotinylated proteins were collected by incubation with immobilized Neutravidin Gel overnight at 4°C. Proteins were eluted by boiling in 5× SDS-PAGE denaturing sample buffer (Pierce) for electrophoresis and Western blotting with the antibody against STIM1. β-Catenin immunoblotting was also examined for verifying equal loading of each lanes.
Measurement of [Ca2+]cyt.
Details of the digital imaging methods employed for measuring [Ca2+]cyt were described in our previous publications (28, 32, 34, 35). Briefly, cells were plated on 25-mm coverslips and incubated in culture medium containing 3.3 μM fura-2 AM for 30 min at room temperature (22–24°C) under an atmosphere of 10% CO2 in air. The fura-2 AM-loaded cells were then superfused with standard bath solution for 20–30 min at 22–24°C to wash away extracellular dye and permit intracellular esterases to cleave cytosolic fura-2 AM into active fura-2. Fura-2 fluorescence from the cells and background fluorescence were imaged using a Nikon Diaphot microscope equipped for epifluorescence. Fluorescent images were obtained using a microchannel plate image intensifier (Amperex XX1381; Opelco, Washington, DC) coupled by fiber optics to a Pulnix charge-coupled device video camera (Stanford Photonics, Stanford, CA). Image acquisition and analysis were performed with a Metamorph Imaging System (Universal Imaging). The ratio imaging of [Ca2+]cyt was obtained from fura-2 fluorescent emission excited at 380 and 340 nm (34, 35).
Measurement of cell migration.
Migration assays were carried out as described in our earlier publications (26, 28, 32, 34, 35). Cells were plated at 6.25 × 104/cm2 in DMEM containing FBS on 60-mm dishes thinly coated with Matrigel according to the manufacturer's instructions (BD Biosciences) and were incubated as described for stock cultures. Cells were fed on day 2, and cell migration was assayed on day 4. To initiate migration, the cell layer was scratched with a single edge razor blade cut to ∼27 mm in length. The scratch was made over the diameter of the dish and extended over an area 7–10 mm wide. The migrating cells in six contiguous 0.1-mm squares were counted at ×100 magnification beginning at the scratch line and extending as far out as the cells had migrated. All experiments were carried out in triplicate, and the results were reported as number of migrating cells per millimeter of scratch.
Immunofluorescence staining.
Immunofluorescence was carried out according to the method of Vielkind and Swierenga (49) with minor changes (27). After cells were fixed using 3.7% formaldehyde in phosphate-buffered saline, rehydrated samples were incubated overnight with a primary antibody against STIM1 diluted (1:300) in blocking buffer at 4°C and then incubated with secondary antibody conjugated with Alexa Fluor-594 (Molecular Probes, Eugene, OR) for 2 h at room temperature. Finally, the slides were washed, mounted, and viewed through a Zeiss confocal microscope (model LSM410). Images were processed using Photoshop software (Adobe, San Jose, CA).
Statistical analysis.
All data are expressed as means ± SE from six dishes. Immunoprecipitation and immunoblotting results were repeated three times. The significance of the difference between means was determined by analysis of variance. The level of significance was determined using the Duncan's multiple-range test (11).
RESULTS
STIM1 translocation to the plasma membrane increases significantly after wounding.
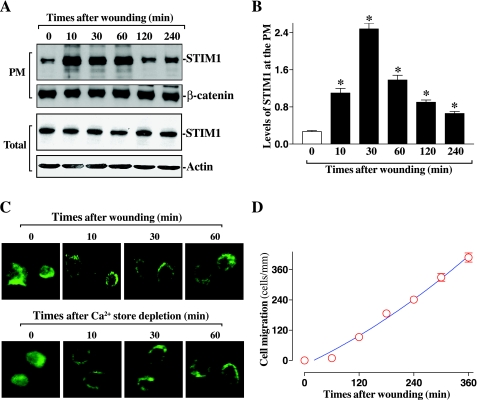
To determine the involvement of STIM1 in the regulation of intestinal epithelial restitution after wounding, differentiated IEC-Cdx2L1 cells, which represent differentiated IECs from the surface of the mucosa (26, 46, 47), were used. Consistent with our previous studies (26, 47), ectopic expression of the Cdx2 gene by exposure of stable IEC-Cdx2L1 cells to 4 mM IPTG for 16 days resulted in a differentiated phenotype. These differentiated IEC-Cdx2L1 cells were polarized, showed lateral membrane interdigitations, a well-demarcated basal lamina, and microvilli at the apical pole and also expressed brush-border enzymes such as sucrase-isomaltase (data not shown). This line of differentiated IEC-Cdx2L1 cells is extensively used and widely accepted as an in vitro model system for cell division-independent stage of epithelial restitution (18, 26, 29, 34, 35, 42). As shown in Fig. 1A, levels of STIM1 at the plasma membrane increased dramatically after wounding as measured by surface biotinylation assays, although there were no significant changes in levels of total STIM1. The increase in STIM1 translocation to the plasma membrane occurred within 10 min after wounding, peaked between 30 and 60 min, and then gradually declined (Fig. 1B). Maximum increases in the levels of plasma membrane STIM1 after wounding were approximately four to sixfold the prewounding control level. The levels of STIM1 at the plasma membrane were returned to near-normal level at 240 min after wounding. β-Catenin, a membrane-associated protein, served as negative control in this study and exhibited no changes in its levels at the plasma membrane region after wounding. Consistent with the immunoblotting results, STIM1 immunostaining in the plasma membrane was also increased after wounding. Relocalization of STIM1 to the plasma membrane region occurred rapidly after wounding, and it remained elevated at the plasma membrane region for >60 min (Fig. 1C, top). Similar to those observations obtained from other cell types (2, 12, 44, 56), STIM1 translocation to the plasma membrane region in IECs was also induced by Ca2+ store depletion after exposure to 10 μM cyclopiazonic acid (CPA) (Fig. 1C, bottom), which served as a positive control in this study. Interestingly, induced relocalization of STIM1 to the plasma membrane occurred before the beginning of epithelial restitution as indicated by an induction of cell migration after wounding (Fig. 1D). Cell migration over the denuded area occurred at 120 min after wounding, whereas the remarkable increases in cell migration were observed between 240 and 360 min. In addition, DNA synthesis was also measured in parallel samples by the technique of [3H]thymidine incorporation to determine the involvement of cell proliferation in this model. There were no changes in DNA synthesis within 6 h after wounding (data not shown). Furthermore, inhibition of cell proliferation by treatment with mitomycin C (2 μg/ml) also failed to alter cell migration when measured 6 h after wounding (data not shown), indicating that cell division did not participate in this short process. These findings indicate that STIM1 rapidly translocates to the plasma membrane region after wounding, which is paralleled by an increase in cell migration.
