Abstract
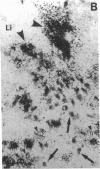
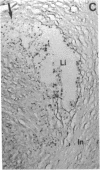
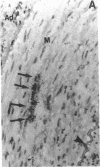
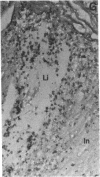
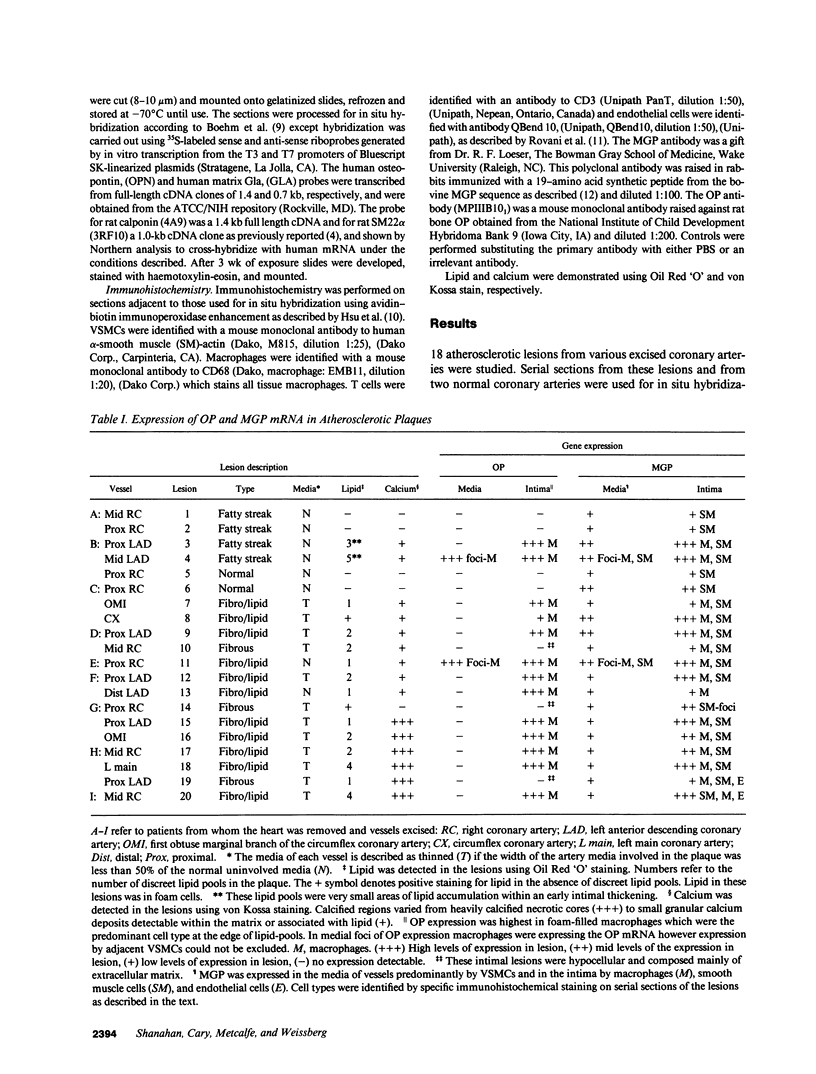
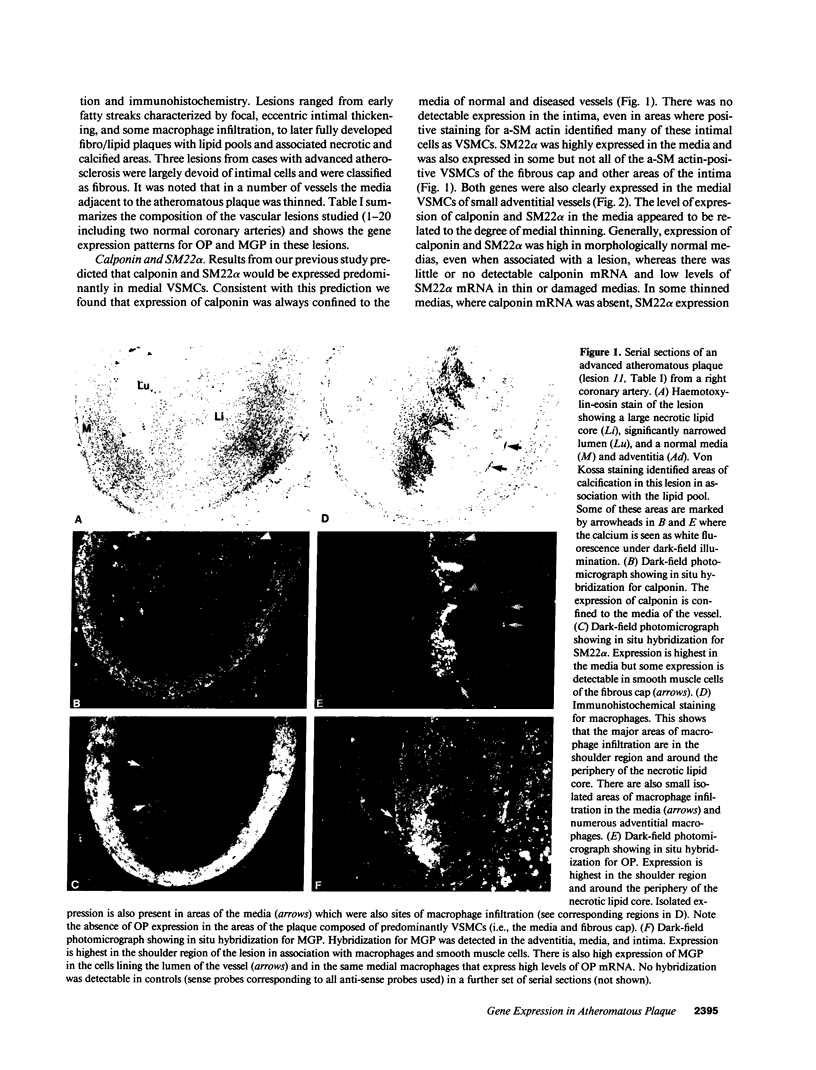
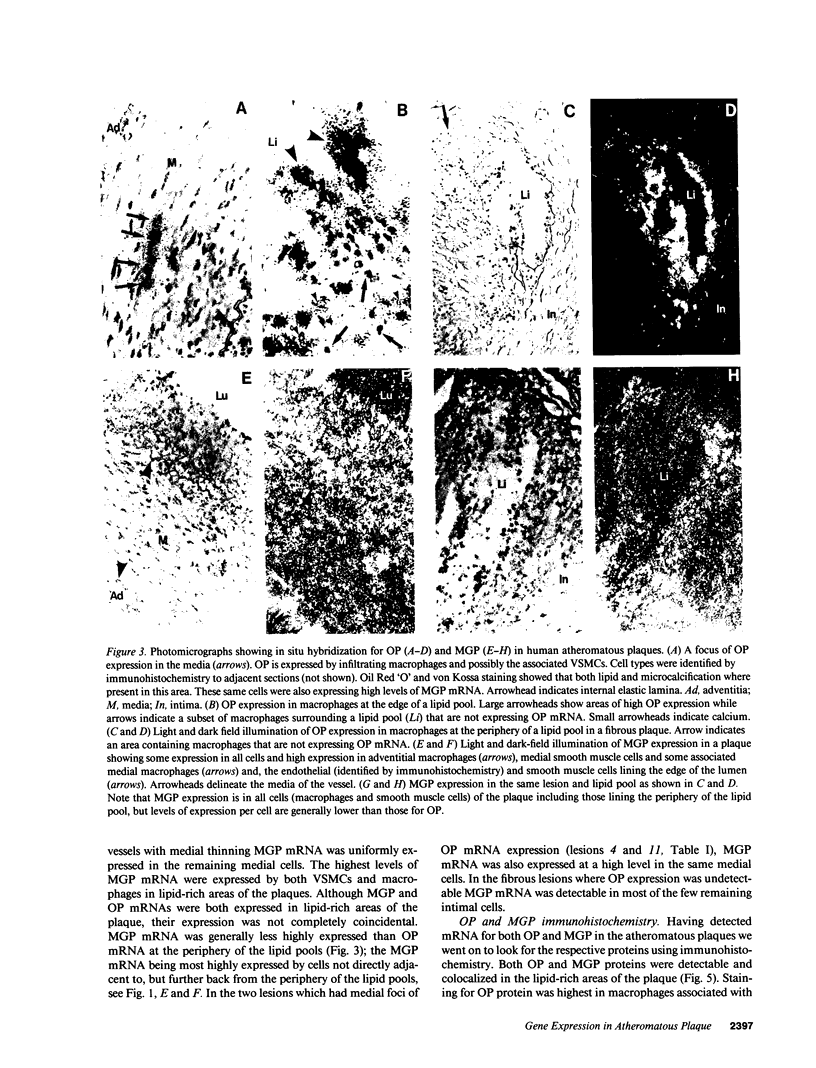
Calcification is common in atheromatous plaques and may contribute to plaque rupture and subsequent thrombosis. However, little is known about the mechanisms which regulate the calcification process. Using in situ hybridization and immunohistochemistry we show that two bone-associated proteins, osteopontin (OP) and matrix Gla protein (MGP), are highly expressed in human atheromatous plaques. High levels of OP mRNA and protein were found in association with necrotic lipid cores and areas of calcification. The predominant cell type in these areas was the macrophage-derived foam cell, although some smooth muscle cells could also be identified. MGP was expressed uniformly by smooth muscle cells in the normal media and at high levels in parts of the atheromatous intima. Highest levels of this matrix-associated protein were found in lipid-rich areas of the plaque. The pattern of expression of these two genes contrasted markedly with that of calponin and SM22 alpha, genes expressed predominantly by differentiated smooth muscle cells and whose expression was generally confined to the media of the vessel. The postulated function of OP and MGP as regulators of calcification in bone and the high levels and colocalization of both in atheromatous plaques suggest they have an important role in plaque pathogenesis and stability.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Calcific diseases. A concept. Arch Pathol Lab Med. 1983 Jul;107(7):341–348. [PubMed] [Google Scholar]
- Barone L. M., Owen T. A., Tassinari M. S., Bortell R., Stein G. S., Lian J. B. Developmental expression and hormonal regulation of the rat matrix Gla protein (MGP) gene in chondrogenesis and osteogenesis. J Cell Biochem. 1991 Aug;46(4):351–365. doi: 10.1002/jcb.240460410. [DOI] [PubMed] [Google Scholar]
