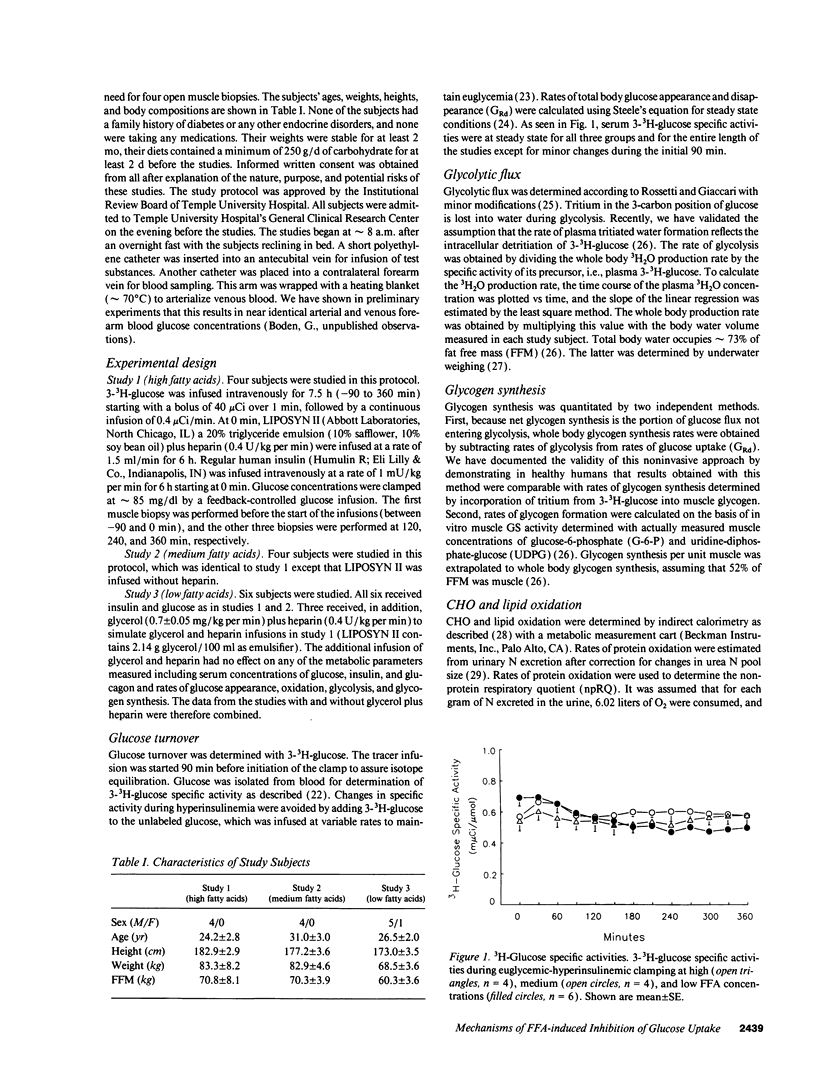
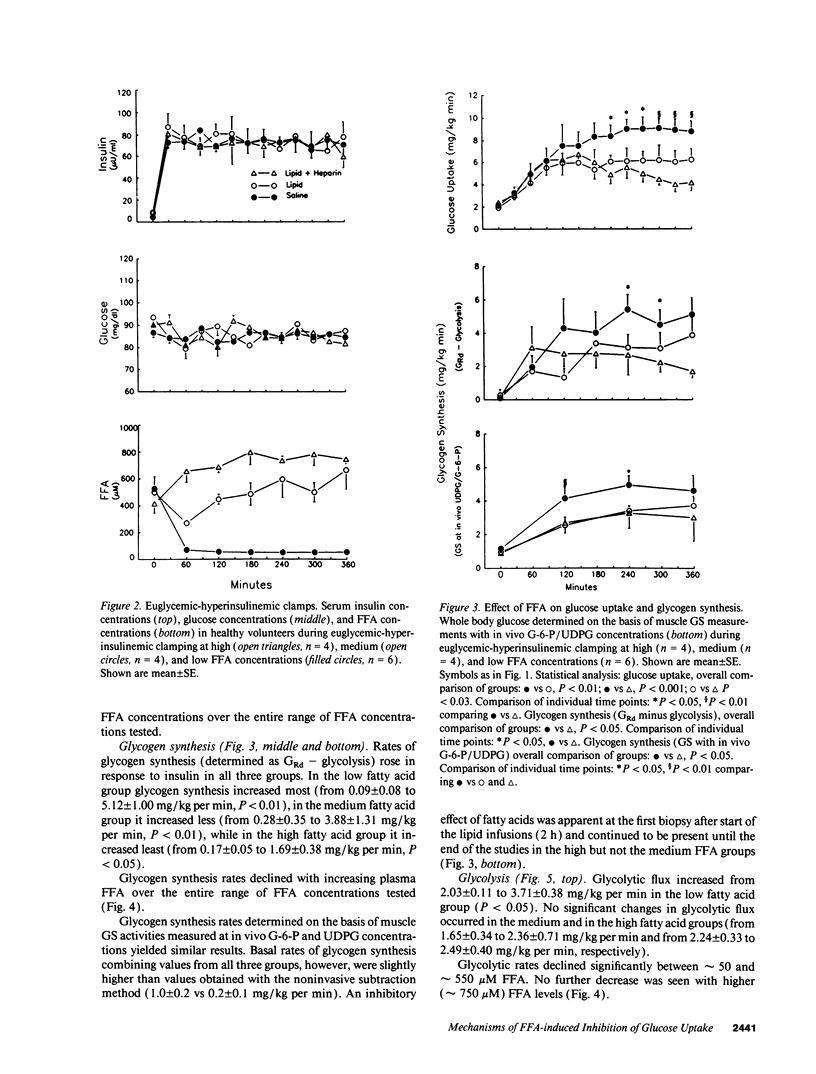
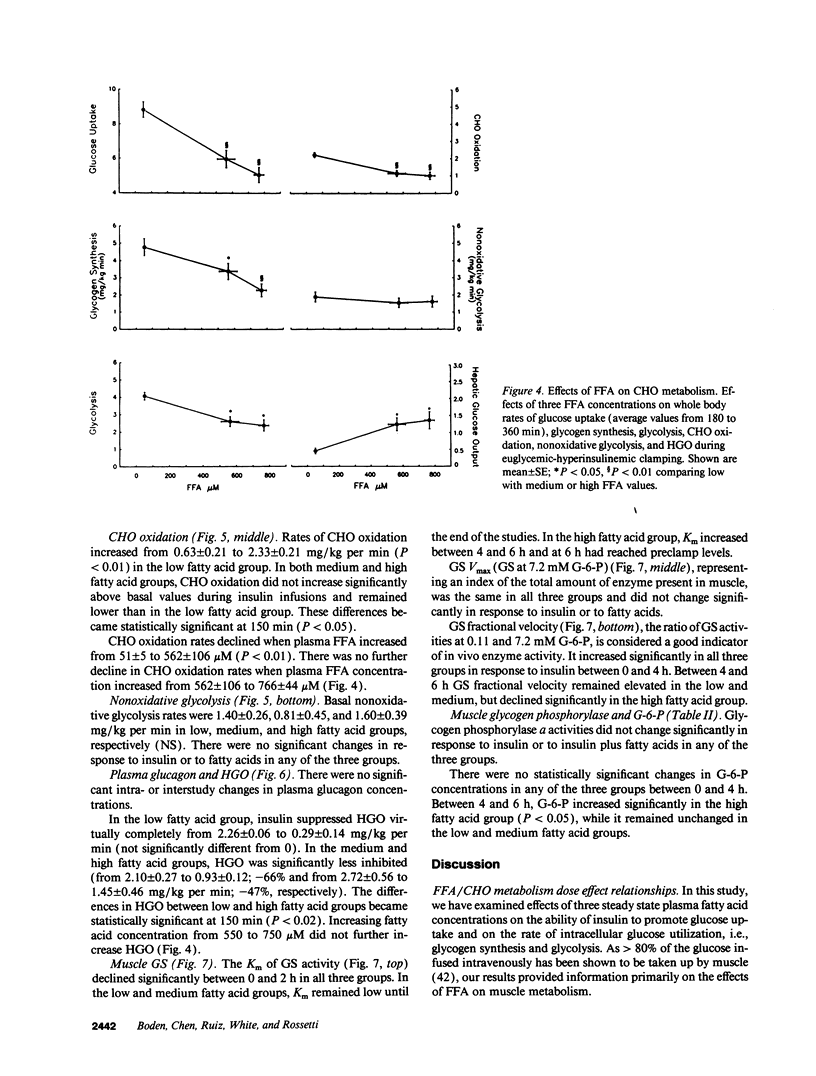
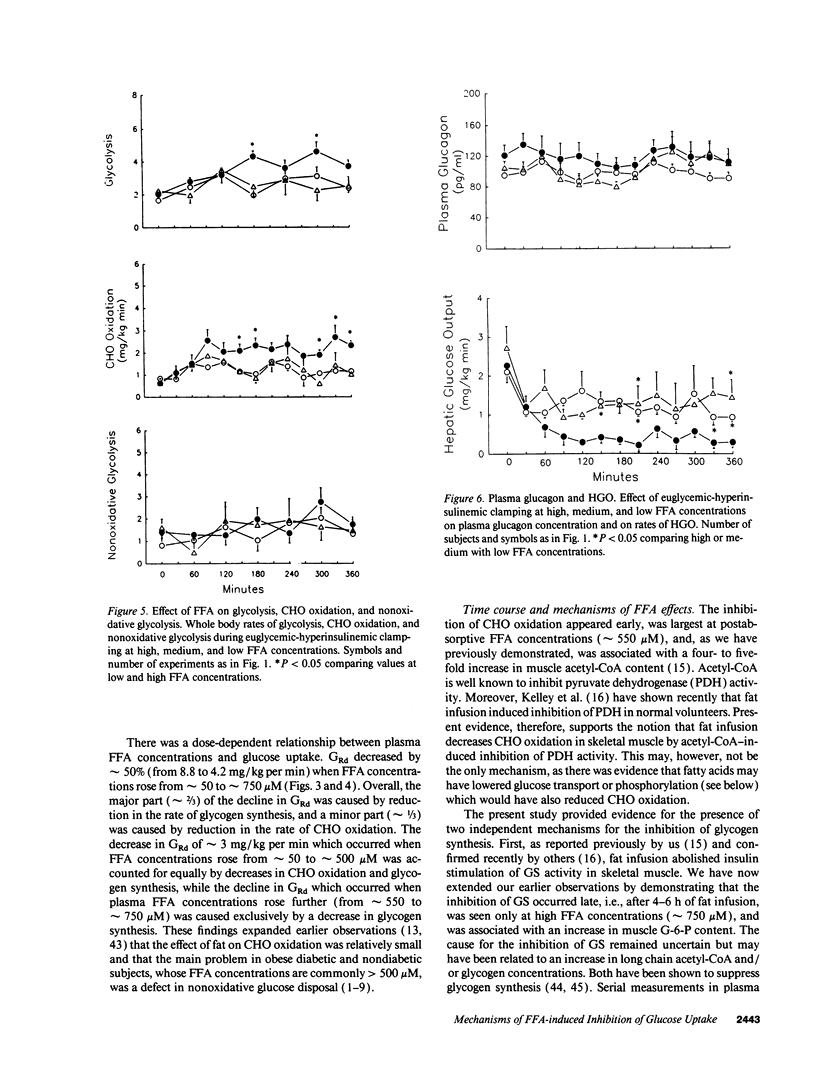
Abstract
Increased plasma FFA reduce insulin-stimulated glucose uptake. The mechanisms responsible for this inhibition, however, remain uncertain. It was the aim of this study to determine whether the FFA effect was dose dependent and to investigate its mechanism. We have examined in healthy volunteers (13 male/1 female) the effects of three steady state plasma FFA levels (approximately 50, approximately 550, approximately 750 microM) on rates of glucose uptake, glycolysis (both with 3-3H-glucose), glycogen synthesis (determined with two independent methods), carbohydrate (CHO) oxidation (by indirect calorimetry), hepatic glucose output, and nonoxidative glycolysis (glycolysis minus CHO oxidation) during euglycemic-hyperinsulinemic clamping. Increasing FFA concentration (from approximately 50 to approximately 750 microM) decreased glucose uptake in a dose-dependent fashion (from approximately 9 to approximately 4 mg/kg per min). The decrease was caused mainly (approximately 2/3) by a reduction in glycogen synthesis and to a lesser extent (approximately 1/3) by a reduction in CHO oxidation. We have identified two independent defects in glycogen synthesis. The first consisted of an impairment of muscle glycogen synthase activity. It required high FFA concentration (approximately 750 microM), was associated with an increase in glucose-6-phosphate, and developed after 4-6 h of fat infusion. The second defect, which preceded the glycogen synthase defect, was seen at medium (approximately 550 microM) FFA concentration, was associated with a decrease in muscle glucose-6-phosphate concentration, and was probably due to a reduction in glucose transport/phosphorylation. In addition, FFA and/or glycerol increased insulin-suppressed hepatic glucose output by approximately 50%. We concluded that fatty acids caused a dose-dependent inhibition of insulin-stimulated glucose uptake (by decreasing glycogen synthesis and CHO oxidation) and that FFA and/or glycerol increased insulin-suppressed hepatic glucose output and thus caused insulin resistance at the peripheral and the hepatic level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua S., Bonadonna R., Buzzigoli G., Boni C., Ciociaro D., Maccari F., Giorico M. A., Ferrannini E. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987 May;36(5):502–506. doi: 10.1016/0026-0495(87)90051-5. [DOI] [PubMed] [Google Scholar]
- Boden G., Ray T. K., Smith R. H., Owen O. E. Carbohydrate oxidation and storage in obese non-insulin-dependent diabetic patients. Effects of improving glycemic control. Diabetes. 1983 Nov;32(11):982–987. doi: 10.2337/diab.32.11.982. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjahan N., Gerich J. E., Mandarino L. J. Skeletal muscle is a major site of lactate uptake and release during hyperinsulinemia. Metabolism. 1992 Feb;41(2):176–179. doi: 10.1016/0026-0495(92)90148-4. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990 Dec;86(6):2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H. GLYCOGEN SYNTHETASE ACTIVITY IN SKELETAL MUSCLE. INTERCONVERSION OF TWO FORMS AND CONTROL OF GLYCOGEN SYNTHESIS. J Biol Chem. 1965 Feb;240:588–593. [PubMed] [Google Scholar]
- Damsbo P., Vaag A., Hother-Nielsen O., Beck-Nielsen H. Reduced glycogen synthase activity in skeletal muscle from obese patients with and without type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991 Apr;34(4):239–245. doi: 10.1007/BF00405082. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widén E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989 Aug 10;321(6):337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Fanelli C., Calderone S., Epifano L., De Vincenzo A., Modarelli F., Pampanelli S., Perriello G., De Feo P., Brunetti P., Gerich J. E. Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest. 1993 Oct;92(4):1617–1622. doi: 10.1172/JCI116746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., Shank M., Petrides A. S., DeFronzo R. A. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest. 1991 Jan;87(1):83–89. doi: 10.1172/JCI115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli G., Ferrannini E., Stern M., Haffner S., DeFronzo R. A. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes. 1992 Dec;41(12):1575–1586. doi: 10.2337/diab.41.12.1575. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Ladenson J. H., Henriksen E. J., Holloszy J. O., McDonald J. M. Palmitate stimulates glucose transport in rat adipocytes by a mechanism involving translocation of the insulin sensitive glucose transporter (GLUT4). Biochem Biophys Res Commun. 1991 May 31;177(1):343–349. doi: 10.1016/0006-291x(91)91989-p. [DOI] [PubMed] [Google Scholar]
- Johnson A. B., Argyraki M., Thow J. C., Broughton D., Jones I. R., Taylor R. Effects of intensive dietary treatment on insulin-stimulated skeletal muscle glycogen synthase activation and insulin secretion in newly presenting type 2 diabetic patients. Diabet Med. 1990 Jun;7(5):420–428. doi: 10.1111/j.1464-5491.1990.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Mandarino L. J. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990 Dec;86(6):1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Mokan M., Simoneau J. A., Mandarino L. J. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993 Jul;92(1):91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan R. G., Lamb D. R., Lutz S. A., Perrill C. V., Reimann E. M., Schlender K. K. Glycogen synthase activation in human skeletal muscle: effects of diet and exercise. Am J Physiol. 1979 Jun;236(6):E660–E666. doi: 10.1152/ajpendo.1979.236.6.E660. [DOI] [PubMed] [Google Scholar]
- Kolaczynski J. W., Boden G. Effects of oleate and fatty acids from omental adipocytes on insulin uptake in rat liver cells. Endocrinology. 1993 Dec;133(6):2871–2874. doi: 10.1210/endo.133.6.8243313. [DOI] [PubMed] [Google Scholar]
- MARSH W. H., FINGERHUT B., MILLER H. AUTOMATED AND MANUAL DIRECT METHODS FOR THE DETERMINATION OF BLOOD UREA. Clin Chem. 1965 Jun;11:624–627. [PubMed] [Google Scholar]
- McGarry J. D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992 Oct 30;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Baron A. D., Edelman S. V., Brechtel G., Wallace P., Olefsky J. M. Use of a variable tracer infusion method to determine glucose turnover in humans. Am J Physiol. 1990 Jan;258(1 Pt 1):E16–E23. doi: 10.1152/ajpendo.1990.258.1.E16. [DOI] [PubMed] [Google Scholar]