Fig. 1.
Redistribution of stromal interaction molecule 1 (STIM1) to the plasma membrane (PM) during epithelial restitution after wounding in differentiated intestinal epithelial cells (IECs) (IEC-Cdx2L1 line). Before experiments, stable IEC-Cdx2L1 cells were grown in DMEM containing 4 mM isopropyl β-d-thiogalactopyranoside (IPTG) for 16 days to induce cell differentiation. Epithelial restitution was induced by removing part of the monolayer, as described in MATERIALS and METHODS. A: levels of STIM1 protein at the PM as measured by surface biotinylation assays. The PM fractions were isolated at various times after wounding and biotinylated proteins were detected by Western blot analysis using the antibody against STIM1. After the blot was stripped, β-catenin immunoblotting was performed as an internal control for equal loading. For measurement of total STIM1 protein, actin immunoblotting was performed as an internal control for equal loading. Three experiments were performed that showed similar results. B: quantitative analysis of Western immunoblots by densitometry from cells described in A (to panel). Values are means ± SE of data from 3 separate experiments; relative levels of STIM1 at the PM were corrected for loading as measured by densitometry of β-catenin. *P < 0.05 compared with unwounded controls (0 min). C: subcellular localization of STIM1 in cells described in A (top) and cells exposed to 10 μM cyclopiazonic acid for depleting Ca2+ store (bottom). Cells were fixed, permeabilized, and incubated with the anti-STIM1 antibody, and then with anti-IgG conjugated with FITC. Original magnification, ×400. D: changes in levels of cell migration after wounding in cells described in A. Values are means ± SE of data from 6 dishes.
Increased STIM1 levels at the plasma membrane by overexpressing its EF-hand motif mutants promotes Ca2+ influx and enhances cell migration after wounding.
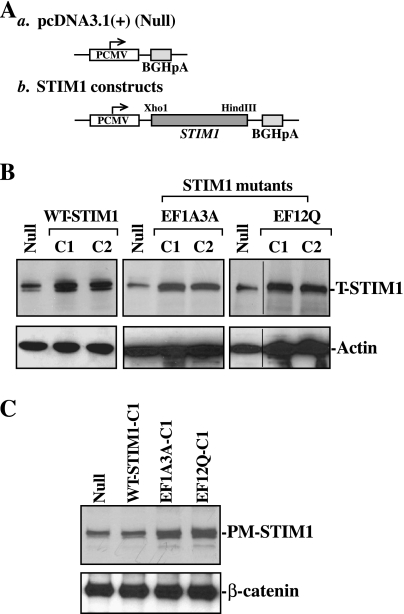
To determine whether STIM1 functions within the plasma membrane to induce cell migration after wounding by controlling Ca2+ influx, stable WT or EF-hand mutant STIM1-transfected IECs were developed and characterized. The STIM1 expression vectors were prepared from pcDNA3.1(+) to express either WT-STIM1 or STIM1 carrying specific NH2-terminal EF-hand motif mutations (EF1A3A and EF12Q) as shown in Fig. 2A. Previous studies demonstrated that STIM1 protein has several distinct functional domains, and that mutation of the EF-hand motif caused a translocation of STIM1 from the ER toward the plasma membrane without store depletion, a movement similar to the translocation of the WT-STIM1 protein in response to store emptying (8, 12, 56). As shown in Fig. 2B, six clones (two of each STIM1 expression vector) resistant to the selection medium containing 0.6 mg/ml G418 expressed high levels of either WT- or EF-hand mutated STIM1. Levels of STIM1 protein were approximately eightfold the value of cells that were transfected with the control vector containing no STIM1 cDNA (Null) but also exposed to the same dose of G418. There were no significant differences in levels of STIM1 protein between parental IEC-6 cells and the clone that was transfected with Null (data not shown). Although ectopic overexpression of WT-STIM1 failed to alter STIM1 levels within the plasma membrane, there were dramatic increases in the levels of STIM1 at the plasma membrane region in cells overexpressing EF-hand mutant STIM1 protein EF1A3A or EF12Q under unstimulated conditions (Fig. 2C). Levels of STIM1 at the plasma membrane in the clone of cells stably expressing EF1A3A or EF12Q were approximately sevenfold the value of Null-transfected cells or the clone that was transfected with the WT-STIM1.