- Boehm T., Gonzalez-Sarmiento R., Kennedy M., Rabbitts T. H. A simple technique for generating probes for RNA in situ hybridization: an adjunct to genome mapping exemplified by the RAG-1/RAG-2 gene cluster. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3927–3931. doi: 10.1073/pnas.88.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström K., Watson K. E., Horn S., Wortham C., Herman I. M., Demer L. L. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993 Apr;91(4):1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela L., Hsieh C. L., Francke U., Price P. A. Molecular structure, chromosome assignment, and promoter organization of the human matrix Gla protein gene. J Biol Chem. 1990 Sep 5;265(25):15040–15048. [PubMed] [Google Scholar]
- Engelhard D., Cohen D., Strauss N., Sacks T. G., Jorczak-Sarni L., Shapiro M. Randomised study of myringotomy, amoxycillin/clavulanate, or both for acute otitis media in infants. Lancet. 1989 Jul 15;2(8655):141–143. doi: 10.1016/s0140-6736(89)90192-x. [DOI] [PubMed] [Google Scholar]
- Fraser J. D., Price P. A. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J Biol Chem. 1988 Aug 15;263(23):11033–11036. [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale J. E., Williamson M. K., Price P. A. Carboxyl-terminal proteolytic processing of matrix Gla protein. J Biol Chem. 1991 Nov 5;266(31):21145–21149. [PubMed] [Google Scholar]
- Hirsch D., Azoury R., Sarig S., Kruth H. S. Colocalization of cholesterol and hydroxyapatite in human atherosclerotic lesions. Calcif Tissue Int. 1993 Feb;52(2):94–98. doi: 10.1007/BF00308315. [DOI] [PubMed] [Google Scholar]
- Hirsch D., Landis W. J., Azoury R., Sarig S. Liposome interactions with hydroxyapatite crystals: a possible mechanism in the calcification of atherosclerotic plaques. Calcif Tissue Int. 1992 Mar;50(3):261–265. doi: 10.1007/BF00296291. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hunter G. K., Goldberg H. A. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. M. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc. 1976 Feb;35(2):156–162. [PubMed] [Google Scholar]
