Abstract
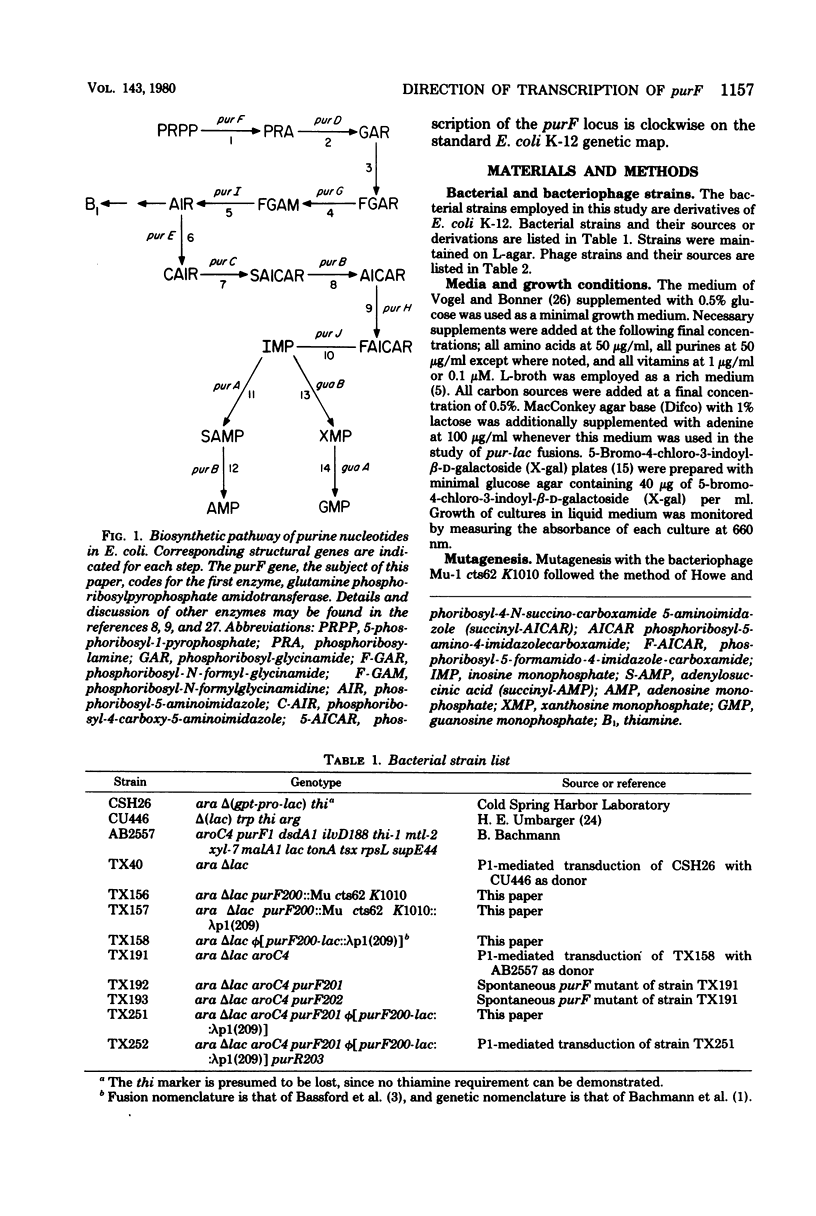
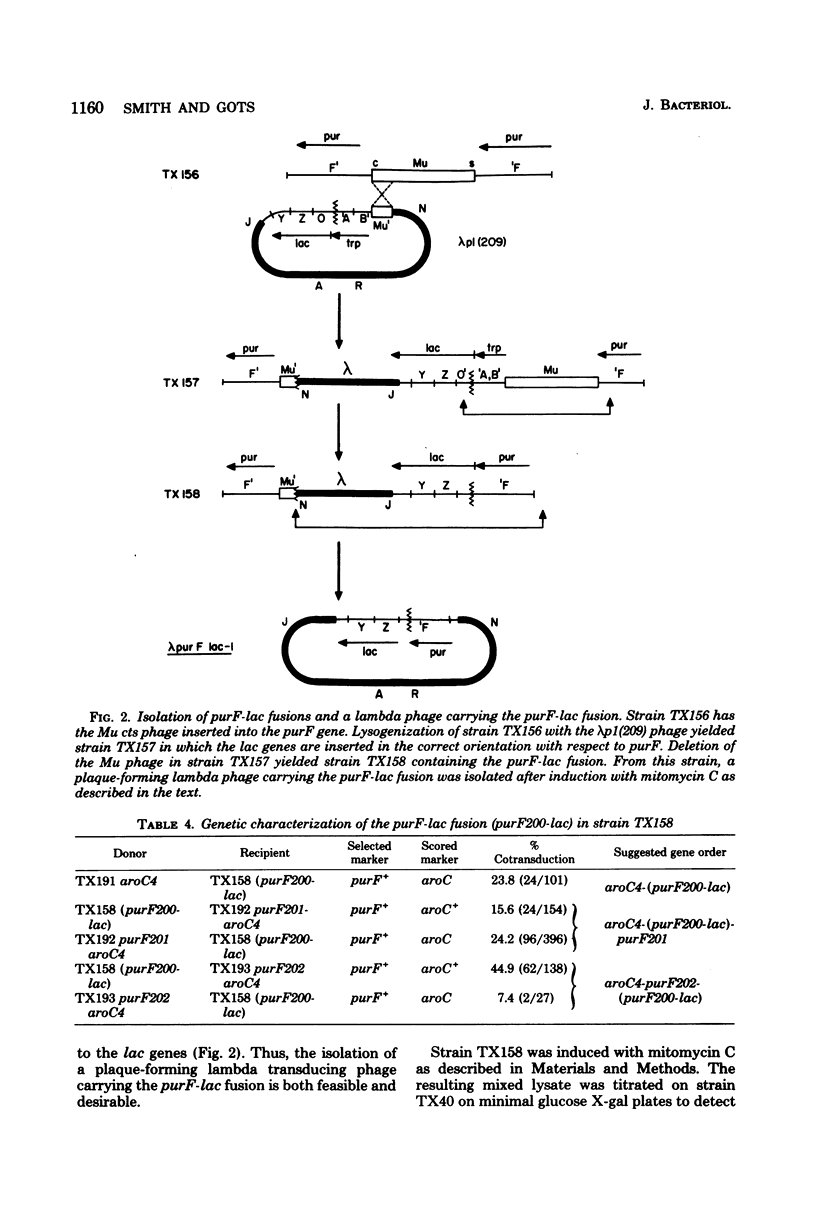
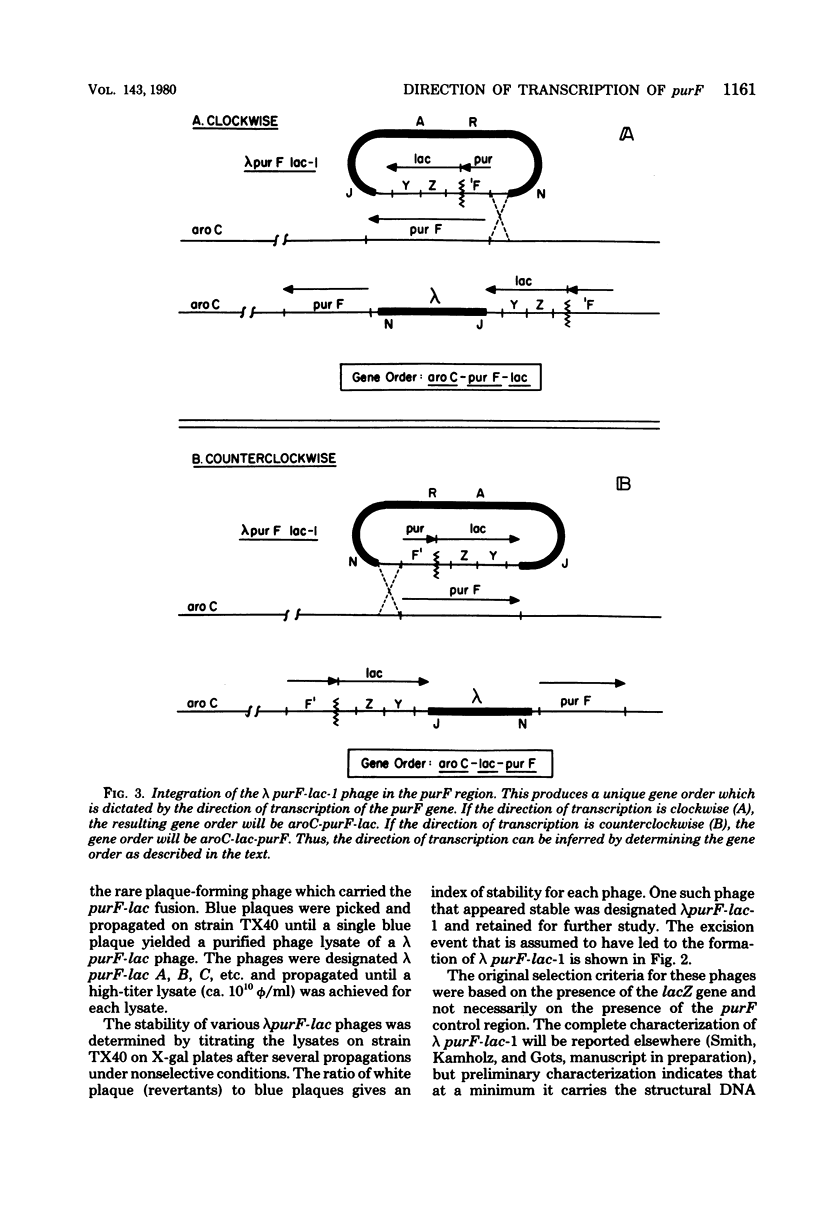
The purF locus codes for the first enzyme, glutamine phosphoribosylpyrophosphate amidotransferase, of the purine biosynthetic pathway. A strain of Escherichia coli K-12 was isolated in which the lac structural genes were fused to the control region of the purF locus. This purF-lac fusion was shown to respond to purine-specific regulatory signals. A plaque-forming lambda transducing phage bearing this purF-lac fusion was isolated. This phage was used to genetically determine the direction of transcription for the pufF locus by two independent means. Results from both methods agreed that the direction of transcription of the purF locus was clockwise on the standard Escherichia coli K-12 genetic map.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson C. E., Gots J. S. Occurrence of a regulatory deficiency in purine biosynthesis among pur A mutants of Salmonella typhimurium. Mol Gen Genet. 1976 Apr 23;145(1):31–36. doi: 10.1007/BF00331554. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. Use of argA-lac fusions to generate lambda argA-lac bacteriophages and to determine the direction of argA transcription in Escherichia coli. J Bacteriol. 1977 Oct;132(1):60–66. doi: 10.1128/jb.132.1.60-66.