Fig. 2.
Characterization of stable STIM1-transfected IEC cells. A: structure of expression vector. a, control vector (Null); b, STIM1 expression vector. The complete open reading frame of wild-type (WT)-STIM1 or each of two STIM1 EF-hand motif mutants EF1A3A and EF12Q cDNA was subcloned into the expression vector pcDNA3.1(+) under control of cytomegalovirus immediate-early promoter (PCMV). B: representative STIM1 immunoblots in various clones (C) of stable STIM1-transfected cells. IEC-6 cells were transfected with the STIM1 expression vector or Null by the LipofectAMINE 2000, and clones resistant to the selection medium containing 0.6 mg/ml G418 were isolated and screened for STIM1 expression by Western immunoblotting analysis. Equal loading was monitored by actin immunoblotting. The internal dividing line indicates that irrelevant sections were removed from the same immnoblots in assembling the data. C: levels of STIM1 at the PM as measured by surface biotinylation assays in cells described in B. The PM fractions were isolated from different stable STIM1-transfected clones, whereas PM-STIM1 levels were measured by Western blot analysis. β-Catenin immunoblotting was performed as an internal control for equal loading. Three experiments were performed that showed similar results.
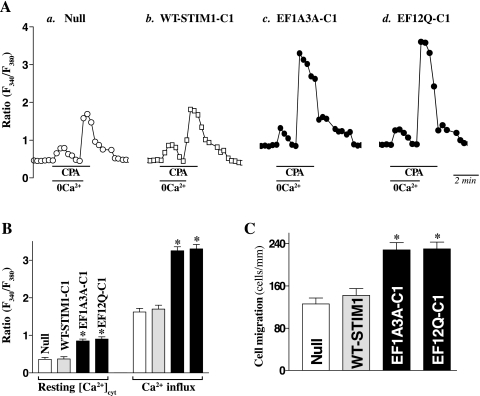
Results presented in Fig. 3 further show that stable ectopic expression of EF-hand motif mutant STIM1 not only increased store depletion-induced Ca2+ influx but also stimulated IEC migration after wounding. Cells transfected with the EF-hand mutant STIM1 exhibited a significant increase in levels of both resting [Ca2+]cyt and Ca2+ influx after store depletion by the exposure to CPA. Levels of resting [Ca2+]cyt in stable clones expressing EF1A3A or EF12Q were increased by ∼85% when compared with cells transfected with the Null or the clone that was transfected with the WT-STIM1. Although transient Ca2+ release from the store depletion were not changed, sustained Ca2+ influx after the Ca2+ store depletion by CPA was increased by approximately twofold in the clone of cells that were transfected with either EF1A3A or EF12Q. On the other hand, there were no significant differences in levels of resting [Ca2+]cyt and Ca2+ influx induced by CPA between Null-transfected cells and the clone of cells overexpressing WT-STIM1. We also examined changes in [Ca2+]cyt levels in other independently transfected clones such as EF1A3A-C2 and EF12Q-C2 and demonstrated that increased levels of resting [Ca2+]cyt and Ca2+ influx through SOCs were similar to those observed in the clone of EF1A3A-C1 or EF12Q-C1 (data not shown). Importantly, overexpression of the activating EF-hand motif mutant STIM1 also significantly stimulated cell migration after wounding (Fig. 3C). The numbers of cells migrating over the wounded edge in cells stably expressing EF-hand motif mutant STIM1 were increased by ∼45% at 6 h after wounding. In contrast, overexpression of WT-STIM1 did not induce cell migration after wounding. We also examined changes in cell proliferation in stable WT-STIM1 or EF-hand mutant STIM1-transfected IECs and demonstrated that increased levels of mutant STIM1 (EF1A3A-C1 or EF12Q-C1), rather than the WT-STIM1, marginally induced cell proliferation. Cell numbers in stable EF-hand mutant STIM1-transfected IECs were increased by ∼30% on day 6 after initial plating compared with parental cells and cells overexpressing WT-STIM1. However, this induction in cell proliferation had no significant effect on epithelial restitution, because there was no an increase in DNA synthesis in stable EF1A3A and EF12Q cells at 6 h after wounding and since inhibition of cell proliferation by mitomycin C did not prevented an increased cell migration in cells overexpressing EF-hand mutant STIM1 protein (data not shown). These results strongly suggest that induced STIM1 translocation to the plasma membrane plays a critical role in activating SOCs, thus increasing [Ca2+]cyt influx and cell migration after wounding.
Fig. 3.
Effect of ectopic overexpression of WT- or EF-hand mutant STIM1 on store-operated Ca2+ influx and cell migration after wounding. A: representative records showing the time course of cytosolic free Ca2+ concentration ([Ca2+]cyt) changes after exposure to 10 μM cyclopiazonic acid (CPA) in the absence (0 Ca2+) or presence of extracellular Ca2+ in cells described in Fig. 2. a, cells transfected with Null; b, clones of cells expressing WT-STIM1; c, clones of cells expressing STIM1 mutant EF1A3A; and d, clones of cells expressing STIM1 mutant EF12Q. B: summarized data showing resting [Ca2+]cyt (left) and the amplitude of CPA-induced Ca2+ influx (right) from cells described in A. Values are means ± SE; n = 20. *P < 0.05 compared with cells transfected with the Null or cells expressing WT-STIM1. C: summarized data showing cell migration 6 h after wounding by removal of part of the monolayers in cells described in A. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with cells transfected with the Null or cells expressing WT-STIM1.