- Kohri K., Suzuki Y., Yoshida K., Yamamoto K., Amasaki N., Yamate T., Umekawa T., Iguchi M., Sinohara H., Kurita T. Molecular cloning and sequencing of cDNA encoding urinary stone protein, which is identical to osteopontin. Biochem Biophys Res Commun. 1992 Apr 30;184(2):859–864. doi: 10.1016/0006-291x(92)90669-c. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Lian J. B., Gallop P. Atherocalcin, a gamma-carboxyglutamic acid containing protein from atherosclerotic plaque. Biochem Biophys Res Commun. 1979 Nov 14;91(1):41–49. doi: 10.1016/0006-291x(79)90580-1. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Prien E. L., Jr, Glimcher M. J., Gallop P. M. The presence of protein-bound gamma-carboxyglutamic acid in calcium-containing renal calculi. J Clin Invest. 1977 Jun;59(6):1151–1157. doi: 10.1172/JCI108739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loecker T. H., Schwartz R. S., Cotta C. W., Hickman J. R., Jr Fluoroscopic coronary artery calcification and associated coronary disease in asymptomatic young men. J Am Coll Cardiol. 1992 May;19(6):1167–1172. doi: 10.1016/0735-1097(92)90319-i. [DOI] [PubMed] [Google Scholar]
- Loeser R. F., Wallin R. Cell adhesion to matrix Gla protein and its inhibition by an Arg-Gly-Asp-containing peptide. J Biol Chem. 1992 May 15;267(14):9459–9462. [PubMed] [Google Scholar]
- Loeser R., Carlson C. S., Tulli H., Jerome W. G., Miller L., Wallin R. Articular-cartilage matrix gamma-carboxyglutamic acid-containing protein. Characterization and immunolocalization. Biochem J. 1992 Feb 15;282(Pt 1):1–6. doi: 10.1042/bj2820001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990 Aug 25;265(24):14432–14438. [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Wang Z. L., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985 Nov;53(5):556–562. [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstone J. R., Weber M., Lees-Miller J. P., Carpenter M. R., Smillie L. B. Amino acid sequence of chicken gizzard smooth muscle SM22 alpha. J Biol Chem. 1987 May 5;262(13):5985–5991. [PubMed] [Google Scholar]