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Jochimsen B., Koduri K. R. Microbial models and regulatory elements in the control of purine metabolism. Ciba Found Symp. 1977;(48):23–41. doi: 10.1002/9780470720301.ch3. [DOI] [PubMed] [Google Scholar]
- Howe M. M., Bade E. G. Molecular biology of bacteriophage mu. Science. 1975 Nov 14;190(4215):624–632. doi: 10.1126/science.1103291. [DOI] [PubMed] [Google Scholar]
- Howe M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology. 1973 Jul;54(1):93–101. doi: 10.1016/0042-6822(73)90118-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger L. J., Zalkin H. Glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Purification and properties. J Biol Chem. 1979 May 10;254(9):3382–3392. [PubMed] [Google Scholar]
- NIERLICH D. P., MAGASANIK B. REGULATION OF PURINE RIBONUCLEOTIDE SYNTHESIS BY END PRODUCT INHIBITION. THE EFFECT OF ADENINE AND GUANINE RIBONUCLEOTIDES ON THE 5'-PHOSPHORIBOSYL-PYROPHOSPHATE AMIDOTRANSFERASE OF AEROBACTER AEROGENES. J Biol Chem. 1965 Jan;240:358–365. [PubMed] [Google Scholar]
- Ohnishi S. T., Barr J. K. A simplified method of quantitating protein using the biuret and phenol reagents. Anal Biochem. 1978 May;86(1):193–200. doi: 10.1016/0003-2697(78)90334-2. [DOI] [PubMed] [Google Scholar]