STIM1 interacts with TRPC1 thus inducing Ca2+ influx through SOCE.
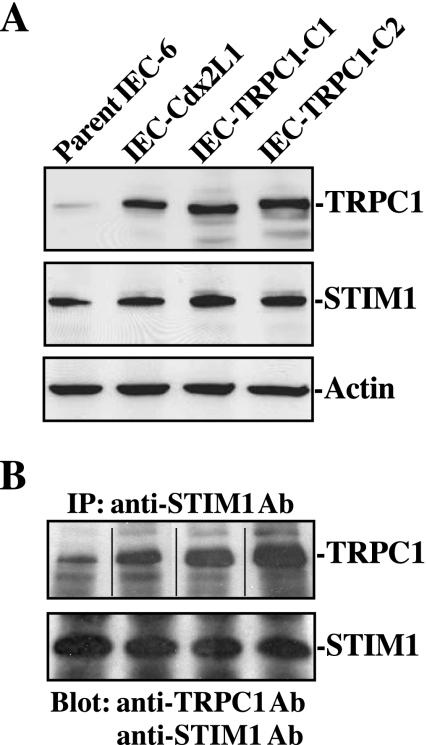
Our previous studies have demonstrated that TRPC1 functions as a SOC in IECs and mediates Ca2+ influx after store depletion (16, 17, 32). To test the possibility that STIM1 regulates Ca2+ influx through interaction with TRPC1 channels after injury, the following two sets of experiments were carried out. First, we examined changes in physical interaction of STIM1 with TRPC1 in various lines of IECs including parental IEC-6 cells, differentiated IEC-Cdx2L1 cells, and two clones of stable TRPC1-expressing cells (IEC-TRPC1) recently developed in our laboratory (16, 32). These stable TRPC1-transfected cells exhibited sixfold increase in TRPC1 protein (Fig. 4A, top) and also displayed increased Ca2+ influx after store depletion (data not shown). Similar results have been reported previously (32). Although the levels of TRPC1 varied among different lines of IECs, STIM1 expression were consistent in all lines of IECs (Fig. 4A, middle). To determine whether STIM1 forms STIM1/TRPC1 complex, whole cell lysates were immunoprecipitated with the specific anti-STIM1 antibody, and then these precipitates were examined by Western blot analysis using the antibody against TRPC1 or STIM1. As shown in Fig. 4B, immunoprecipitation of STIM1 resulted in coimmunoprecipitation of TRPC1 in parental IEC-6 cells, differentiated IEC-Cdx2L1 cells, and both clones of IEC-TRPC1 cells, but the levels of TRPC1 protein in IEC-TRPC1 cells were higher than those observed in parental IEC-6 and IEC-Cdx2L1 cells. These results indicate that STIM1 physically interacts with TRPC1 in the intestinal epithelium.
Fig. 4.
Levels of transient receptor potential 1 (TRPC1) and STIM1 proteins and their interactions in different lines of IECs. A: representative immunoblots for TRPC1 and STIM1 proteins in IEC-6 cells, differentiated IEC-Cdx2L1 cells, and two clones (C) of IECs overexpressing TRPC1 (IEC-TRPC1). Levels of total TRPC1 and STIM1 were examined by Western blot analysis, and actin immunoblotting was performed as an internal control for equal loading. B: levels of TRPC1 and STIM1 proteins in the complex immunoprecipitated (IP) by the anti-STIM1 antibody (Ab) in cells described in A. After whole cell lysates (500 μg) were immunoprecipitated by the specific antibody against STIM1, precipitates were separated by performing SDS-PAGE (10% acrylamide) gels. Levels of TRPC1 and STIM1 proteins were measured using Western blot analysis with the antibody against TRPC1 or STIM1. The internal dividing line indicates that immunoblotting bands were derived from the same blot but reordered in assembling the data. Three separate experiments were performed that showed similar results.
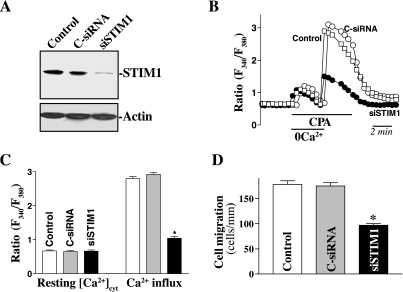
Second, we determined the role of STIM1/TRPC1 complex in the regulation of Ca2+ influx through SOCs and epithelial restitution after wounding. siRNA targeting the STIM1 mRNA (siSTIM1) was used to specifically block endogenous STIM1 in IECs stably overexpressing TRPC1. Initially, we determined the transfection efficiency of the siRNA nucleotides in IEC-TRPC1 cells and demonstrated that >95% of cells were positive when they were transfected with a fluorescent FITC-conjugated C-siRNA for 24 h (data not shown). As shown in Fig. 5A, exposure to siSTIM1 for 48 h decreased levels of STIM1 protein by ∼85%, whereas transfection with C-siRNA at the same concentrations showed no inhibitory effects. STIM1 silencing by the transfection with siSTIM1 inhibited Ca2+ influx after store depletion in stable IEC-TRPC1 cells (Fig. 5, B and C), although it had no effect on the levels of resting [Ca2+]cyt. The level of CPA-induced Ca2+ influx was decreased by ∼65% in STIM1-silenced cells compared with those observed in controls and cells transfected with C-siRNA. Furthermore, inhibition of STIM1 expression and subsequent decrease in Ca2+ influx by transfection with siSTIM1 also impaired intestinal epithelial restitution after wounding in IEC-TRPC1 cells (Fig. 5D). The number of cells migrating over the denuded area in cells transfected with siSTIM1 was decreased by ∼45% 6 h after wounding. We also examined the effect of STIM1 silencing on Ca2+ influx and epithelial restitution in other line of IECs and demonstrated that decreased levels of STIM1 by transfection with siSTIM1 also inhibited Ca2+ influx through SOCs and repressed cell migration after wounding in differentiated IEC-Cdx2L1 cells (see Supplemental Fig. S1 for this article online at the Journal website). In addition, neither siSTIM1 nor C-siRNA affected cell viability as measured by Trypan blue staining (data not shown). These findings indicate that STIM1 is necessary for the induction in TRPC1-mediated Ca2+ influx thus enhancing cell migration after wounding.