- Natali A., Buzzigoli G., Taddei S., Santoro D., Cerri M., Pedrinelli R., Ferrannini E. Effects of insulin on hemodynamics and metabolism in human forearm. Diabetes. 1990 Apr;39(4):490–500. doi: 10.2337/diab.39.4.490. [DOI] [PubMed] [Google Scholar]
- Nurjhan N., Campbell P. J., Kennedy F. P., Miles J. M., Gerich J. E. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986 Dec;35(12):1326–1331. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Trapp V. E., Reichard G. A., Jr, Mozzoli M. A., Smith R., Boden G. Effects of therapy on the nature and quantity of fuels oxidized during diabetic ketoacidosis. Diabetes. 1980 May;29(5):365–372. doi: 10.2337/diab.29.5.365. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Newsholme E. A., Hales C. N. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann N Y Acad Sci. 1965 Oct 8;131(1):324–333. doi: 10.1111/j.1749-6632.1965.tb34800.x. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Hollenbeck C., Jeng C. Y., Wu M. S., Chen Y. D. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988 Aug;37(8):1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Hansen S. A., Hansen B. F. Mechanisms limiting glycogen storage in muscle during prolonged insulin stimulation. Am J Physiol. 1988 Nov;255(5 Pt 1):E621–E628. doi: 10.1152/ajpendo.1988.255.5.E621. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Farrace S., Choi S. B., Giaccari A., Sloan L., Frontoni S., Katz M. S. Multiple metabolic effects of CGRP in conscious rats: role of glycogen synthase and phosphorylase. Am J Physiol. 1993 Jan;264(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1993.264.1.E1. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990 Jun;85(6):1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lee Y. T., Ruiz J., Aldridge S. C., Shamoon H., Boden G. Quantitation of glycolysis and skeletal muscle glycogen synthesis in humans. Am J Physiol. 1993 Nov;265(5 Pt 1):E761–E769. doi: 10.1152/ajpendo.1993.265.5.E761. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Saloranta C., Koivisto V., Widén E., Falholt K., DeFronzo R. A., Härkönen M., Groop L. Contribution of muscle and liver to glucose-fatty acid cycle in humans. Am J Physiol. 1993 Apr;264(4 Pt 1):E599–E605. doi: 10.1152/ajpendo.1993.264.4.E599. [DOI] [PubMed] [Google Scholar]