- Parker J., Fishman S. E. Mapping hisS, the structural gene for histidyl-transfer ribonucleic acid synthetase, in Escherichia coli. J Bacteriol. 1979 Apr;138(1):264–267. doi: 10.1128/jb.138.1.264-267.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Margarita D. Fine structure analysis of the threonine operon in Escherichia coli K-12. Mol Gen Genet. 1978 Jun 1;162(1):101–107. doi: 10.1007/BF00333856. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Smith F. J., Umbarger H. E. Mutations affecting the formation of acetohydroxy acid synthase II in Escherichia coli K-12. Mol Gen Genet. 1979 Feb 1;169(3):299–314. doi: 10.1007/BF00382276. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Smolin D. E., Umbarger H. E. Polarity and the regulation of the ilv gene cluster in Escherichia coli strain K-12. Mol Gen Genet. 1976 Oct 18;148(2):111–124. doi: 10.1007/BF00268374. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vales L. D., Chase J. W., Murphy J. B. Orientation of the guanine operon of Escherichia coli K-12 by utilizing strains containing guaB-xse and guaB-upp deletions. J Bacteriol. 1979 Jul;139(1):320–322. doi: 10.1128/jb.139.1.320-322.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby C. A., Gots J. S. Genetic blocks and unique features in the biosynthesis of 5'-phosphoribosyl-N-formylglycinamide in Salmonella typhimurium. J Biol Chem. 1969 Apr 25;244(8):2095–2102. [PubMed] [Google Scholar]