Fig. 5.
Changes in the levels of store-operated Ca2+ influx and cell migration after STIM1 silencing in stable IEC-TRPC1 cells. A: representative STIM1 immunoblots. After cells were transfected with either small interfering RNA (siRNA) targeting the STIM1 mRNA coding region (siSTIM1) or control siRNA (C-siRNA) for 48 h, whole cell lysates were harvested for Western blot analysis to monitor the expression of STIM1 and loading control actin. Three separate experiments were performed that showed similar results. B: representative records showing the time course of [Ca2+]cyt changes after exposure to CPA in the absence (0 Ca2+) or presence of extracellular Ca2+ in cells described in A. C: summarized data showing resting [Ca2+]cyt (left) and the amplitude of CPA-induced Ca2+ influx (right) from cells described in B. Values are means ± SE; n = 20. *P < 0.05 compared with controls and cells transfected with C-siRNA. D: changes in cell migration after wounding in cells described in A. Cell migration was assayed 6 h after part of the monolayer was removed. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with controls and cells transfected with C-siRNA.
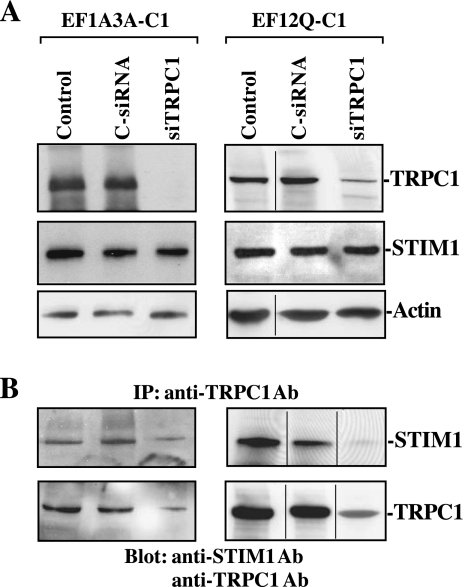
TRPC1 silencing decreases Ca2+ influx and represses restitution in stable EF-hand mutant STIM1-transfected IECs.
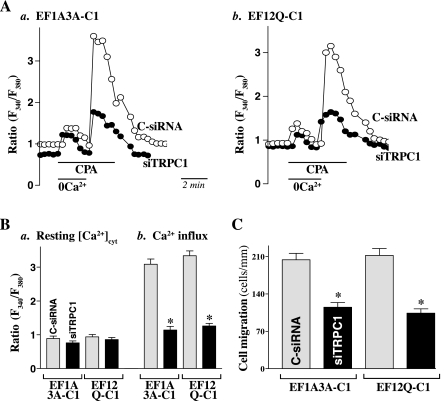
To further determine whether increased STIM1 at the plasma membrane requires TRPC1 for the stimulation of Ca2+ influx, we examined the influence of TRPC1 silencing on Ca2+ influx after store depletion in stable EF-hand mutant STIM1-transfected IECs. As shown in Fig. 6A, transfection with the siRNA specifically targeting the coding region of TRPC1 mRNA (siTRPC1) for 48 h decreased TRPC1 protein by ∼80% in stable EF1A3A-C1 and EF12Q-C1 cells, but it did not affect STIM1 protein levels. Decreased TRPC1 by siTRPC1 transfection reduced the levels of STIM1/TRPC1 complex as measured by immunoprecipitation assays using the anti-TRPC1 antibody (Fig. 6B). Importantly, TRPC1 silencing also reduced store depletion-induced Ca2+ influx in both EF1A3A-C1 and EF12Q-C1 cells (Fig. 7, A and B). Although there were no significant changes in the levels of resting [Ca2+]cyt between cells transfected with siTRPC1 and cells transfected with C-siRNA, the levels of store depletion-mediated Ca2+ influx were diminished by ∼65% after TRPC1 silencing. Furthermore, TRPC1 silencing also inhibited cell migration after wounding in stable EF1A3A- and EF12Q-transfected cells (Fig. 7C). The numbers of cells migrating over the wounded edge were suppressed by ∼40% in TRPC1-silenced cells compared with those observed in cells transfected with C-siRNA. These findings indicate that STIM1-induced Ca2+ signaling is mediated by TRPC1 channels, thus inducing epithelial restitution after wounding.
Fig. 6.