- Price P. A., Fraser J. D., Metz-Virca G. Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8335–8339. doi: 10.1073/pnas.84.23.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Lane T. F., Iruela-Arispe M. L., Ross R., Sage E. H. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani P., Bradley N. J., Fletcher C. D. QBEND/10, a new monoclonal antibody to endothelium: assessment of its diagnostic utility in paraffin sections. Histopathology. 1990 Sep;17(3):237–242. doi: 10.1111/j.1365-2559.1990.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Shanahan C. M., Weissberg P. L., Metcalfe J. C. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993 Jul;73(1):193–204. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- Shiraga H., Min W., VanDusen W. J., Clayman M. D., Miner D., Terrell C. H., Sherbotie J. R., Foreman J. W., Przysiecki C., Neilson E. G. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., DeVouge M. W., Mukherjee B. B. Physiological properties and differential glycosylation of phosphorylated and nonphosphorylated forms of osteopontin secreted by normal rat kidney cells. J Biol Chem. 1990 Oct 25;265(30):18696–18701. [PubMed] [Google Scholar]
- Takahashi K., Nadal-Ginard B. Molecular cloning and sequence analysis of smooth muscle calponin. J Biol Chem. 1991 Jul 15;266(20):13284–13288. [PubMed] [Google Scholar]
- Tanimura A., McGregor D. H., Anderson H. C. Matrix vesicles in atherosclerotic calcification. Proc Soc Exp Biol Med. 1983 Feb;172(2):173–177. doi: 10.3181/00379727-172-41542. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Toda T., Leszczynski D. E., Kummerow F. A. The role of 25-hydroxy-vitamin D3 in the induction of atherosclerosis in swine and rabbit by hypervitaminosis D. Acta Pathol Jpn. 1983 Jan;33(1):37–44. doi: 10.1111/j.1440-1827.1983.tb02098.x. [DOI] [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- Young M. F., Kerr J. M., Termine J. D., Wewer U. M., Wang M. G., McBride O. W., Fisher L. W. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics. 1990 Aug;7(4):491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]