- Shimoyama R., Ray T. K., Savage C. R., Jr, Owen O. E., Boden G. In vivo and in vitro effects of antiinsulin receptor antibodies. J Clin Endocrinol Metab. 1984 Nov;59(5):916–923. doi: 10.1210/jcem-59-5-916. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Tappy L., Owen O. E., Boden G. Effect of hyperinsulinemia on urea pool size and substrate oxidation rates. Diabetes. 1988 Sep;37(9):1212–1216. doi: 10.2337/diab.37.9.1212. [DOI] [PubMed] [Google Scholar]
- Thiébaud D., DeFronzo R. A., Jacot E., Golay A., Acheson K., Maeder E., Jéquier E., Felber J. P. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Brechtel G., Henry R. R. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. J Clin Invest. 1991 Feb;87(2):489–495. doi: 10.1172/JCI115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaag A., Henriksen J. E., Beck-Nielsen H. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Mar;89(3):782–788. doi: 10.1172/JCI115656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard H., Bjørbaek C., Andersen P. H., Bak J. F., Pedersen O. Impaired expression of glycogen synthase mRNA in skeletal muscle of NIDDM patients. Diabetes. 1991 Dec;40(12):1740–1745. doi: 10.2337/diab.40.12.1740. [DOI] [PubMed] [Google Scholar]
- Wells A. M., Sutcliffe I. C., Johnson A. B., Taylor R. Abnormal activation of glycogen synthesis in fibroblasts from NIDDM subjects. Evidence for an abnormality specific to glucose metabolism. Diabetes. 1993 Apr;42(4):583–589. doi: 10.2337/diab.42.4.583. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wititsuwannakul D., Kim K. H. Mechanism of palmityl coenzyme A inhibition of liver glycogen synthase. J Biol Chem. 1977 Nov 10;252(21):7812–7817. [PubMed] [Google Scholar]
- Wolfe B. M., Klein S., Peters E. J., Schmidt B. F., Wolfe R. R. Effect of elevated free fatty acids on glucose oxidation in normal humans. Metabolism. 1988 Apr;37(4):323–329. doi: 10.1016/0026-0495(88)90131-x. [DOI] [PubMed] [Google Scholar]