Changes in the levels of STIM1/TRPC1 complex after TRPC1 silencing in IECs stably overexpressing EF-hand motif mutant STIM1. A: representative immunoblots of TRPC1 and STIM1 in TRPC1-silenced cells. After stable EF1A3A- (left) and EF12Q-transfected cells (right) were grown in control DMEM medium for 2 days, they were transfected with either siRNA targeting the TRPC1 mRNA coding region (siTRPC1) or control siRNA (C-siRNA), and whole cell lysates were harvested 48 h thereafter. Levels of TRPC1 and STIM1 proteins were measured by Western blot analysis, and equal loading was monitored by actin immunoblotting. The internal dividing line indicates that immunoblotting bands were derived from the same blot but reordered in assembling the data. B: changes in the levels of STIM1 and TRPC1 proteins in the complex IP by anti-TRPC1 antibody (Ab) in cells described in A. Levels of STIM1 and TRPC1 were measured using Western blot analysis, and three separate experiments were performed that showed similar results.
Fig. 7.
Effect of TRPC1 silencing on store-operated Ca2+ influx and cell migration in cells described in Fig. 6. A: representative records showing the time course of [Ca2+]cyt changes after exposure to 10 μM CPA in the absence (0 Ca2+) or presence of extracellular Ca2+ in TRPC1-silenced cells. a, stable EF1A3A-transfected cells; and b, stable EF12Q-transfected cells. After cells were transfected with either siTRPC1 or C-siRNA for 48 h, Ca2+ influx was measured. B: summarized data showing resting [Ca2+]cyt (a) and the amplitude of CPA-induced Ca2+ influx (b) from cells described in A. Values are means ± SE; n = 20. *P < 0.05 compared with cells transfected with C-siRNA. C: summarized data showing cell migration 6 h after wounding by removal of part of the monolayers in cells described in A. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with cells transfected with C-siRNA.
DISCUSSION
In the response to acute injury, damaged IECs are sloughed, and remaining viable cells from areas adjacent to or just beneath the injured surface migrate to quickly cover the denuded area (43, 51). In the present study, we established the importance of STIM1 in the control of IEC migration after wounding, thus advancing our understanding of the mechanism of intestinal epithelial restitution. Experiments aimed at characterizing the molecular aspects of this process indicate that STIM1 directly interacts with and regulates TRPC1 channel activity in IECs and that formation of the STIM1/TRPC1 complex is necessary for the stimulation of Ca2+ influx through SOCs after wounding. Increased levels of the plasma membrane STIM1 by ectopic overexpression of its EF-hand motif mutants promoted Ca2+ influx after store depletion and enhanced epithelial restitution, which was prevented by TRPC1 silencing.
Results reported here clearly show that relocalization of STIM1 to the plasma membrane increased after wounding, although there were no changes in the levels of total STIM1. This wounding-induced STIM1 redistribution appears to have a functional role in the regulation of intestinal epithelial restitution, because subcellular reorganization of STIM1 alters Ca2+ influx through SOCs (38, 54, 56) and since it occurred before an induction in cell migration after injury (Fig. 1). STIM1 was originally identified as a tumor suppressor (40, 41), but it has been recognized as a central regulator of SOC-mediated Ca2+ influx since several studies revealed that increased translocation of STIM1 to the plasma membrane plays a critical role in the activation of SOCs in Drosophila S2 cells (38, 56) and HEK cells (13). Depletion of luminal Ca2+ within the ER triggers aggregation and translocation of STIM1 into junctions closely associated with the plasma membrane, where they activate the highly selective SOCs in cell type-dependent manner via conformational coupling (38, 45, 54). Reduction in the levels of STIM1 at the plasma membrane represses SOC activation and decreases Ca2+ influx in response to store depletion (1, 22). In support of the involvement of STIM1 in the regulation of epithelial restitution, our previous studies have demonstrated that increased Ca2+ influx through SOCs is crucial for the stimulation of IEC migration after wounding and that reduction in [Ca2+]cyt delays epithelial restitution in vitro (28, 29, 32, 53) as well as in vivo (33, 51).
The data from the current study further show that stable transfection with the EF-hand mutant STIM1 not only induced its translocation toward the plasma membrane (Fig. 2C) but also increased both Ca2+ influx through SOCs and cell migration after wounding (Fig. 3). Interestingly, overexpression of WT-STIM1 did not increase STIM1 levels at the plasma membrane and also failed to induce Ca2+ influx and cell migration, indicating that STIM1 by itself is not a functional SOC channel in IECs and that increased STIM1 translocation to the plasma membrane, rather than an induction in total STIM1 level, activates SOC-mediated Ca2+ influx and stimulates cell migration. Consistent with our current findings, Jurkat or S2 cells transfected with the EF-hand mutant STIM1 also exhibit increased STIM1 levels at their plasma membrane and display an increase in SOC-mediated Ca2+ influx (38, 56). STIM1 is also shown to regulate SOC influx in several lines of cells including HEK293, SH-SY5Y, and HeLa cells (12, 13, 38, 45). Although the exact reasons causing subcellular reorganization of EF-hand mutant STIM1 proteins remain unknown, several studies suggest that the altered localization of EF-hand mutants to the plasma membrane could result from their inability to bind Ca2+ within the intracellular store (54, 56). It is likely that STIM1 is initially located in the membrane of the Ca2+ store, with its low-affinity EF-hand inside the lumen-sensing Ca2+ and stabilized by bound Ca2+ when the store is full. When the store is depleted in response to stressful stimuli such as injury, Ca2+ dissociates and STIM1 translocates to the plasma membrane. Mutation of the EF-hand domain mimics Ca2+ store depletion, and it initiates STIM1 translocation and activates SOCs, thus stimulating cell migration after wounding.
The current studies also indicate that increased STIM1 at the plasma membrane after wounding enhances Ca2+ influx by directly interacting with and activating TRPC1 channels. First, STIM1 physically interacted with TRPC1 and formed a STIM1/TRPC1 complex in IECs (Fig. 4). Second, decreased formation of the STIM1/TRPC1 complex by STIM1 silencing reduced Ca2+ influx through SOCs and repressed cell migration after wounding in stable IEC-TRPC1 cells (Fig. 5) and in differentiated IEC-Cdx2L1 cells (supplemental Fig. S1). Third, TRPC1 silencing also decreased SOC-mediated Ca2+ influx and inhibited epithelial restitution in IECs stably overexpressing EF-hand mutant STIM1 (Fig. 7). These findings are consistent with results from others (1, 4, 12, 22, 54) who have demonstrated that STIM1 directly binds to TRPC1 and determines its function as SOCs in different types of cells. STIM1 also directly interacts with TRPC4 and TRPC5 and regulates their channel activity, but it does not bind to TRPC3 and TRPC6 (55). It has been reported that STIM1 regulates TRPC3 and TRPC6 through a distinct mechanism (54, 55). However, IECs do not express TRPC3, TRPC4, and TRPC6 (29, 32). Although TRPC5 is expressed in IECs (32), it is unclear whether TRPC5 forms native SOCs and plays a role in Ca2+ influx after store depletion. In salivary gland cells, a dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is necessary for activation of SOC channel in response to internal Ca2+ store depletion (22).
In summary, these results indicate that induced STIM1 translocation to the plasma membrane promotes intestinal epithelial restitution by enhancing TRPC1-mediated Ca2+ signaling. Levels of STIM1 at the plasma membrane increase after wounding, whereas STIM1 silencing decreases Ca2+ influx through SOCs and suppresses cell migration. Ectopic expression of the EF-hand mutant STIM1 increases its levels at the plasma membrane, enhances SOC-mediated Ca2+ influx, and promotes restitution after wounding. Our study further shows that STIM1 directly interacts with TRPC1 in IECs and that the formation of STIM1/TRPC1 complex is critical for activation of SOC-induced Ca2+ signaling. TRPC1 silencing prevents store depletion-induced Ca2+ influx and inhibits cell migration after wounding in cells overexpressing EF-hand mutant STIM1. These findings suggest that STIM1 functions as an upstream signal initiating TRPC1 activation after mucosal injury and that STIM1/TRPC1-induced Ca2+ influx plays an important role in the maintenance of intestinal epithelial integrity under physiological and various pathological conditions.
GRANTS
This work was supported by Merit Review Grant from the Department of Veterans Affairs and by National Institutes of Health Grants DK-57819, DK-61972, and DK-68491. J-Y. Wang is a Research Career Scientist, Medical Research Service, Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Alicia S, Angélica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium 44: 479–491, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA 103: 16704–16709, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol 9: 81–88, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourdheuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol 298: C993–C1005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bootman MD, Lipp P, Berridge MJ. The organization and functions of local Ca2+-signals. J Cell Sci 114: 213–222, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Chiu WT, Tang MJ, Jao HC, Shen MR. Soft substrate up-regulates the interaction of STIM1 with store-operated Ca2+ channels that lead to normal epithelial cell apoptosis. Mol Biol Cell 19: 2220–2230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dignass AU, Tsunekawa S, Podolsky DK. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology 106: 1254–1262, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L, Hanke P, Schropp P, Mühlstedt S, Zorn C, Huber M, Schmittwolf C, Jagla W, Yu P, Kerkau T, Schulze H, Nehls M, Nieswandt B. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest 117: 3540–3550, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X, Rao JN, Liu L, Rizvi M, Turner DJ, Wang JY. Polyamines regulate β-catenin tyrosine phosphorylation via Ca2+ during intestinal epithelial cell migration. Am J Physiol Cell Physiol 283: C722–C734, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Guo X, Rao JN, Liu L, Zou T, Turner DJ, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: novel function for polyamines. Am J Physiol Cell Physiol 285: C1174–C1187, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Harter JL. Critical values for Duncan's new multiple range test. Biometrics 16: 671–685, 1960 [Google Scholar]
- 12.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res 103: e97–e104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Li L, Rao JN, Zou T, Zhang HM, Boneva D, Bernard MS, Wang JY. Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol 288: C89–C99, 2005 [DOI] [PubMed] [Google Scholar]
- 15.López JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem 281: 28254–28264, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Marasa BS, Rao JN, Zou T, Liu L, Keledjian KM, Zhang AH, Xiao L, Chen J, Turner DJ, Wang JY. Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-κB activation through Ca2+ influx. Biochem J 397: 77–87, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marasa BS, Xiao L, Rao JN, Zou T, Liu L, Wang J, Bellavance E, Turner DJ, Wang JY. Induced TRPC1 expression increases protein phosphatase 2A sensitizing intestinal epithelial cells to apoptosis through inhibition of NF-κB activation. Am J Physiol Cell Physiol 294: C1277–C1287, 2008 [DOI] [PubMed] [Google Scholar]
- 18.McCormack SA, Viar MJ, Johnson LR. Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am J Physiol Gastrointest Liver Physiol 264: G367–G374, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Moore TM, Brough GH, Babal P, Stevens T. Store-operated calcium entry promotes shape change in pulmonary endothelial cells expressing Trp1. Am J Physiol Lung Cell Mol Physiol 275: L574–L582, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates Orai1 channel activation. J Biol Chem 283: 8014–8022, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution: characterization of cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 89: 1501–1511, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem 282: 9105–9116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 77: 901–930, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Quaroni A, Wands J, Trelstat RL, Isselbacher KJ. Epithelial cell cultures from rat small intestine. J Cell Biol 80: 248–265, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao JN, Li L, Li J, Bass BL, Wang JY. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol Gastrointest Liver Physiol 277: G1149–G1158, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Rao JN, Li L, Bass BL, Wang JY. Expression of the TGF-β receptor gene and sensitivity to growth inhibition following polyamine depletion. Am J Physiol Cell Physiol 279: C1034–C1044, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Rao JN, Li L, Golovina VA, Platoshyn O, Strauch ED, Yuan JXJ, Wang JY. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 280: C993–C1007, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, Yuan JXJ, Wang JY. Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol 282: C885–C898, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Rao JN, Wang JY. Ca2+ signaling in epithelial restitution. In: Gastrointestinal Mucosal Repair and Experimental Therapeutics, edited by Cho CH, Wang JY, Switzerland: Karger, 2002, p29–42 [Google Scholar]
- 31.Rao JN, Guo X, Liu L, Zou T, Murthy KS, Yuan JXY, Wang JY. Polyamines regulate Rho-kinase and myosin phosphorylation during intestinal epithelial restitution. Am J Physiol Cell Physiol 284: C848–C859, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, Marasa BS, Turner DJ, Yuan JXJ, Wang JY. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol 290: G782–G792, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Rao JN, Wang JY. Regulation of Kv channel activity and intracellular junctions by polyamines in intestinal epithelial cells. In: Polyamine Cell Signaling: Physiology, Pharmacology and Cancer Research, edited by Wang JY, Casero RA, Jr, Totowa, NJ: Humana, 2006, p363–381 [Google Scholar]
- 34.Rao JN, Liu L, Zou T, Marasa BS, Boneva D, Wang SR, Malone DL, Turner DJ, Wang JY. Polyamines are required for phospholipase C-γ1 expression promoting intestinal epithelial cell restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335–G343, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Rao JN, Liu S, Zou T, Liu L, Xiao L, Zhang X, Bellevance E, Yuan JXJ, Wang JY. Rac1 promotes intestinal epithelial restitution by increasing Ca2+ influx through interaction with phospholipase C-γ1 after wounding. Am J Physiol Cell Physiol 295: C1499–C1509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao JN, Zou T, Liu L, Wang P, Timmons JA, Wang JY. Stromal interaction molecule 1 (STIM1) regulates intestinal epithelial restitution by modulating store-operated Ca2+ influx after wounding. Gastroenterology 136: A411, 2009 [Google Scholar]
- 37.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Borisy G, Parson JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutten MJ, Ito S. Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol Gastrointest Liver Physiol 244: G171–G182, 1983 [DOI] [PubMed] [Google Scholar]
- 40.Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: a gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res 57: 4493–4497, 1997 [PubMed] [Google Scholar]
- 41.Sabbioni S, Veronese A, Trubia M, Taramelli R, Barbanti-Brodano G, Croce CM, Negrini M. Exon structure and promoter identification of STIM1 (alias GOK), a human gene causing growth arrest of the human tumor cell lines G401 and RD. Cytogenet Cell Gene 86: 214–218, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Santos MF, McCormack SA, Guo Z, Okolicany J, Johnson LR. Rho proteins play a critical role in cell migration during the early phases of mucosal restitution. J Clin Invest 100: 216–225, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silen W, Ito S. Mechanism for rapid-epithelialization of the gastric mucosal surface. Annu Rev Physiol 47: 217–229, 1985 [DOI] [PubMed] [Google Scholar]
- 44.Smyth JT, Dehaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci 121: 762–772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 135: 110–122, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Suh E, Chen LL, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene-transcription. Mol Cell Biol 14: 7340–7351, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 16: 619–625, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traber PG, Wu GD. Intestinal development and differentiation: In: Gastrointestinal Cancers: Biology, Diagnosis, and Therapy, edited by Rustgi AK. Philadelphia, PA: Lippincott Raven, 1995, p21–43 [Google Scholar]
- 49.Vielkind U, Swierenga SH. A simple fixation procedure for immunofluorescent detection of different cytoskeletal components within the same cell. Histochemistry 91: 81–88, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol Gastrointest Liver Physiol 259: G584–G592, 1990 [DOI] [PubMed] [Google Scholar]
- 51.Wang JY, Johnson LR. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 100: 333–343, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Wang JY, McCormack SA, Viar MJ, Wang HL, Tzen CY, Scott RE, Johnson LR. Decreased expression of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol 265: G331–G338, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Wang JY, Wang J, Golovina VA, Li L, Platoshyn O, Yuan JXJ. Role of K+ channel expression in polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 278: C303–C314, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium 42: 205–211, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Mancarella S, Wang Y, Yue C, Ritchie M, Gill DL, Soboloff J. The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J Biol Chem 284: 19164–19168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.